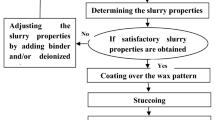
Currently, the use of ceramic shells for casting metal melts is a common practice in most machine-building enterprises. The primary method of producing ceramic molds involves investment casting, followed by the removal of the pattern material. The formation of ceramics from a ceramic suspension via a repeated application method necessitates the use of processing additives with a number of physico-chemical properties that ensure the quality of the fabricated shells. Processing additives based on water are characterized by a rather complex composition, including several organic compounds. This results in their compatibility issues and a reduction in the long-term performance under continuous mixing of the ceramic suspension susceptible to sedimentation. Furthermore, the composition of the suspension varies due to uneven removal of components during operation. It is evident that in order to develop an efficient processing additive, it is necessary to carry out an in-depth study of the physico-chemical properties of the components and their combined aqueous solutions, which would allow the selection of components and their concentration to be optimized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fabrication of ceramic molds for the casting of high-temperature alloys involves the formation of ceramic shells through the repeated application of an aqueous suspension of high-temperature fine-grained metal oxide materials. This process is followed by the intermediate drying of the layers, which are surfaced with coarse-grain powders. The number of layers reaches 10 – 12, with the first two or three layers being responsible for accurately reproducing the shape of the future casting within the allowed deviations of size dimensions. The subsequent layers typically provide strength to the mold, ensuring its preservation during metal casting and crystallization [1, 2]. The strength of the resulting ceramic product depends largely on the composition of the ceramic suspension, which includes aqueous colloidal processing additives. A commonly used component that is introduced to increase the strength of the products is aluminum powder, in particular of the ASD-4 type [3, 4]. It is important to note that the introduction of this component into the composition of an aqueous ceramic suspension results in the formation of explosive hydrogenas a result of the interaction of aluminum with water. Therefore, the development of a method to prevent this undesirable reaction is of importance.
Given the diverse functions of the shell layers and the type of casting, such as equiaxed casting or directed crystallization casting, the compositions of ceramic powders and aqueous colloidal processing additives used at different stages of mold manufacturing are typically distinct [1,2,3,4,5,6].
Processing additives are highly concentrated colloidal solutions of silica or alumina sol, characterized by the required size and sufficiently high value of the zeta potential of the micelles [7,8,9], as well as the stability of the potential over a longtime interval. The studies showed that the zeta potential of silica sols manufactured by Kompas LLC, Kazan, has remained at a high level for over a year. The composition of aqueous processing additives includes various organic components such as surfactants to ensure the wettability of the wax pattern, antifoaming agents to prevent foaming during continuous stirring of the suspension, which is characterized by sedimentation instability, binders (adhesives) to provide the primary strength of the green shell, bactericides, and other components [10, 11]. Since the first three components are of key importance in the development of the processing additive composition, they can be selected based on the study of the physico-chemical properties of their aqueous solutions.
Mutual compatibility of introduced organic additives, which can be evaluated experimentally, is essential. One method is the calorimetric measurement of the heat of mixing of components. Since the enthalpy of mixing (∆Hm) is one of the variables of the Gibbs free energy in the process of mutual dissolution of components, its positive value can exceed the configurational entropy component of their mixing, resulting in a positive change in the Gibbs free energy [12,13,14,15]. In this case, the system may become unstable, potentially leading to the segregation of individual phases.
The chemical stability of fine ASD-4 aluminum powder in aqueous surfactant solutions is of paramount importance in the development of a processing additive for reinforced ceramics. This stability can be evaluated both using calorimetry and directly by studying the gas emission.
Prior to the actual development of the additive composition, the scope of physico-chemical studies involves the selection of an optimal foaming agent, including its optimal concentration, and a binder (adhesive) that provides sufficient strength of the green ceramic shell to ensure precise reproduction of the shape of a product. It is important to note that the mutual influence of organic components on the physico-chemical properties of their aqueous solutions is frequently nonlinear and non-additive, and on occasion, synergistic. This necessitates the use of specific methodologies in the development of processing additives.
Results
Calorimetric studies
The heat of mixing was determined using an adiabatic calorimeter (Fig. 1) [16].
Scheme of calorimetric system: 1) syringe; 2) tube; 3) air outlet tube; 4) test tube for supplying a solution for mixing; 5) test tube for mixing two solutions; 6) sealed test tube with one of the differential thermocouple junctions; 7) isothermal shell; T, ∆T) outputs of temperature and temperature difference signals of a thermocouple to the corresponding devices.
