The effect of the form of the silicon-containing raw material on the silicate formation and crystallization properties of glasses in the lithium-aluminum silicate system is examined. It is shown that amorphous silicon can be used effectively to increase the uniformity of sital glass produced under industrial conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Glass ceramic materials based on the lithium-aluminum silicate system possess a unique combination of high optical transparency and ultralow linear thermal expansion coefficient (CLTE). The optically transparent sitals CERAN and ZERODUR (Schott, Germany), KERALITE/PYROCERAM-III (Corning, US) and CLEARCERAM (OHARA, Japan) with near zero CLTE have been produced since the mid-1960s [1–4]. The same group of materials includes CO-115M sital glass, which LZOS JSC has been producing now for more than 40 years and enjoys stable demand in the international market [5].
The continuing work on improving the composition and properties of transparent sitals is aimed at solving a number of problems: development of the most technologically acceptable conditions for melting and producing glass, controlling the magnitude of and stabilizing the CLTE in different temperature ranges, increasing the optical uniformity and transparency and so on. The melting temperature of sital glass must not exceed about 1600°C, while in practice all transparent sitals developed in the system Li2O–Al2O3–SiO2 are low-alkali with a high content of silicon and aluminum oxides. This predetermines the high viscosity of the melt formed and requires elevated melting temperatures, fining and homogenization, which results in premature wear of the refractory of the melting tank in the glassmaking furnace and difficulties in mixing the melt and makes it difficult of produce optical quality glass.
Ordinarily, the melting temperature of the glass is lowered by changing the sequence of silicate formation reactions depending on the composition of the raw materials and by introducing small amounts of modifying low-melting additives.
In the present work we discuss the results of investigations aimed at lowering the melting temperature of and fining glasses in the system Li2O–Al2O3–SiO2 and improving their production properties by replacing the main siliconcontaining raw materials (quartz grit) by amorphous silica.
For glass melting and heat treatment we used laboratory electric furnaces with SiC heaters, equipped with equipment for mechanical mixing and bubbling in the molten glass. The glass was cooked in corundum crucibles with capacity 800 ml at temperatures no higher than 1620°C with soaking times to 7 h followed by pouring into heated metal molds. After the molten glass was poured out, the mold with the glass was placed for rough firing in a muffle furnace heated up to 450°C and soaked for 4 h, after which the furnace was allowed to cool to room temperature.
Glasses with the following compositions in the system Li2O–Al2O3–SiO2 were studied (%): 50 – 62 SiO2; 6 – 10 P2O5; 22 – 26 Al2O3; 0.6 – 2 MgO; 0.5 – 2 ZnO; 0.3 – 4 CaO; 0.5 – 4 BaO; 1 – 4 TiO2; 1 – 4 ZrO2; 0 – 2 As2O3 and variable Li2O content from 3.5 to 5.0%. The structure and phase transformations in the glasses were studied by means of DSC (Netzsch STA 449 differential-scanning calorimeter) and IR spectroscopy (Nicole 380 IR Fourier spectrometer). The phase composition of the crystallized materials was determined by XPA (Bruker D2 Phaser diffractometer).
The particularities of the silicate formation processes were studied by comparing the phase composition of the batches heat-treated in the temperature interval from 1000 to 1400°C in 100°C steps and soaking at each temperature for 1 h in a laboratory electric furnace with carbide-silicon heaters. The batches were prepared using ultrapure raw materials for making optical glass, and quartz grit or amorphous silicon was used to introduce silicon oxide.
The content of the main component in the quartz grit used, which was a product of comminution of vein quartz [6], was 99.86%. Amorphous silicon oxide is a synthetic material characterized by higher reactivity [7], and according to 14-4TU6-09-5016–82 contains 99.99% of the main component. The results of heat treatment of the batches are presented in Table 1.
Sintering of batch based on amorphous silicate with different lithium oxide content is accompanied by compaction and shrinkage. Loose, uniform, microporous, white sinters, which easily crumble in hand, are formed in the temperature range 1000 – 1300°C. Heating to 1400°C significantly increases the strength of the liquid phase, whose presence cannot be determined visually.
Sinters of batches containing crystalline quartz possess a more nonuniform and large-pore structure. The strength of the sinters and the pore size increase as temperature increases from 1000 to 1300°C. The appearance of a liquid phase and associated shrinkage by approximately 50% are observed at 1400°C.
On the whole the strength and vitrification of sinters with quartz grit are greater than that obtained with amorphous silicon. The looser and more uniform structure of batches with amorphous silica facilitate the removal of the gas phase formed as a result of the decomposition of raw materials.
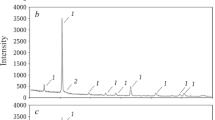
The results of the XPA investigation of the phase composition of the sinters are presented in Fig. 1.
At 1000°C lithium meta- and orhtosilicates and traces of two high-temperature modifications of quartz, viz., α-cristobalite and α-tridimite, are present in batches with lithium oxide content >3 wt.%. At 1000°C lithium silicates start to melt and dissolve tridimite and silicates and aluminum phosphate become the main crystalline phase. In addition, the reflections associated with the existence of polymorphic modifications of silica become weaker and new reflections attesting to the appearance of lithium aluminate and aluminum silicate appear. An increase of the heat-treatment temperature to 1300°C is accompanied by the final recrystallization of tridimite and cristobalite into a high-temperature modification of quartz (d =2.70 Å), and at 1400°C aluminum phosphate and a high-temperature modification of quartz, distinguished from the usual modifications by the crystallographic characteristics (see Fig. 1 a), are detected in the sinter.
