The article investigates the high-temperature interaction and sintering processes of samples of low-temperature firing high-alumina for technical ceramics based on the kaolin – alumina-containing component – dolomite ternary system and establishes the areas of formation of high-alumina body for technical ceramics on the ternary diagram. It is shown that as a result of high-temperature interaction, the formation of new crystalline phases of minerals occurs, including mullite, corundum, β-cristobalite, as well as in a small amount of anorthite and an amorphous glassy phase, which impart the necessary physical and mechanical properties to the fired sample. The area of optimal compositions for obtaining low-temperature sintering high-alumina ceramic bodies based on the kaolin – alumina-containing waste – dolomite ternary system was determined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is known [1,2,3] that many industries use grinding equipment with corresponding ceramic grinding bodies to produce substances characterized by fine and superfine milling. The advantages of grinding bodies made of high-alumina mixtures include high strength and low attrition value, resulting in minimal pollution of the crushed product during operation. Commonly used are high-alumina grinding bodies in the form of balls and cylinders.
According to the production technology, the main operational requirement for grinding bodies to obtain fine ceramic mixtures is their high wear resistance [4,5,6], which can be achieved by using the densely sintered material with significantly higher hardness and density compared to the materials being crushed.
In addition, the phase composition of high-aluminagrinding bodies should exhibit the maximum content of crys-talline phases of corundum and mullite minerals characterized by increased hardness. Therefore [7,8,9,10], to obtain adensely sintered corundum-mullite material of the required density and hardness from enriched kaolin and alumina waste without mineralizers and fluxing additives, it is necessary to apply a production temperature exceeding 1600°C.
In this context, the potential for reducing the temperature of synthesis and sintering of corundum-mullite material with the highest possible content of Al2O3was investigated. To achieve this, compositions containing a sintering additive of dolomite from the Dehkanabad deposit, comprising a natural, homogeneous carbonate rock with minimal impurities, were considered.
It is important to note that identifying the concentration range, in which the aforementioned aluminate and alumino-silicate minerals occur, allows the properties of the compositions based on the kaolin – alumina-containing waste – dolo-mite ternary mutual system to be generalized.
Materials and Methods
The starting materials for experimental studies included samples of enriched kaolin from the Alliansky deposit, dolomite from the Dehkanabad deposits, spent alumina-containing catalyst from the Shurtan Gas Chemical Complex (ShGCC) of the Republic of Uzbekistan, and concentration compositions based on the investigated ternary system of kaolin – alumina-containing waste – dolomite.
The material composition of the initial components and experimental mixtures was analyzed at the Accredited Analytical Laboratory “STROM” of the Institute of General and Inorganic Chemistry (IGIC), Academy of Sciences of the Republic of Uzbekistan. The mineralogical composition was determined by x-ray diffraction analysis (XRD) [11, 12]. Mineralogical analysis was carried out by the powder diffraction method using a Shimadzu x-ray diffractometer (Japan) with copper radiation. X-ray diffraction patterns were recorded at 0.05° intervals at the current mode and tube voltage of 30 mA and 40 kV, respectively. The total x-ray power was 2 kW. In order to identify mineral phases in the x-ray diffraction patterns and analyze the results, widely recognized reference books and international databases on x-ray diffraction analysis were consulted [13, 14].
In order to investigate the phase transformations occurring during the synthesis of corundum-mullite materials with the addition of dolomite, the conventional method of annealing and quenching samples was used, followed by phase identification using XRD.
The melting point of the prepared mixtures was determined visually for a formed cone by a conventional method using a platinum-platinum-rhodium thermocouple in tubular high-temperature furnaces in order to establish the newly formed crystalline phases of minerals following heat treatment. It should be noted that tests with temperatures above 1600°C were carried out using the flame of an acetylene-oxygen torch, which allows for testing at high temperatures (up to 1800°C). For this purpose, the experimental cones were mounted on a highly refractory corundum substrate and introduced into the combustion zone of an acetylene-oxygen flame. Temperature control was carried out using an optical pyrometer OPPIR-017E by the hot-wire method at an average heating rate of the test cone of 30 – 35 K/min within the temperature range of 1600 – 2000°C.
The melting point of the compositions was determined by the cone fusion method [15, 16]. In order to ensure accurate results, the synthesis of samples based on a ternary system was carried out using calcined raw materials.
Experimental
The study focused on a region within a ternary system, which consisted of compositions prepared from calcined samples of raw material components, including enriched kaolin from the Allians deposit, alumina waste from the Shurtan Gas Chemical Complex, and dolomite from the Dehkanabad deposit.