The calorimetric experiment is divided into three stages:
– 1 stage — a uniform variation in temperature difference ∆T between test tubes 5 and 6 over time is established and recorded, resulting from steady-state heat exchange with shell 7 and secondary thermal processes within the calorimeter (temperature drift);
– 2 stage — mixing of solutions. Upon pressing syringe 1, the solution from test tube 4 is forced by air through tube 2 into test tube 5. Consequently, the differential thermocouple reacts to the release/absorption of heat of mixing, while any deviation from the temperature baseline established during the initial “free run” stage is recorded on the scale of the M17/2 string galvanometer. The sensitivity of the system was 0.002°C per scale division (5 mm) of the galvanometer;
– 3 stage — upon the completion of the studied dissolution process and release of the heat of mixing, the temperature drift of the calorimeter returns to uniformity. The main criterion for the energy of interaction of the components, the height of a single temperature pulse recorded relative to the baseline is proportional to the heat of mixing (Table 1). It is important to note that the heat of mixing and the enthalpy of mixing are characterized by opposite signs.
The obtained results indicate that in most cases the enthalpy of mixing is positive, which suggests the potential for segregation of the mixture when the permissible concentration limits of the components are exceeded. In two cases, the enthalpy of mixing exhibits a significant negative value. This indicates the chemical reaction that occurs during the interaction of the components, which can have adverse effects during the development of processing additive formulations. Although these results are qualitative rather than quantitative, they provide useful information on the properties of mixtures.
Significantly different are the data on the interaction of ASD-4 powder with aqueous surfactant solutions. Figure 2 shows the test results of the interaction of aqueous solutions of three surfactants with aluminum powder [17].
Figure 2 illustrates that the chemical interaction of aluminum with water persists for 25 min when alkyl polyglucoside (APG) and fatty alcohol sulfoethoxylate surfactants are used, while for synthanol AE-7 surfactants, the reaction is terminated following 15 min of the experiment. In the authors’ opinion, the molecules of synthanol AE-7 adsorbed on the particles of ASD-4 powder prevent the access of water molecules to the aluminum surface, which results in the termination or retardation of the reaction between them. In addition, these findings were confirmed by the direct investigation of gas emission in sealed cells with a gas outlet tube, as evidenced by the data presented in Table 2.
Selection of antifoaming agent
As mentioned earlier, the antifoaming agent is used to eliminate a side effect resulting from the use of surfactant wetting agents. This is achieved by ensuring the rapid degradation of foam on the surface of the agitated ceramic slurry. This is necessary to prevent the trapping of foam bubbles on the surface of the forming ceramic shell, which is especially relevant for the first face layer of ceramics on the surface of the wax pattern [18, 19]. The wetting agent and antifoaming agent should be thermodynamically compatible. Furthermore, it is essential to ensure that the concentration of the antifoaming agent be always in excess in order to prevent foaming at any concentration of the wetting agent. This is particularly important when considering the gradual removal of the wetting agent and other components from the suspension volume due to their adsorption on the surface of the ceramic powders introduced into the suspension during the production of a series of ceramic products.
Consequently, following the selection of the basic components, the optimal concentration of the antifoaming agent should be determined empirically, as it is no longer a matter of concentration selection.
Table 3 provides an example of selecting the concentration of the additional penta-475 antifoaming agent, used in combination with the basic antifoaming agent laprol 6003, in the development of the processing additive composition for the face layer.
Determination of a concentration of a binder (adhesive)
A fundamental requirement for this component is the ability to wet the powder material that forms the ceramic slurry. For each type of adhesive, it is necessary to determine the concentration range that would ensure the desired strength of the green ceramic product, taking into account the assumed additive components.
Development of Formulations for Processing Additives
One of the requirements for the processing additive is its performance at varying compositions within certain limits, since during the use of ceramic slurry for layer-by-layer formation of casting molds or other products, the components of the additive are removed from the volume unevenly. The second requisite is the precise reproduction of the pattern shape during the fabrication of the ceramic shell.
Processing additive composition for a face layer
The development of a processing additive composition for a face layer of ceramics without the reinforcing component ASD-4 is presented as an example. The development was carried out by a combined method that integrated elements of mathematical planning for a two-parameter experiment. The concentrations of a LABSA wetting agent and a modified KLEO starch binder (adhesive) were used as axes on the coordinate plane. The contact angle of the wax surface was selected as a response function as the most crucial and sensitive characteristic of the processing additive. The contact angle was measured using a KRUSS 100 tensiometer. A more detailed description of the measurement technique can be found elsewhere [22]. The experiment was carried out using five combinations of solution concentrations, as detailed in Table 4.
