The particulars of mullite crystallization during the synthesis of the ceramic composite material ‘mullite – zirconium oxide,’ obtained from a two-phase gel by co-precipitation combined with sol-gel synthesis, were studied. Powders of the ceramic composite material ‘mullite – ZrO2‘ were obtained. They were heat treated at 1150 and 1350°C. Samples of the ceramic composite ‘mullite – zirconium oxide’ were made from the obtained powders by semi-dry pressing. The microstructure and phase composition of the ceramic were studied.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mullite 3Al2O3·2SiO2 is a stable crystalline phase in the binary phase diagram aluminum oxide – silicon oxide. It has the following properties making it a suitable material for structural applications: low thermal expansion coefficient, low density, high-temperature strength, stability under temperature gradients, chemical stability, and low creep rate. Mullite-based ceramic composite materials are considered candidates for the development of heat-shielding and heat-insulating materials in hot industries (petrochemical, porcelain, metallurgical), for example, in the structural elements of electric furnaces, hot metal mixers, low-frequency induction furnaces, furnace mounting plates, and linings of high-temperature reactors, as well as filters for hot gasses and metal melts, heat-loaded components of power plants, heat exchangers and other products exposed to high temperatures and corrosive environments. Mullite is also considered as a matrix material for the development of reinforced composites [1,2, – 3].
Three main types of polycrystalline mullite ceramics are used: monolithic mullite ceramics, mullite coatings, and mullite composites. Monolithic mullite ceramics are used in the manufacture of wares, porcelain, refractories, structural elements of furnaces, catalytic converters, and so on. Mullite coatings are used to protect metals and ceramics from chemical degradation at high temperatures. This type of surface coating is known as environmental protection coatings, which make the material stable under specific conditions [4]. Mullite composites include mullite matrices and reinforcing fillers that increase strength and reduce material brittleness. They are used in components and structures of gas turbine engines, structural elements of high power furnaces, burner tubes and spacecraft, and also as radio transparent windows [5].
Currently, there is an interest in ceramic synthesis and mechanisms of crystallization of a ceramic. As a rule, all methods of obtaining mullite involve the preparation of a mixture of aluminum and silicon oxides or their precursors and subsequent heat treatment, which results in the formation of a single-phase mullite material. In technical products the formation of solid solutions with fluctuations in the ratios of aluminum and silicon oxides from 3Al2O3·2SiO2 to 2Al2O3·SiO2 is possible. Moreover, 3 : 2 mullite is thermodynamically stable, and the 2 : 1 composition decomposes into 3 : 2 mullite and corundum during long-time heat treatments; but, during rapid synthesis from melt or highly active powders solid-solution crystals are formed in the range from 3 : 2 to 2 : 1.
In obtaining mullite by the solid-phase method natural clays such as kaolinite Al8[Si4O10](OH)8 are used as initial components, which decompose at temperature to form active compounds capable of forming crystalline mullite at high temperatures [6, 7]. These methods were developed several decades ago, but now active research is also underway to study the processes of thermal decomposition of natural aluminosilicates and the nature of mullite formation reactions [8, 9]. The problem with this method is that a high temperature must be provided in order to complete the reaction in the solid state. And as long as mineral springs are used as the starting material, the mullite inevitably contains impurities which form a glass phase along the grain boundaries, resulting in strength reduction and lowering of the maximum temperature of use of the material.
In obtaining mullite powders by chemical synthesis methods such as sol-gel, precipitation, hydrolysis, spray pyrolysis, and chemical vapor deposition (CVD) are used. Mullite preparation from chemically synthesized precursors is of great interest to developers of ceramic materials. In particular, sol-gel methods are widely used to obtain mullite powders and bulk samples at relatively low processing temperatures [10,11,12,13,14, – 15]. In sol-gel processing, colloidal particles or molecules in suspension or sol undergo a chemical change (e.g. change in pH) that causes them to form a continuous network called a gel. The sol-gel process makes it possible to obtain a uniform and reactive gel that can be sintered at low temperature, and hence a uniform submicron microstructure can be obtained. The chemical synthesis of mullite includes methods for mullite preparation using starting materials in the form of a mixture of sols or a mixture of salts and sols.
