Enamel coatings for protecting ozonizer electrodes were obtained in the system PbO–B2O3–Sb2O3–SiO2 with variable content of silicon oxide and antimony oxide. The results showed that enamel with antimony oxide content 5 wt.% possesses the best combination of thermal, thermomechanical, and electrical properties in combination with the required covering power.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ozonation is currently a universal method of water treatment, since it completely exterminates all microbes, significantly reduces the content of organic matter dissolved in water, and purifies water from phenols and some chemical compounds that are not amenable to other chemical reagents. Ozone, being the strongest oxidizer, eliminates phenolic compounds much more effectively than chlorine and unlike the latter does not engender toxic compounds during reactions.
Pesticides and hydrocarbons, requiring activated carbon to be removed without ozonation, are effectively eliminated from water at heightened ozone concentration (compared to conventional treatment).
Currently, the most widespread method of ozone production is generation in the zone of a barrier discharge in an ozonizer. However, the performance of these setups directly depends on the life time of the dielectric barrier, which is primarily determined by the intensity of the direct impact of the electric field acting on the dielectric and the high-temperature effect of the microdischarge channels as well as the effect of the dielectric barrier material itself with insulating enamels playing this role.
Operating Principle of an Ozonizer
Large ozonizers are used to purify effluents from oil refineries as well as chemical and metallurgical industry enterprises and to bleach pulp at pulp-and-paper mills [1].
Ozonizers can be represented simply as a pair of electrodes to which a high voltage is applied. A gap is maintained between the electrodes; it forms a discharge space through which a gas is passed. In the interelectrode space or on the surface of one or both electrodes, a dielectric material with high resistivity and sufficient electrical strength is secured so as to create a dielectric barrier that excludes the formation of spark or arc discharges. An alternating voltage capable of forming a barrier discharge is applied to the electrodes. In the interelectrode space, conditions are created for intense electron bombardment of oxygen molecules, as a result of which ozone molecules are formed.
To increase the efficiency and life time of dielectric barriers, the most stable materials and improved designs are being developed for them, improved designs are used for the ozonizer units, and a system for preliminary preparation of a gas mixture is used for ozone generation [2]. One of the most important problems in developing ozonizers is choosing a dielectric material that meets the following requirements: high dielectric constant, large volume and surface electrical resistance, and low dielectric losses. In addition, the dielectric material must have high chemical stability, heat resistance, and mechanical strength. In addition, the coating must be continuous and of equal thickness over the entire surface of the electrode and good adhesion to the metal. The requirements of chemical stability are imposed due to the fact that corrosive substances can be present in the discharge zone — ozone and atomic oxygen, which are strong oxidants, as well as various nitrogen oxides formed during the synthesis of ozone from air, and nitric acid formed in the presence of moisture [3,4,5,6, – 7].
The electrosynthesis of ozone with an electric discharge in ozonizers is one of the classic chemical reactions in an electric discharge. Currently, in an ozonizer, insulating enamel is used as a dielectric barrier, which makes it possible to significantly reduce the thickness of the dielectric barrier, increase the power and performance of the ozonizer, improve the conditions for cooling the electrodes, and make it possible to create high-frequency ozonizers operating at high-frequency currents (up to 10 kHz) [8].
Other important advantages of ozonizers with enameled electrodes are simplicity of design, small dimensions, and low metal consumption. The mass of such an installation with the same performance is 10 times smaller than that of a low-frequency installation.
An example of a plate ozonizer with a dielectric coating (enamel) is presented in Fig. 1.
Compositions and Insulating Properties of Insulating Enamel
The development of enamel coatings involves an entire series of requirements: attainment and matching of the linear thermal expansion coefficient, high gas-tightness coefficient, and adhesion of the coating to the metal. Glass enamel coatings combine high CLTE values with gas-tightness and relatively low refractoriness but their electrical insulating properties are lower than that of ceramic coatings. Problems of the synthesis of insulating enamels on steel lie in the need to achieve high dielectric properties and CLTE values. The difficulty of solving this problem lies in the fact that the compounds conventionally used to increase the CLTE contain alkali metal cations, which are current carriers and sharply lower the electrical resistance of enamels [9,10, – 11].
An example of a dielectric enamel is an enamel with heightened adhesion strength equal to magnitude 5, high heat resistance, impact strength, and spreadability of the compound (weight content, %): 29 – 35 SiO2; 20 – 24 Na2O; 8 – 12 Al2O3; 12–16 B2O3; 6 – 10 TiO2; 1 – 3 NiO; 1.7 – 6.0 Fe2O3; 3 – 9 CaF2; 0.1 – 1.0 CuO; 0.1 – 2.0 Cr2O3; 0.1 – 1.0 Mo2O5 (SU No. 1470685) [12].