The step of concentration variation, determined by the sensitivity of the response function, should ensure that its alteration be achieved with a confidence probability exceeding 95%. The concentration of the Laprol 6003 antifoaming agent was 10% higher than the concentration of the wetting agent [23].
Each solution was examined twice: for a degreased wax pattern in a 10% Metalin solution and a non-degreased wax pattern pre-lubricated with a solution of castor oil in alcohol. In both scenarios, the technology in production was imitated. The results of the mathematical processing of data on contact angle are presented in Figs. 3 and 4.
The parameters of the empirical equations and the results of the mathematical processing of the experimental data are presented in Figs. 3 and 4. The resulting equations allow the contact angle to be calculated over the entire specified range of variation of the equation parameters.
The research findings indicated that the intervals for varying the concentrations of processing additive components were selected in an appropriate manner. This was evidenced by a sufficiently large shift in the contact angle when transitioning to different points in the experiment plan. From the graphical representation shown in Fig. 4, it can be seen that the lowest point of the theoretical curve is above the lowest possible values shown on the x and y axes, which indicates that the theoretical minimum of the response function is located below the plane of 10°. In this regard, another composition of the processing additive was investigated — LAKESIL® 40 + 200 ppm of LABSA + 220 ppm of Laprol 6003 + 20 ppm of KLEO — which was obtained by calculation according to the equation shown in Fig. 4. The direct experimental measurement confirmed that the contact angle for this composition is lower than the calculated value and is equal to zero.
Composition of a processing additive for subsequent ceramic layers containing ASD-4 reinforcing agent
The method for developing the processing additive composition was described in the previous example. Five solutions were used for the experiment: the concentration of the wetting agent synthanol AE-7 in the solution was varied in 90 ppm increments (20 – 200 ppm), the concentration of the PVA adhesive was varied in 50 ppm increments (50 – 150 ppm), the concentration of the antifoaming agent penta-475 was 50 ppm in all solutions. The experimental plan is presented in Table 5 and Fig. 5.
The measurement results of the contact angle for the two types of wax plates in solution No. 2 are shown in Figs. 6 and 7.
As the plate is immersed, the contact angle decreases to zero, indicating that the wax model is well-wetted by the solution in both cases (Figs. 6 and 7). The contact angle is zero over the entire immersion depth of the plate.
The results of this experiment demonstrate that the intervals for varying the concentrations of additives were selected in an appropriate manner. In addition, the solution with the lowest concentration (No. 2) exhibited the highest wettability of the wax pattern [24].
The research was carried out using the scientific equipment at the Center for Science-Intensive Chemical Technologies and Physical-Chemical Research of the Perm National Research Polytechnic University under the project of the Perm Research Center “Rational Subsurface Management (RFMEFI62120X0038).
References
K. V. Martynov, Ceramic Molds Based on a Silica Binder for Investment Casting, Author’s Abstract of Candidate’s Thesis [in Russian], St. Petersburg (2005).
E. N. Kablov, Cast Blades of Gas Turbine Engines (Alloys, Technology, and Coatings) [in Russian], MISiS, Moscow (2001).
S. I. Repyakh, Background Technology of Investment Casting [in Russian], Lira, Dnepropetrovsk (2006).
N. Y. Parshukova, “Use of additives to improve the properties of ceramic molds for investment casting,” Izv. Vissh. Uchebn. Zaved., Chern. Metall. [in Russian], No. 56(2), 69 – 70 (2013).
M. K. Romanov and L. I. Zhuravleva, “Analysis of the technological and economic feasibility of using additive technologies in the manufacture of ceramic parts,” Steklo Keram., 92(9), 9 – 16 (2019). [M. K. Romanovand L. I. Zhuravleva, “Analysis of the technological and economic feasibility of using additive technologies in the manufacture of ceramic parts,” Glass Ceram., 76(9 – 10), 328 – 333 (2020).]
E. Dean, M. Darnis, S. Nilsson, and A. Flodin, “Patent RF 2754261,” Byull. Izobr. Polezn. Modeli, No. 25 (2021).
L. G. Maksyutina, A. V. Shilov, V. L. Zvezdin, and A. S. Koryakovtsev, Investment Casting for Aerospace Purposes: Handbook [in Russian], PSTU, Perm (2005).