In developing a method for the synthesis of mullite ceramics, it is important to resolve the problem of guaranteeing high product strength and at the same time lower the cost of the technological process. Technological methods for increasing the strength of monolithic oxide ceramics at moderate temperatures are based on two main approaches: obtaining a structure with sufficiently small crystals that increases strength by reducing the size of initial defects comparable to the grain size, and introducing hardening additives that lower the sensitivity of ceramics to crack propagation. At high temperatures, creep resistance, traditionally improved by grain coarsening or the introduction of large crystals of creep-resistant phases, becomes a critical factor determining the performance of most materials.
In the present work the process of crystallization during sintering of mullite ceramics with addition of zirconium oxide was studied as a function of the degree of defectiveness of the initial powders. Powders were obtained from a two-phase gel by a method combining co-deposition and sol-gel synthesis. In this ceramic zirconium oxide acted as a sintering additive and crack blocker simultaneously.
MATERIALS AND METHODS
Aluminum, zirconium, and silicon salts and alcoholates were used as starting materials for obtaining samples.
The powder was obtained by co-precipitation, and a blank was formed by semi-dry pressing. The resulting semi-finished products and materials were studied by x-ray diffraction analysis as well as optical and scanning electron microscopy.
EXPERIMENT
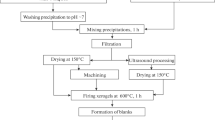
The process of obtaining a composite mullite – zirconium oxide powder included the preparation of an aqueous solution of aluminum, zirconium, and yttrium salts as well as a hydrolyzed solution of TEOS in acetone, mixing the prepared solutions, hydrolysis of the resulting solution, followed by washing and calcining the precipitate [16]. The calculated mass content of zirconium oxide in the ceramic material was up to 10%.
The precipitated mixture was calcined at 1150 and 1350°C (Fig. 1), chosen for determining the separation of the targeted phases. The onset time of the separation of the targeted phases and the degree of their structural perfection were fixed by means of XPA.
The calcined precipitate was comminuted in a ball mill. On the basis of the resulting powder, a press-powder was prepared for fabricating experimental samples of structural ceramic material based on the mullite-zirconium oxide system by semi-dry pressing.
On the basis of the phase composition, the powder obtained after firing at 1350°C was a mixture of mullite solid solutions with tetragonal zirconium oxide, and after firing at 1150°C it was a mixture of mullite with tetragonal zirconium oxide and intermediate Al2O3 phases; in this case, XPA-detected impurities of other phases were absent. This suggests that at 1150°C the dissolution of aluminum oxide in mullite has not yet occurred.
A study of the dispersion and morphology of powders (Fig. 2) showed that the particles of powders ground in a ball mill have an approximately equiaxial shape with no obvious signs of cleavage; the particles are optically isotropic; the size of most particles lies in the range 0.5 – 5 μm. An analysis of the obtained results allows us to conclude that the powder particles obtained as a result of grinding consist of very small (< 0.2 μm) crystals of the main phases. This conclusion follows from the optical isotropy of the particles, which, according to XRD data, consist of birefringent crystals.
It should be noted that when using synthesized mullite as an initial powder to fabricate ceramics of the classical type the technological and operational properties of the resulting material are not unique functions of the degree of perfection of semi-finished product crystals and the sizes of their aggregates.
On the one hand, as the particle size of the powder and the degree of perfection of its crystals decrease, the temperature decreases significantly, the sintering rate increases, and the grain size in the structure of the resulting ceramic decreases to a certain extent. On the other hand, the use of powders with extremely high sintering activity leads to a sharp increase in shrinkage and the appearance of a significant number of small closed pores (the ‘zonal sintering’ effect). As a result, the indicated phenomena tighten the requirements for the accuracy of maintaining the temperature and uniformity of its distribution over the volume of the fired blank, which significantly degrades the manufacturability of the materials. Moreover, ceramic materials obtained from highly active powders have a significantly greater proneness to collective recrystallization, which limits their use at high (close to the limiting value for a given composition) temperatures. In this case, for the same phase composition, the powder fired at 1150°C had a significantly higher degree of structural disorder, which is obvious from the extremely large width of the reflections of the main phases. To determine the influence of this factor on the technological properties of the obtained powders, control samples of sintered ceramics were made from them.
Sample blanks were formed by semi-dry pressing, which provides the highest and most stable characteristics of ceramic materials in products with a simple geometric shape. Pressing was performed in a cylindrical steel mold on a hydraulic press. The resulting compacts were dried to constant weight and fired at 1850°C. After firing, the sintered blanks were cut into 5 × 10 × 50 mm samples, using a diamond cutting wheel, and machined on flat surfaces by grinding with a loose abrasive on a metal plate. After mechanical processing, samples of the ceramic material were obtained in order to determine its density and study the structural features.