In optimizing the combination of physicochemical and technological properties of enamels, there arise the questions of developing a formulation of complex compositions with partial replacement of silica by low-melting oxides (Li2O, Na2O, K2O, PbO, B2O3), oxides playing the role of fluids (CaO, SrO, BaO, ZnO, FeO, MnO, TiO2), as well as fluorides (CaF2, NaF, NasAiFe, Na2SiF6). Besides silica, refractory oxides, such as Al2O3, Cr2O3, ZrO2, SnO2, and so on, are often used [13].
Low-alkali and alkali-free glasses and enamels containing a minimum amount of current carriers — their electrical resistance is higher and dielectric losses are lower than in alkaline compositions—have the highest dielectric properties. So, the enamel formulation containing (weight content, %) 11 – 13.8 SiO2; 3.9 – 4.5 Al2O3; 5.3 – 7.0 B2O3; 0.8 – 3.0 TiO2; 0.3 – 0.6 Cr2O3; 56.5 – 58.2 BaO; 13.9 – 18.2 CdO; 0.5 – 0.7 CoO; 0.1 – 0.4 MnO; 0.5 – 0.8 V2O5 at 400°C is characterized by volumetric resistivity 108 – 109 Ω · cm and quite high CLTE (105 – 122) × 10–7 K–1. The melting temperature of this enamel is equal to 1400°C (SU 1673550) [14].
A dielectric coating for stainless steel is known for use as an electrode coating in an ozone generator (weight content, %): 29.5 – 38.7% SiO2; 0.5 – 1.5% Li2O; 2.0 – 9.0% Na2O; 9.0 – 15.0% Ka2O; 15 – 22% BaO; 9.0 – 13.0% B2O3; 1.5 – 7.0% CaO; 4.0 – 10.0% SrO; 2.0 – 8.0% CdO; 0.1 – 1.0% CoO; 0.1 – 1.0% Mn2O3; 0.1 – 1.0% NiO. The dielectric constant of this enamel is equal to 7.1 – 7.8, dielectric loss angle tangent 0.001 – 0.002, CLTE (132 – 139) × 10–7 K–1, and dielectric strength 27 – 33 kV/mm. This enamel provides high ozone output and ozonizer durability [15].
The following insulating enamel is given in [16] (weight content, %): 25 – 35% SiO2; 3 – 8% SrO; 0.5 – 1.0% CoO; 0.5 – 10% NiO; 1 – 4% Na2O; 3 – 5% K2O; 3 – 18% Bi2O3; 0.2 – 10% CuO; 1 – 3% Sb2O3; 1 – 6% V2O5; 4 – 7% CaO; 4 – 7% CdO; remainder — BaO. It is characterized by heightened CLTE (156 × 10–7 K–1), dielectric constant 13.2, dielectric loss angle tangent 0.013.
Also known is an insulating enamel with heightened adhesion to metal and an extended temperature zone of stability of the glass matrix from 700 to 900°C (weight content, %): 20 – 35 SiO2; 3 – 7 B2O3; 4 – 7 CaO; 1.5 – 3.0 SrO; 0.5 – 1.0 CoO; 2.5 – 6.0 CdO; 0.2 – 1.0 MnO; 0.5 – 1.0 NiO; 0 – 2 Na2O; 1 – 5 K2O; 0 – 6 CaF2; 0.5 – 1.0 Cr2O3; 0.5 – 1.0 MoO3; 5 – 8 Na3AlF6; 0.1 – 1.5 Al2O3; 45 – 55 BaO [17].
The results of studies on obtaining high-lead protective glass-like coatings for steel based on the system PbO–B2O3–Sb2O3–Bi2O3–SiO2 are described in [18]. Typical values of the CLTE for enamels are (104 – 115) × 10–7 K–1.
The resistance of enamel increases sharply when one alkaline oxide is partially replaced by another. This effect is most pronounced when three alkaline oxides are present simultaneously in the ratio Li2O: Na2O: 2K2O. The more alkaline oxides present in the enamel, the greater the magnitude of the effect is. As temperature rises, the magnitude of the effect decreases. The resistance increases when bivalent metal oxides are introduced into the enamel composition. In terms of the degree to which oxides influence the enamel resistance, the oxides can be arranged in the following series: BeO > ZnO > MgO > CaO > SrO2 > PbO > BaO.