V. N. Ivanov, V. N. Volkov, S. A. Kazennov, et al., Investment Casting [in Russian], Mashinostroenie, Moscow (1984).
P. G. Kudryavtsev, “Obtaining inorganic composites and ceramics using colloidal suspensions,” Inzh. Vestn. Dona: Electronic Journal, No. 4 (2018). URL: http://www.ivdon.ru/ru/magazine/archive/n4y2018/5333
D. A. Ordin, V. L. Zvezdin, A. V. Shilov, V. Z. Poylov, and N. P. Uglev, “Patent RF 2670115, IPC 51 B 22 C 1/18 (2006.01), applicant and patent holder — JSC ODK–Aviadvigatel (RU),” Byull. Izobr. Polezn. Modeli, No. 29 (2018).
A. S. Maksyutin, N. A. Zotov, and N. S. Petelkina, “Patent RF 2446910, IPC 51 B 22 C 1/18, applicant and patent holder — STC Kompas LLC (RU),” Byull. Izobr. Polezn. Modeli, No. 10 (2012).
V. A. Kirillin, A. E. Sheindlin, and E. E. Shpilrain, Thermodynamics of Solutions [in Russian], Energiya, Moscow (1979).
A. A. Tager, “Thermodynamics of polymer mixing and thermodynamic stability of polymer compositions,” Vysokomol. Soed., 19(8), 1659 – 1669 (1977).
A. V. Kiryanov, Calorimetric Research Methods: Handbook for Advanced Training Program “Advanced Research Methods for New Materials in Electronics And Optoelectronics for Information and Telecommunication Systems,” [in Russian], Nizhny Novgorod (2007).
M. M. Popov, Thermometry and Calorimetry, Vol. 2 [in Russian], Izd. Mosk. Univ., Moscow (1954).
E. N. Novokreschennykh, V. Z. Poilov, N. P. Uglev, and D. A. Ordin, “Thermodynamic compatibility of components in water-colloidal binders,” Vestn. PNIPU, No. 3, 104 – 115 (2017).
E. R. Melnikova, E. N. Novokreschennykh, and N. P. Uglev, “Investigation of the chemical stability of aluminum in aqueous solutions of organic binder components,” Khim. Ekol. Urban., No. 2, 343 – 346 (2019).
I. R. Mukhamadeev, O. B. Demenok, A. A. Ganeev, et al., “Selection of water-based processing additives for investment casting of titanium alloys,” Vestn. YuUrGU, Ser. Metallurgiya, 15(3), 95 – 104 (2015).
V. S. Bessmertny, V. I. Stadnichuk, N. I. Bondarenko, et al., “Kinetics of oxidation of aluminum powder used in alumina-sillimanite ceramics,” Vestn. BGTU im. V. G. Shukhova, No. 1, 151 – 154 (2015).
K. M. Myrzina, E. N. Novokreshchennykh, D. A. Ordin, and N. P. Uglev, “Comparative study and selection of a wetting agent for aqueous colloidal processing additives,” Vestn. PNIPU, No. 4, 550 – 553 (2017).
N. P. Uglev, V. Z. Poilov, M. S. Dyakov, and E. N. Novokreshchennykh, “Development of an express method for determining the mechanical strength of cast ceramics,” Mezhd. Nauch.-Issled. Zh., No. 11(53), Pt. 4, November, 126 – 129 (2016).
E. N. Novokreschennykh, A. S. Gulyaeva, and N. P. Uglev, “Improving the quality of foundry ceramic molds by improving the wettability of wax models with a water based ceramic suspension,” Steklo Keram., 95(3), 32 – 37 (2022). [E. N. Novokreschennykh, A. S. Gulyaeva, and N. P. Uglev, “Improving the quality of foundry ceramic molds by improving the wettability of wax models with a water based ceramic suspension,” Glass Ceram., 79, 103 – 106 (2022).]
E. N. Novokrestennykh, K. M. Myrzina, D. A. Ordin, et al., “Research and selection of reagents in the development of compositions of aqueous colloidal processing additives for cast ceramics,” Mezhd. Nauch.-Issled. Zh., No. 10(64), Pt. 2, October, 14 – 18 (2017).
N. P. Uglev and E. N. Novokreshchennykh, “Patent RF 2792535, IPC B 22 C 9/04 (2023.01), patent holder — the Federal State Budgetary Educational Institution of the National Research University (RU),” Byull. Izobr. Polezn. Modeli, No. 9 (2023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 4, pp. 45 – 54, April, 2024.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Novokreshchennykh, E.N., Uglev, N.P. Development of Processing Additive Compositions for Ceramic Suspension Based on the Study of the Physico-Chemical Properties of the Components. Glass Ceram 81, 168–173 (2024). https://doi.org/10.1007/s10717-024-00677-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-024-00677-y