Abstract
Collapse occurs in soils as a result of inter-particle bond breakage caused by wetting and/or stressing. Inter-particle bond is usually provided by either suction, clay, calcium carbonate or other salts but upon wetting and/or loading they undergo repacking due to bond softening/weakening. Despite the large body of research in this subject, there is still poor understanding of the process of softening/weakening and the collapse mechanism of certain bond elements, particularly CaCO3, considering its low solubility in water. Because CaCO3 is common in natural soils, reaching 1–30% contents in most commonly known natural collapsible soil, understanding its influence on the collapse phenomenon is crucial for geotechnical characterization of soils. In this study, the impact of calcite bond content and wetting fluid type on the collapse potential (CP) and rate of collapse of calcareous silty-clay soils are investigated. CP was estimated by the percentage decrease in height of an oedometer specimen due to wetting. Distilled water and a 5% acid solution (AS) were used as wetting fluids. Wetting was mainly done at 300 kPa overburden stress. Results reveal that the magnitude and rate of collapse are controlled mainly by the calcite content, and pH of wetting fluid. Both magnitude and rate of collapse decrease with increasing CaCO3 content. Increasing clay content resulted in higher CP for non-calcareous samples but resulted in lower CP for calcareous samples. Wetting with acid solution demonstrated higher CP and tends to prolong time to reach complete collapse resulting also in long-term collapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Unlike consolidation where the reduction in volume (or void ratio) is the consequence of time-dependent discharge of excess porewater, the reduction in volume for a collapsible soil is rapid and the result of water ingress into the soil structure and/or bond breaking caused by increased stresses (Jefferson et al. (2005); Fattah et al. (2012); Vandanapu et al. (2017)). Collapsible soils are usually capable of sustaining substantial high-applied vertical stress in their in situ conditions without significant volume change but when wetted, they undergo rapid and large reduction in volume (Alan and Robert 1988; Lawton et al. 1989; Rogers 1995; Assallay 1998). The water ingress into the soil is believed to destroy existing inter-particle bonds (that effectively support a relatively open metastable structure, the main prerequisite to collapse according to reference in Jefferson and Rogers (2012), resulting in densification of the soil through collapse. Common bond materials reported include suction, clay, and calcium carbonate (CaCO3) (Rogers et al. 1994, 1995; Osipov and Sokolov 1995; El Howayek et al. (2011); Jefferson and Rogers (2012); Milodowski et al 2015). Gypsum is another bond material common in Iraq and other regions (Fattah et al. 2012). Wetting, reduces the bonding and sudden restructuring occurs (Rogers et al. (1994); Mansour et al. (2008); Li et al. (2016); Ayeldeen et al. (2017)).
Collapsible soils are extremely common globally, presenting structural and geotechnical engineering problems. They form through both natural processes and human activity (Opukumo et al. 2022a). Those containing significant amounts of CaCO3 content can be classified as being calcareous in nature (references in Kishchuk (2000); Thompson (2007); Delage et al. (2008)).
In natural collapsible soils CaCO3 seems to dominate the literature as the principal bond element (reference in Li et al. 2016; Demars and Chaney 1982; Feda 1995; Lamas et al. 2002; Mansour et al. 2008; Milodowski et al. 2015). Calcareous (also known as carbonate) soils span a large range of soils widely distributed globally with the arid and semi-arid climates favouring more of their production (Houston et al. 2001; Seiphoori and Zamanian 2022 and references therein). For example, there is extensive occurrence of calcareous clays in the majority of countries of the Middle East Region and Australia (Kadry 1973; Mansour et al. 2008). In the UK, sections of the brickearth deposits of south Essex and Kent are described as calcareous silty-clays (Bell and Culshaw 2001; Milodowski et al. 2015; Culshaw et al. 2020). Wetted-state unstable marls occur in Austria, Canada, U.S., France, Iran, Switzerland, and Egypt [references in Yong and Ouhadi (2007)].
Despite the large amounts of CaCO3 content found in collapsible soils, its influence on the collapse phenomenon has continued to be debated. There are those who believe that the solubility of CaCO3 was too slow to demonstrate the sudden volume reduction mechanism (e.g., Smalley 1971; Mellors 1995; Osipov and Sokolov 1995; Mansour et al. 2008), whereas others think CaCO3 bonding enhances collapsibility, e.g., Milodowski et al (2015). In some cases, a soil containing CaCO3 raises arguments among investigators due to differences in study approach. The role of CaCO3 in the collapsibility of the Pegwell Bay brickearth for example, was questioned by Mellors (1995) while Milodowski et al (2015) emphasized its potential to influence collapse. Milodowski et al. (2015) based their argument on fabric studies of SEM images that revealed CaCO3 as the particle-to-particle connector.
These arguments are valid but may have resulted from a disregard that CaCO3 occurs in different polymorphs (calcite, aragonite or vaterite), with each capable of exhibiting different chemical behaviours under different environmental conditions. Secondly, laboratory collapse tests were traditionally carried out using only tap or distilled water as wetting fluid. These facts are almost ignored in the assessment of soil collapsibility despite recommendation by ASTM (D 5333 - 03) that field anticipated pore fluids should be simulated in laboratory testing regime for assessing soil collapsibility. The present work adopted a 5% acetic solution (pH 2.26 at room temperature) as wetting fluid to compare with the traditionally used distilled water (pH 6.70 at room temperature), in investigating the much less studied impact of calcite bond dissolution on the collapsibility of collapsible clayey samples innovatively manufactured in Opukumo et al. (2022b). It is common knowledge that acidic (pH < 5) precipitations are common in many regions of the world due to atmospheric pollution (Grennfelt et al. 2020). This study aimed at providing an insight on the need to include soil mineralogy, especially dissolvable minerals, in the geotechnical characterisation of soils and to further consider potential wetting fluid type in assessing their collapse potential (CP). The wetting load of samples was largely restricted to a maximum of 300 kPa (an equivalent of a 15 m high embankment load) in order to prevent crushing of inter-particle bond prior to the assessment of CP.