Materials and Procedures
Glasses in the chemical system SiO2–PbO–B2O3–Sb2O3 were selected, based on the published data, as objects of research in order to obtain lead-silicate enamels characterized by good spreadability and high adhesion to steel as well as high chemical resistance. Lead oxide contains the highly polarizable cation Pb2+, which lowers the glass softening point and the melt viscosity. Four compositions of the glass with Sb2O3 weight content varying from 0 to 7.5% due to SiO2 content reduction from 7.5 to 0% were investigated. Antimony oxide was introduced as an adhesion activator into the enamel composition in order to secure strong adhesion of the enamel coating to steel. This oxide also helps to increase the CLTE of the glass.
The aim of the present work was to obtain an insulating enamel for steel and to study the effect of the Sb2O3 weight content in the enamel on its thermophysical, dielectric, and adhesive properties.
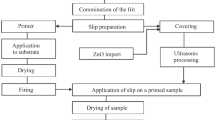
Quartz sand, boric acid, lead oxide, and antimony oxide were chosen as the initial components for synthesizing the enamels. The calculation of the batch was performed on the basis of the composition of the raw materials, taking into account the volatilization of boric acid (10%) and lead oxide (5%). Quartz sand was sieved through a No. 025 sieve. The prepared raw materials were weighed on a laboratory technical balance with accuracy 0.01 g; the components were manually mixed for 15 – 20 min to a uniform state. Glass melting was conducted in corundum crucibles in electric furnaces. The maximum melting temperature was equal to 650°C. The molten glass was poured onto a steel plate, as a result of which specimens in the form of disks and beams were obtained; the glasses were annealed in a muffle furnace at 200°C.
The enamel was applied by the electrostatic (powder) method. The enamel samples were fired in an electric oven at 400 – 450°C.
The density of the enamel was determined by hydrostatic weighing (GOST 24409–80), CLTE — in an air atmosphere (heating rate 5°C/min) using a high-temperature horizontal dilatometer DIL 402 PC (Netzsch), dielectric properties — using a device (Rohde & Schwarz ZNB20) for measuring the dielectric properties of materials, breakdown voltage — on a high-voltage setup with polished samples in the form of disks with a spherical depression, with a silver coating, contact angle — on an automated optical analyzer after heat treatment of glass samples located on a substrate made of material with respect to which the wetting angle is determined, strength of adhesion of the enamel to the substrate — by the thermal shock method.
Differential thermal analysis was performed with a STA 449 C Jupiter synchronous thermal analysis system (Netzsch) in an oxidizing environment with heating rate 10 K/min.
Results and Discussion
The temperature parameters for the formation of an enamel coating (onset temperature of melting) were determined by means of a simultaneous thermal analysis (STA) performed in the temperature range 20 – 600°C.
The results of the studies are presented in Fig. 2. Analysis of the obtained data showed that the softening temperature of the enamel decreases with increasing antimony oxide content in the enamel composition. This can be explained by the fact that in the glass composition this oxide acts as a flux, lowering the degree of connectivity of the silicon-oxygen framework.
Based on the results of the determination of the density of the enamel, the density was found to vary in the range 3246 – 3310 kg/m3, depending on the composition. The enamel density versus the chemical composition is displayed in Fig. 3.
The results show that due to the replacement of Si4+ cations with the heavier Sb3+ cations the density of the material increases with increasing antimony oxide content in the enamel composition.
In order to exclude cracking and chipping in the coating during its application and during the operation of the ozonizer the CLTE of the enamel must be equal to at least (130 – 140) × 10–7 K–1. The study of the CLTE of the obtained enamels established that it increased from 118 × 10–7 to 165 × 10–7 K–1 with the content of Sb2O3 in the enamel increasing due to SiO2. The CLTE increases because the Sb3+ cations have a strong polarizability due their large radius, i.e. the electron density of a neighboring atom is pulled toward them, so that the structure is disordered (cationic field strength 1.76 — Sb3+, 1.57 — Si4+, 0.32 — Pb2+, 1.45 — B3+). Located in the free planes of the structural network the Sb3+ cations compensate for the excess negative charge of the complex anion. Since the oxygen environment of the modifier cations is formed in accordance with their coordination requirements, an increase in the content of Sb–O bonds leads to weakening of the Pb–O bond, so that the mobility of lead molecules increases with increasing temperature and the CLTE increases in consequence.