The initial raw materials were calcined in a silicon carbide furnace at 1300°C for one hour until the complete decomposition of their constituent minerals containing lattice water and carbonates. Subsequently, the calcination products were finely ground in anuralite ball mill. The resulting powders were then sieved through sieve No. 2 (mesh size of 0.2 mm). To prevent the hydration of the solid solution of calcined dolomite (calcium and magnesium oxides), the ground powder of the solid solution was placed in a desiccator filled with calcium chloride prior to preparing the charge mixtures.
Test ceramic mixtures based on a ternary system were prepared using the conventional method of ceramic technology. For this purpose, the charge components in the required ratios were crushed in a laboratory ball mill equipped with uralite balls. At this fineness of grinding, the residue on the 0.07 mm sieve was no more than 1%, with a moisture content of about 35%. Further, the obtained mixtures were dehydrated on filter cloth with a humidity of 19 – 22% to subsequently achieve a plastic state. The plastic mixture, molded into cylinders measuring 30 × 40 mm, was subjected to atmospheric drying followed by electric drying until it reached an air-dry state. The dried test samples were sintered at a firing temperature of 1400°C with a heating rate of 100°C every 10 min. The isothermal exposure lasted for 1.0 – 1.5 h. A total of 12 test samples, each weighing 50 g, were prepared. The basic physical and mechanical characteristics were determined for the fired test samples of ceramic mixtures.
Results and Discussion
As a result of the calcination process, adsorbed and lattice water were removed from the initial components of the system, particularly kaolin. The product obtained following calcination was sintered chamotte with a mineralogical composition including mullite, β-quartz, and a glass phase. Following the calcination of alumina-containing waste, x-ray diffraction analysis revealed the formation of aluminum oxide in the form of α-corundum (high-temperature form) and a small amount (less than 3 wt.%) of impurities consisting of silicates and aluminates of calcium and iron. During the calcination of dolomite, a solid solution based on calcium and magnesium oxides was obtained.
The material composition of calcined test samples of raw materials based on the kaolin – alumina-containing waste – dolomite ternary system is shown in Table 1.
For further research, test mixtures were prepared from the calcined powder samples. These compositions corresponded to several points in the ternary system consisting of Allians enriched kaolin, Dehkanabad dolomite, and alumina-containing waste from ShGCC, limited by the dolomite content of up to 20 wt.%.
The main characteristics of the samples of the raw materials used to develop the composition of the ceramic mixtures are shown in Table 2.
It is important to note that during the development and use of these materials, fundamental scientific principles and data on the composition and structure of the materials were taken into account in order to achieve the desired technological results. The results of the chemical analysis demonstrate that the content of aluminum oxide in kaolins is insufficient to obtain high-alumina mixtures. In order to increase the amount of Al2O3, alumina-containing waste from gas processing was introduced into the charge to obtain the mullite and mullite-corundum ceramic body.
The formulations were developed on the basis of data on phase equilibria and crystallization paths of phases in the high-alumina region of the (CaO, MgO)–Al2O3–SiO2 systems. The region of the studied compositions based on the kaolin – alumina-containing waste – dolomite ternary system is shown in Fig. 1. As can be seen from the ternary diagram, the optimal compositions of high-alumina mixtures lie near the alumina region within the triangle, where its content is maximal. It is important to note that the criteria included the achievement of high density following sintering and other physical and mechanical parameters of the fired ceramic body with the highest possible content of aluminum oxide at technologically acceptable firing temperatures of 1400°C.
Consequently, in order to develop the desired compositions of high-alumina ceramic materials for technical purposes, it is necessary to use enriched kaolins, alumina, or high-alumina rocks, as well as various mineralizers, as the main argillaceous materials.
To increase the amount of aluminum oxide in the ceramic mixture, an alumina-containing spent catalyst of the Shurtan gas chemical complex (ShGCC) was used as the main component. Prior to introducing the test mixtures into the charge, the spent catalyst was pre-fired at a temperature of 110 – 1200°C for 1 h to ensure the formation of gamma-aluminum oxide and the removal of lattice water from the amorphous structure of the initial alumina-containing catalyst.
Calcination at this temperature results in the formation of gamma-alumina. Further increase in the firing temperature to 1300°C leads to the formation of the alpha form of aluminum oxide, a promising corundum mineral that can be used in the production of various types of ceramic materials and technical products. The concentration and chemical compositions of samples based on the kaolin – alumina-containing waste – dolomite system are shown in Tables 3 and 4, respectively.
The chemical analysis of the test concentration compositions indicates that the content of the main components, in particular aluminum oxide and silicon oxide, is above 60.0 wt.% in all samples. As shown in the work [17], the content of aluminum oxide indicates that the test samples contain mullite and mullite-corundum in their mineralogical composition. However, in all samples of high-alumina mixtures, a slight difference in the content of alkaline earth oxides (MgO, CaO) and alkali oxides (Na2O, K2O), along with iron oxides Fe2O3, is noted.