The structure and physical properties of the obtained samples were investigated. The structure of materials was investigated using a (SEM) Hitachi S-405A scanning electron microscope. The choice of an electron microscope was based on the low contrast of the structural elements of such materials when observed in an optical microscope in reflected light and the laboriousness of preparing thin transparent sections of such materials for investigations in polarized light.
In establishing the physical properties of the materials, the open and closed porosity, density as determined by hydrostatic weighing, true density, microstructure and phase composition were determined.
The open porosity and density were determined by hydrostatic weighing. As a result it was found that there is practically no open porosity on the samples (< 1%). The average values obtained for the density by hydrostatic weighing were equal to 3150 kg/m3 for ceramics made from low-fired powder and 3220 kg/m3 for high-fired.
The true density was found by the pycnometric method (GOST 2409–95). The average value of ρtrue for ZrO2-modified mullite was equal to 3390 kg/m3.
The closed porosity Pc (g) for the materials was determined by calculation using the formula
where ρtrue is the true density and ρh is the density as determined by hydrostatic weighing.
According to the calculations the closed porosity was equal to 17% for the material made using low-fired powder and 4.6% for high-fired powder.
An investigation of the structure and distribution of phases in the fractures of the obtained materials performed using SEM at 1350-fold magnification showed that for the samples under consideration the grain size for the main phases was less than 12 μm and the closed porosity with 2 – 8 μm pores was insignificant and uniformly distributed.
The typical appearance of the microstructure of the obtained materials based on mullite is displayed in Fig. 3 for the material obtained using powder fired at 1150°C and in Fig. 4 for 1350°C. Fractography of a fracture was chosen as the method to investigate the microstructure. A scanning electron microscope was used to obtain photographs of the cleavage surfaces of pressed samples. This method reveals the structure of dense materials consisting of anisotropic but chemically homogeneous crystals, since high-percentage mullite ceramics are extremely difficult to selectively etch. The material obtained from a more sintering-active powder, fired at 1150°C, contains separate large and very large lamellar crystals, which we identified as mullite according to their characteristic habit. These crystals are located in the bulk of the matrix with significantly finer grains. In particular, inclusions of large mullite lamellae > 50 μm long can be seen in Fig. 3 (at the right-hand edge of the image), which represents the cleavage surface of a mullite material with a bimodal structure. Segregations of a second phase are observed along the grain boundaries and on the surface of the pores. The light color of the precipitates in the SEM indicates a high content of heavy elements — modifiers (Zr and Y) — in them.
A determination of the mechanical strength of the obtained samples showed that ceramics formed using the initial powder obtained by firing at 1350°C had average bending strength 250 MPa with individual points spread in the range from 210 to 285 MPa and using a more sintering-active low-fired (1150°C) powder — 173 MPa with spread 150 to 215 MPa.
This structural difference can be used to adapt mullite materials for solving various problems: ceramics with a relatively fine-grained homogeneous structure is optimal for obtaining materials with sufficiently high strength (to 250 MPa) at moderate temperatures, while material reinforced with large mullite plates has a higher resistance to creep at very high temperatures for mullite-corundum materials (to 1750°C).
CONCLUSIONS
The process of mullite crystallization during the synthesis of ceramic composite material ‘mullite – zirconium oxide’ obtained from a two-phase gel by combining co-precipitation and sol-gel synthesis was investigated. Powders of ceramic composite material ‘mullite – ZrO2’ were obtained; they were heat treated at 1150 and 1350°C. Samples of the ceramic composite material ‘mullite – zirconium oxide’ were made from the obtained powders by the method of semi-dry pressing. The microstructure and phase composition of ceramics were investigated.
It was determined that the proposed methods for preparing initial powders can be used to obtain thermostable mullite ceramics for various purposes. It was confirmed that in low-temperature firing a high degree of defectiveness of the initial powder structure leads to intense collective recrystallization. It was proposed to use this effect to obtain a ceramic with large-grained size-bimodal microstructure that combines high creep resistance at high temperatures with satisfactory mechanical strength.
The work was performed using equipment at the Climatic Testing Unit of the National Research Center Kurchatov Institute – VIAM.