Conversely, as antimony oxide increases in the formulation, the surface tension of enamels decreases from 0.216 to 0.024 N/m due to the softening of the structure, as a result of which the enamel’s spreadability increases (Fig. 4 shows the surface tension of the enamel versus the Sb2O3 content). The efficiency of ozonizers directly depends on the dielectric properties of the enamels. So, for the optimal performance of ozonizers, the dielectric loss of the enamel should not exceed 0.004 – 0.006, as a result of which the dielectric barrier rises and the dielectric constant remains no lower than 5 – 7. The results of the investigation of the electrical properties of enamels are presented in Table 1.
Such a dependence can be explained by the fact that an increase in the Sb2O3 brings about higher mobility of lead ions, which are charge carriers, i.e. the greater Sb2O3, the more charge carriers and the lower the electrical indices.
One of the most important parameters for enameling is the covering power, which is characterized by the wetting angle (spreadability). After cooling, the steel substrate with the samples was placed on the object stage of an automated optical analyzer, an image of a drop of each sample was recorded, and at least three measurements of the wetting angle were made on the left- and right-hand sides of each drop.
The wetting angle, θ°, was determined by the formula
where θleft and θright are the values of the wetting angle on the left- and right-hand sides, respectively.
The arithmetic mean of the values of the wetting angle of three samples of synthesized enamel is taken as the test result. The temperature dependence of the wetting angle of enamels is shown in Fig. 5.
Based on the results of the investigations, it was shown that the introduction of antimony oxide into the enamel compound in amount 0 to 7.5% (by weight) promotes, due to silicon oxide, a reduction of the enamel’s wetting angle, which is most pronounced at test temperatures from 250 to 450°C. Acting as a flux, antimony oxide helps to improve the enamel’s spreadability on the metal surface. In the formulation 4, which does not contain silicon oxide, good wetting of the metal surface occurs due to lead oxide, which lowers the enamel melting point and surface tension.
The dependence of the surface tension of the synthesized enamels on the chemical composition (percentage of antimony oxide) is displayed in Fig. 6. According to the figure, enamel’s content of lead oxide increases and the content of silicon oxide decreases, the surface tension decreases monotonically from 0.216 to 0.024 N/m on account of the replacement of Si4+ cations with the larger Sb3+ cations.
From the standpoint of the electrochemical hypothesis the adhesion of enamel to a metal substrate is due to the electrolytic deposition of lead on the surface during firing, which intensifies with decreasing surface tension.
In the present work the formulation dependence of the adhesion strength of enamel on metal was studied by the thermal shock method. This consisted of preliminary heating of the samples to 300°C, followed by sharp cooling in water (Twater = 20°C) and weighing of the samples after drying. The test results are presented in Fig. 7.
The strongest enamel-steel adhesion obtains with antimony oxide content equal to 7.5% (weight loss 1% at 220°C and 10% at 300°C, while for formulations 1 – 3 the losses observed at 180 – 200°C and 300°C reach 15 – 20%). This effect is explained by the electrochemical theory of enamel-metal adhesion—in this case Sb2O3 oxide acts as an adhesion activator, semi-anchor adhesion occurs, Sb3+ oxidizes to Sb6+, while the opposite reaction occurs in the metal-enamel junction, Sb6+ is reduced to Sb3+.
Conclusions
Insulating enamels were synthesized in the system SiO2–PbO–B2O3–Sb2O3 with variable weight content Sb2O3 from 0 to 7.5% due to a corresponding decrease in the SiO2 content. It was shown that the best combination of thermophysical, thermomechanical, and electrical properties for use in ozonizers obtains for enamel with antimony oxide weight content 5%, characterized by surface tension 0.07 N/m, and wetting angle 90 to 8° in the temperature range 200 – 400°C.
References
V. V. Lunin, M. P. Popovich, and S. N. Tkachenko, Physical Chemistry of Ozone [in Russian], Izd. MGU, Moscow (1998).
Proceedings of the 1st All-Russia Conference on Ozone and Other Environmentally Friendly Oxidants. Science and Technology, Dedicated to the 250th Anniversary of Lomonosov Moscow State University [in Russian], Knizhnii dom Universitet, Moscow (2005).
E. N. Kablov, Tendencies and Turning Points for the Innovative Development of Russia: Collection of Articles: Collection of Informative Articles [in Russian], VIAM, Moscow (2015).
E. N. Kablov, “New-generation materials: the basis of innovation, technological leadership, and national security of Russia,” Intelekt i Tekhnologii, No. 2(14), 16 – 21 (2016).