At 1000°C a low-temperature modification of quartz (d =3.34 Å) and a poorly resolved doublet near the strongest reflections due to β-eucryptite and β-spodumene solid solutions are present in similar batches with quartz grit. As temperature increases to 1100°C the intensity of the quartz phase remains at the previous level, while two peaks (d =3.51 and 3.47 Å) with almost identical intensity, which belong to β-eucryptite and β-spodumene, appear near the angle 2θ =25°. As temperature continues to increase, the intensity of the reflections due to β-eucryptite and α-quartz gradually decreases while the intensity of the reflections due to β-spodumene solid solutions, which comprise the main crystalline phase at 1400°C (see Fig. 1 b), increases.
As follows from the data presented above, the silicate formation reactions in batches with amorphous silica and quartz grit differ considerably: in batches with amorphous silica they proceed via the formation of predominately double silicates, while triple silicates form in batches with quartz grit. In addition, the intensity of the diffraction peaks of crystalline phases forming in batches with quartz grit is much greater than in batches with amorphous silica (in Fig. 1 b the scale along the intensity axis is compressed by a factor of 2).
Thus, the use of amorphous silica results in the formation of a smaller quantity of crystalline phases and facilitates the removal of the gaseous products of the decomposition reactions of the raw materials and, because the lithium bisilicates melt, forms a lower-melting glass phase at temperatures below 1400°C. This makes it possible to lower the cooking and fining temperature of lithium-aluminum-silicate glasses in industrial glassmaking.
A two-stage technology for making low-alkaline lithium-aluminum-silicate glasses can be proposed on the basis of the investigations of the reactions occurring in batches. At the first stage the initial batch must be heat-treated at temperature about 1200°C for several hours and after cooling the sinter formed is ground and a uniform fine powder with particle size 40 – 60 μm is obtained. At the second stage this powder is used to make glass.
The proposed batch-preparation schedule permits making glass from conventional raw materials and amorphous silica, but in the latter case the molten glass is of higher quality even at lower cooking temperature (1600°C instead of 1620°C for batch with quartz grit) and shorter soaking time: there are no undercooked particles and significantly fewer residual bubbles smaller than 1 mm.
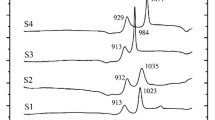
The IR spectrum presented in Fig. 2 is typical for glasses prepared using both types of silicon oxide and it attests that their structure is characteristic for materials belonging to the high-silica region of the ternary diagram of the lithium-aluminum-silicate system.
The intensity ratios and relative arrangement of the absorption bands in the spectrum make it possible to refer them to different groups of vibrations of the bonds in the siliconoxygen tetrahedron [SO4] in the structure of the glass: Si–O–Si stretching vibrations in the range 1100 – 1000 cm – 1 and deformation vibrations of the bonds at 450 cm – 1. The weak absorption band near 720 cm – 1 is probably associated with the vibrations of the Al–O bond in the [AlO4] tetrahedron, since in the presence of titanium ions in the glass aluminum-titanium complexes, in which the aluminum ion occupies the tetrahedral positions for oxygen, are characteristically formed [8].
The crystallization capacity of the glasses made using two forms of silica was determined by means of differential-scanning calorimetry (Fig. 3).
Analysis of the curves presented in Fig. 3 shows that the temperature T g of glass formation and crystallization for glass based on quartz grit is higher than for glass based on amorphous silicon. The glass formation temperature T g varies, as expected, in a 5°C range, and not only the temperature of the maximum of the exothermal effect due to crystallization increases by more than 100°C, but also the intensity of the effect is much lower than for glass based on amorphous silicon. This behavior remains for the entire range of compositions of the experimental glasses.
The experimental results make it possible to recommend replacing quartz grit by amorphous silica and in addition heat-treat the batch at temperature 1200°C followed by grinding for semi-industrial and industrial processes for making optical quality glass at temperatures no higher than 1600°C in order to obtain optically transparent glass ceramic materials in the system Li2O–Al2O3–SiO2.
References
H. Bach and D. Krause, Low Thermal Expansion Glass Ceramics, Springer-Verlag, Berlin (2005).
W. Holand and G. Beall, Glassñeramic Technology, The American Ceramic Society (2002).
A. I. Berezhnoi, Sitals and Photositals [in Russian], Mashinostroenie, Moscow (1966).
Z. Xiao and J. Zhou, “Microstructure and properties of Li2O–Al2O3–SiO2 glass-ceramics,” J. Open Mater. Sci., 5, 45 – 50 (2011).
A. N. Ignatov and E. Yu. Krekhova, “Development of a technology for producing large-size blanks of SO-115M optical sital,” in: Proc. 9th Intern. Conf. on Applied Optics 2010 [in Russian], GOI im. S. I. Vavilova, St. Petersburg (2010), Vol. 2, 80 – 81 (2010).
G. V. Kukolev, Silicon Chemistry and the Physical Chemistry of Silicates [in Russian], Vyssh. Shkola, Moscow (1966).
Technical Conditions 5726-001-11496665–97: Quartz Concentrates from Natural Quartz for Making Optical Glass [in Russian], Ministry of Natural Resources of the Russian Federation, State Geological-Industrial Corporation ‘Kvartssamotsvety,’ Moscow (1997).
R. Ya. Khodakovskaya, Chemistry of Titanium-Containing Glasses and Sitals [in Russian], Khimiya, Moscow (1978).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 7, pp. 3 – 7, July, 2014.
Rights and permissions
About this article
Cite this article
Sigaev, V.N., Savinkov, V.I., Stroganova, E.E. et al. Glass Formation and Crystallization in the Lithium-Aluminum Silicate System: Effect of the Form of the Raw Materials on the Melting and Crystallization Properties. Glass Ceram 71, 225–228 (2014). https://doi.org/10.1007/s10717-014-9657-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-014-9657-3