References
E. N. Kablov, “Next generation materials and digital technologies for their processing,” Vest. Ross. Akad. Nauk, 90(4), 331 – 334 (2020).
V. G. Babashov, V. G. Maksimov, N. M. Varrik, and O. N. Samorodova, “Study of the structure and properties of ceramic composite materials based on mullite,” Aviats. Mater. Tekhnol., No. 1, 54 – 63 (2020), Art. 9; 0/18577/2713-01-93-2021-0-3-94-104
Yu. E. Lebedeva, N. E. Shchegoleva, V. A. Voronov, and S. S. Solntsev, “Ceramic materials based on oxides of aluminum and zirconium obtained by the sol-gel method,” Tr. VIAM: Elektron. Nauch.-Tekhn. Zh., No. 2, Art. 05 (2021); URL: http://www.viam-works.ru (accessed 07/08/2021); https://doi.org/10.18577/2307-6046-2021-0-4-61-73
P. Hou, S. N. Basu, and V. K. Sarin, “Structure and hightemperature stability of compositionally graded CVD mullite coatings,” Int. J. Refr. Met. Hard Mater., 19, 467 – 477 (2001).
J. Anggono, “Mullite ceramics: Its properties, structure, and synthesis,” J. Teknik Mesin., 7(1), 1 – 10 (2005).
S. V. Matrenin and A. I. Slosman, Technical Ceramics [in Russian], Izd. TPU, Tomsk (2004).
V. B. Kulmet’eva, S. E. Porozova, and A. A. Smetkin, Perspektivnye Kompozitsionnye i Keramicheskie Materialy [in Russian], Izd. Permsk. Nats. Issled. Politekh. Universiteta, Perm (2013).
D. Frulli, “Production and characteristics of refractory raw materials based on andalusite and mullite. Influence of impurities on refractory properties,” Novye Ogneupory, No. 3, 93 – 97 (2017).
T. V. Vakalova, A. A. Reshetova, V. M. Pogrebenkov, and V. I. Vereshchagin, “Activation of the synthesis of mullite and sintering of aluminosilicate ceramics based on refractory clay raw materials,” Ogneup. Tekh. Keram., No. 7 – 8, 74 – 80 (2009).
E. Tkalcec, H. Ivankovic, R. Nass, and H. Schmidt, “Crystallization kinetics of mullite formation in diphasic gels containing different alumina components,” J. Eurp. Ceram. Soc., 23(9), 1465 – 1475 (2003).
A. V. Istomin and S. G. Kolyshev, “Electrostatic method of forming ultrathin fibers of refractory oxides,” Aviats. Mater. Tekhnol., No. 2(55), 40 – 46 (2019); https://doi.org/10.18577/2071-9140-2019-0-2-40-46
N. V. Buchilin, G. Yu. Lyulukina, and N. M. Varrik, “Materials based on mullite,” Tr. VIAM: Elektron. Nauch.-Tekhn. Zh., No. 5(53), 32 – 41 (2017), Art. 4; URL: http://www.viamworks.ru (accessed 30.09.2021); https://doi.org/10.18577/2307-6046-2017-0-5-4-4
S. Sandaresan, “Mullitization of diphasic aluminosilicategels,” J. Am. Ceram. Soc., 74(10), 2388 – 2392 (1991).
W.-C. Wei and J. W. Halloran, “Transformation kinetics of diphasic aluminosilicate gels,” J. Am. Ceram. Soc., 71(7), 581 – 587 (1988).
Yu. I. Komolikov, I. D. Kashcheev, and V. R. Khrustov, “Sintering of composite ceramics based on powders of zirconium and aluminum oxides,” Novye Ogneupory, No. 8, 47 – 49 (2015).
E. N. Kablov, B. V. Shchetanov, Yu. A. Ivakhnenko, et al., A Method for Obtaining a Powder of a Ceramic Composite Material, Pat. 2292320 RF, No. 2005125772/03 [in Russian], appl. 08/15/2005, publ. 01/27/2007.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 12, pp. 9 – 14, December, 2021.
Rights and permissions
About this article
Cite this article
Babashov, V.G., Maksimov, V.G. & Varrik, N.M. On Mullite Crystallization in Ceramic Composites. Glass Ceram 78, 471–475 (2022). https://doi.org/10.1007/s10717-022-00434-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-022-00434-z