The G. N. Maslennikova method was used to calculate the oxide contents in test concentration samples during the design of charge compositions for raw materials for high-alumina ceramic mixtures [18].
The calcined test concentration compositions, based on the kaolin – alumina-containing waste – dolomite ternary system, were subjected to physical-mechanical studies in order to determine the main technological parameters, following established procedures in ceramic materials technology.
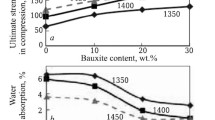
It should be noted that in order to improve the physical and mechanical properties and reduce the sintering temperature, 5 to 20 wt.% of dolomite from the Dehkanabad deposit was added to experimental samples containing kaolin and an alumina-containing component, resulting in the formation of an eutectic composition. An increase in its amount above this limit leads to an increase in the sintering temperature of ceramic mixtures due to the formation of calcium-magnesium solid solutions in the region of high concentration of dolomite. It can be noted that with such concentration compositions, the necessary sintering, high density, low porosity, and water absorption of the test samples are achieved. Molded samples of various concentration compositions based on the ternary system were fired at a temperature of 1400°C for 2 h.
Physical and mechanical properties of calcined samples of concentration compositions based on kaolin – alumina-containing waste – dolomite ternary system are shown in Table 5.
It is important to note that during the high-temperature solid-state reaction in this system, dolomite functions as a fluxing additive, which improves the reactivity of alumina-containing waste and kaolin in the ceramic mixture. Consequently, the addition of dolomite to kaolin-aluminous ceramic mixtures initiates the melting of aluminum oxide, which was synthesized from the alumina-containing waste of the spent ShGCC catalyst. It can also be noted that the introduction of dolomite in the required quantity (5 – 15 wt.%) results in the complete sintering of samples of high-alumina ceramic mixtures due to the formation of a liquid phase between the initial components of the ternary system. The resulting liquid phase improves the high-temperature solid-state interaction between kaolin and alumina-containing waste at relatively low sintering temperatures.
As a result of high-temperature interaction in ceramic samples and an increase in the content of aluminum oxide at an optimal firing temperature of 1400°C of a ceramic mixture based on the ternary system, an enhancement in density and other physical and mechanical parameters is observed.
It has been demonstrated that the developed test concentration compositions meet the requirements of the standard for high-alumina ceramic materials in terms of chemical and mineralogical parameters and physical and mechanical properties, with particular reference to the production of mullite and mullite-corundum grinding bodies.
The x-ray diffraction analysis of the mineralogical phase in calcined samples of a high alumina mixture based on the kaolin – alumina-containing waste – dolomite ternary system showed that following firing at 1400°C, a new phase of mullite minerals 3Al2O3 · 2SiO2 occurs in all samples with a corresponding d-spacing: d = 0,540; 0,375; 0,338; 0,270; 0,267; 0,242; 0,228; 0,220; 0,211; 0,200; 0,170; and 0,168 nm. In addition, mullite is the main crystalline phase of fired samples of high-alumina ceramic mixtures. The x-ray diffraction patterns for a series of fired samples of high-alumina mixtures are presented in Fig. 2.
In addition to the mineral mullite, the observed x-ray patterns exhibit diffraction peaks corresponding to the minerals corundum (d = 0.342 and 0.254 nm) and the high-temperature form of quartz (β-cristobalite) (d = 0.405; 0.316; 0.282; 0.249; 0.209; 0.187; 0.166; 0.161; 0.159; and 0.156 nm), along with low-intensity peaks of α-quartz (d = 0.424; 0.335; 0.247; 0.197; and 0.181 nm).
It can be concluded that the diffraction peaks originating from d-spacings associated with the β-cristobalite mineral exhibit a relatively high intensity when compared to the diffraction peaks of α-quartz.
In addition, x-ray diffraction patterns of fired samples of HAM-6, HAM-8, and HAM-12, containing 15 wt.%, exhibit relatively low-intensity peaks corresponding to the mineral anorthite (d = 0.403; 0.321; 0.316 nm). The x-ray diffraction pattern of the fired HAM-9 sample exhibits distinct diffraction peaks at d-spacings of d = 0.342; 0.254; 0.237; and 0.207 nm, which correspond to the mineral corundum.
X-ray diffraction analysis of fired samples revealed the formation of minerals such as mullite, β-cristobalite, partially corundum, and anorthite. These minerals contribute to the necessary physical, mechanical, and technological properties of high-alumina products, in particular, ceramic grinding bodies.