E. N. Kablov, “Innovative development work at FGUP VIAM GNTs RF on implementation of ‘Strategic directions for the development of materials and their processing technologies for the period up to 2030’,” Aviats. Mater. Tekhnol., No. 1, 3 – 33 (2015); https://doi.org/10.18577/2071-9140-2015-0-1-3-33.
D. V. Grashchenkov, “Development strategy for nonmetallic materials, metal composite materials and thermal protection,” Aviats. Mater. Tekhnol., No. S, 264 – 271 (2017); https://doi.org/10.8577/2071-9140-2017-0-S264-271.
E. N. Kablov, “Role of fundamental research in the creation of new generation materials,” in: Abstracts of the 21st Mendeleev Congress on General and Applied Chemistry, Vol. 2a [in Russian], St. Petersburg (2019).
A. Petzold and G. Peshman, Handbook of Enamel and Enameling [in Russian], Moscow (1990), pp. 72 – 74.
S. S. Solntsev, V. S. Denisova, A. B. Agarkov, and S. V. Gavrilov, “Effect of glass additives from the BaO–Al2O3–SiO2 system on the properties of reaction-cured coatings for protection of nickel alloys,” Tr. VIAM: Elektron. Nauch.-Tekhn. Zh., No. 1, Art. 11 (2018); URL: http://www.viam-works.ru (date of access: 06.23.2021); https://doi.org/10.18577/2307-6046-2018-0-1-11-11.
G. A. Malinina, S. V. Stefanovskii, O. I. Stefanovskaya, et al., “Structural position of samarium in glass-ceramic materials,” Tr. VIAM: elektron. Nauch.-tekhn. Zh., No. 6, Art. 03 (2019); URL: http://www.viam-works.ru (date of access: 06.23.2021); https://doi.org/10.18577/2307-6046-2019-0-6-20-31.
G. A. Malinina, V. S. Denisova, S. S. Solntsev, and M. L. Vaganova, “Study of the effect of oxide additives on the properties of heat-resistant glass-crystalline coating,” Tr. VIAM: Elektron. Nauch.-Tekhn. Zh., No. 5, Art. 09 (2021), No. 5, Art. 09 (2021); URL: http://www.viam-works.ru (date of access: 06.23.2021); https://doi.org/10.18577/2307-6046-2021-0-5-87-95.
A. G. Chigvintsev and T. M. Malygina, “Ground-coat enamel for steel, Pat. SU 1470685 A1 USSR, No. 4244247,” Byull. Izobr. Polezn. Modeli, No. 13 (1989), appl. 05/13/1987, publ. 04/07/1989.
A. S. Eskov,M. I. Oleinik, E. A. Shabrova, et al., “Enamel coating for ozonizer electrodes, Pat. SU 823330 A1 USSR, No. 2761488,” Byull. Izobr. Polezn. Modeli, No. 15 (1981), appl. 04/28/1979, publ. 04/23/1981.
N. M. Bobkova, M. P. Glasova, and E. S. Kashuba, “Frit for enamel coating on steel, Pat. SU 1539174 A1 SSSR, No. 4304341,” Byull. Izobr. Polezn. Modeli (1990), appl. 09/11/1987, publ. 01/30/1990.
M. A. Semin and N. Yu. Khmelnova, “Electrical insulating enamel for stainless steel parts, Pat. 2203233 RF, No. 99107068/03,” Byull. Izobr. Polezn. Modeli (2003), declared 04/15/1999, publ. 04/27/2003.
V. V. Danilin, M. P. Kokurkin, M. A. Semin, and A. I. Smorodin, “Electrical insulating enamel for stainless steel parts, Pat. 2264994 RF, No. 2004112723/03,” Byull. Izobr. Polezn. Modeli, No. 33 (2005), declared 04/26/2004, publ. November 27, 2005.
N. I. Puresev, O. A. Pureseva, E. A. Gordeenya, et al., “Electrical insulating glass enamel for stainless steel products, Pat. 2526445 RF, No. 2012149064/03,” Byull. Izobr. Polezn. Modeli, No. 23 (2014), declared 11/20/2012, publ. 08/20/2014.
A. V. Dorofeeva and M. A. Semin, “Protective glass coatings for steel,” Usp. Khim. Khim. Tekhnol., No. 8, 43 – 46 (2014).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 11, pp. 16 – 22, November, 2021.
Rights and permissions
About this article
Cite this article
Kulikova, O.V., Shchegoleva, N.E., Lebedeva, Y.E. et al. Insulating Enamel Based on Lead-Borate Glass. Glass Ceram 78, 436–441 (2022). https://doi.org/10.1007/s10717-022-00427-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-022-00427-y