Conclusions
The article examined the high-temperature interaction and sintering processes within ceramic samples based on the kaolin – alumina-containing waste – dolomite ternary system for the production of grinding bodies by low-temperature firing. The regions of formation of high-alumina mixtures for technical ceramics are determined in a ternary diagram. X-ray diffraction analysis of high-alumina mixtures revealed the mineralogical composition of the fired samples. The results demonstrated the formation of crystalline phases of minerals mullite, corundum, and β-cristobalite, as well as a small amount of anorthite and an amorphous vitreous phase, which ensured the necessary physical and mechanical properties of the fired sample. The regions of optimal compositions to produce high-alumina ceramic mixtures based on the kaolin – alumina-containing waste – dolomite ternary system by low-temperature sintering were determined.
References
A. M. Salakhov, Advanced Ceramic Materials: Handbook [in Russian], Izd. KFU, Kazan (2016).
V. Ya. Shevchenko and S. M. Barinov, Technical Ceramics [in Russian], Nauka, Moscow (1993).
O. A. Sergievich, E. M. Dyatlova, and R. Yu. Popov, “Synthesis of ceramic materials based on an iron-aluminum silicate system for industrial use,” Ogneup. Tekh. Keram. [in Russian], No. 7 – 8, 3 – 11 (2017).
N. A. Goryachev, I. B. Panteleev, and N. A. Andreeva, “Certification of structural ceramics by mechanical properties,” Ogneup. Tekh. Keram. [in Russian], No. 10, 45 – 49 (2018).
I. D. Kashcheev and K. G. Zemlyanoi, “Possibilities of obtaining high-alumina raw materials from industrial waste for the ceramic and refractory industry (Review),” Novye Ogneupory, No. 5, 83 – 89 (2019).
A. S. Tolkacheva and I. A. Pavlova, Ceramics Technology for Electronic Industry Materials. Part 1: Handbook [in Russian], Izd. Ural Univ., Ekaterinburg (2016).
I. A. Pavlova, K. G. Zemlyanoi, and E. P. Farafontova, Fundamentals of Technology of Nonmetallic and Silicate Materials [in Russian], Izd. Ural Univ., Ekaterinburg (2020).
Al. A. Eminov, Z. R. Kadyrova, and M. I. Iskandarova, “Gas processing waste: promising raw material for designing the composition of ceramic grinding bodies,” Glass Ceram., 78(1 – 2), 35 – 39 (2021).
A. M. Eminov, Z. R. Kadyrova, A. A. Eminov, et al., “Study of synthesis kinetics and features of mullite solid-phase formation,” Refract. Ind. Ceram., 62(4), 394 – 398 (2021).
B. T. Sabirov, Z. R. Kadyrova, and S. S. Tairov., “Development of optimal compositions of ceramic tiles using dune sand,” Glass Ceram., 75(9 – 10), 363 – 365 (2019).
L. M. Kovba and V. K. Trunov, X-Ray Diffraction Analysis [in Russian], Izd. Mosk. Univ., Moscow (1969).
S. S. Tolkachev, Tables of Lattice Spacings [in Russian], Khimiya, Leningrad (1968).
L. I. Mirkin, Handbook of X-Ray Diffraction Analysis of Polycrystals [in Russian], Izd. Fiz.-Mat. Liter., Moscow (1961).
ASTM Standards. Pt 17. Refractories, Glass, Ceramic Materials, Carbon and Graphite Products, ASTM, Philadelphia (2005), pp. 7 – 9, 51 – 61.
A. S. Tolkacheva and I. A. Pavlova, General Issues on the Technology of Fine Ceramics: Handbook [in Russian], Izd. Ural Univ., Ekaterinburg (2018).
N. T. Andrianov, V. L. Balkevich, A. V. Belyakov, et al., Chemical Technology of Ceramics: Handbook [in Russian], RIF “Stroimaterialy,” Moscow (2012).
V. L. Balkevich, Technical Ceramics: Handbook [in Russian], Stroyizdat, Moscow (1984).
G. N. Maslennikova, F. Ya. Kharitonov, and I. V. Dubov, Calculations in Ceramics Technology [in Russian], Stroyizdat, Moscow (1984).
Author information
Authors and Affiliations
Additional information
Translated from Steklo i Keramika, No. 2, pp. 23 – 32, February, 2024
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eminov, A.A., Tairov, S.S., Sabirov, B.T. et al. Ceramic Body Based on Kaolin – Alumina-Containing Waste – Dolomite System. Glass Ceram 81, 62–67 (2024). https://doi.org/10.1007/s10717-024-00660-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-024-00660-7