2 Materials and Method
2.1 Materials
Calcareous silty-clay samples manufactured by the precipitation of calcite into silty-clay mixes, through an innovative system (Opukumo et al. 2022b) were used here, because this present paper is a follow up of that study. Details of the procedures for manufacture are presented in the said reference. The method of sample production was carbonation by CO2 gassing of hydrated lime-soil mixes prepared to a predetermined density of about 1.0 Mg/m3 by volume controlled compaction. Lime contents were designed to yield predetermined quantities of CaCO3 based on the principle that, a gram of Ca(OH)2 completely carbonated (by CO2) was expected to be converted to CaCO3 in the ratio of 1:1.35, representing a 35% increase in mass. Generally, about 90% carbonation was recorded following checks by calculated mass gain, and experimental techniques (Thermogravimetric analysis and determination of organic carbon and total carbon after dry combustion). Finally, x-ray diffraction was used to identify calcite as the precipitated CaCO3 polymorph.
The materials used were quartz-silt extracted from a Lanton alluvium collected from Lanton River, Northumberland, UK; kaolin, a brand of English-China clay (Polwhite E grade) supplied by Imerys Performance Minerals Ltd, UK; hydrated lime supplied by Lafarge Tarmac, UK; and a pure CO2 gas supplied by SIP Industrial, Italy. Kaolin and quartz silt were chosen because of the chemical inertness while clay/silt proportions were merely chosen for convenience.
Table 1 shows a summary of the composition and physical characteristics of the samples produced directly into conventional oedometer rings. The calcite content (%) of samples were controlled to be within bands described as moderately (5–15), strongly (15–25), and very strongly (25–40) calcareous (reference in Kishchuk (2000)).
Samples exhibited very low dry densities (1 ± 0.1 Mg/m3) and exaggerated void ratios ranging between 1.5–1.8 and estimated porosities of 60 – 65%. These values are typical of young sedimentary deposits or normally consolidated clay (see Kokusho et al. (1982) and Shibuya et al. (1998)).
2.2 Methods
2.2.1 Test Specimen Preparation
Test samples were manufactured directly into oedometer rings; therefore, no further preparation was needed. The physical properties of samples, such as volume, dry density, void ratio and porosity were determined following Eqs. 1, 23 and 4.
where V is volume, r is radius, h is height, ρd is dry density, e is void ratio, ρs is particle density, and n is porosity.
2.2.2 Collapse Testing Apparatus
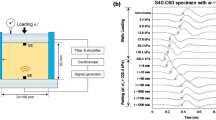
The one-dimensional oedometer method for determining collapse potential (CP) was adopted. The conventional oedometer apparatus used, its components and data acquisition system are shown below as Fig. 1. Calibration of both the conventional oedometer apparatus and transducers for measuring the vertical compression was previously carried out during routine laboratory maintenance by the technical staff at Geotechnical lab of the Newcastle University, Newcastle upon Tyne, UK where testing was carried out. The elastic deformation characteristics were determined in accordance with method described in BS 1377 (1990).
Two testing processes referred to as the “single oedometer” test (SOT) and “double oedometer” test (DOT) were adopted. In both, specimens prepared into oedometer rings were assembled into the oedometer cell. Two air-dried porous discs were placed; one at the base and the other on top of specimen. Dried porous discs were preferred to avoid altering moisture condition of specimens, as well as preventing further dilution of wetting fluid (acidic solution in particular). The assembled oedometer cell with specimen was then placed under the oedometer loading frame. A displacement transducer attached to the equipment and connected to a data acquisition system, was lowered onto the top hanger of the loading frame lever arm.
GDSLAB, a control and data acquisition software for geotechnical laboratory applications manufactured by Global Digital Systems Ltd was used to collect displacement data at intervals of 10 s. However, only total deformation at the end of each stage was desirable (Alan and Robert 1988). Data collection by the software was started simultaneously with loading. Because of the low compressibility of the calcareous specimens tested, loading was started at a 100 kPa. Incremental loadings of 100 up to 500 kPa were applied to all testing while unloading in reverse order was applied in a few cases to investigate any possible rebound or swelling of specimen. A few specimens were also loaded to beyond 500 kPa to a maximum of 860 kPa.
Loading increments by doubling was not considered because 300 kPa had been predetermined as the wetting stress. However, it is not unusual as Alan and Robert (1988) had recommended that what was important is that wetting stress is close to estimated foundation stresses of proposed project.
Collapse was triggered by wetting specimens with either distilled water or a 5% acetic acid solution at different overburden stresses. Against the traditionally used distilled water, an acidic solution was adopted for two reasons. Firstly, as an anticipated pore fluid in environments where acidic precipitations may be common, and secondly, the effect of dissolution of the particle bonding mineral (CaCO3) on the collapse phenomenon may not be seen if only distilled water was used. ASTM (D 5333 - 03) recommends that field anticipated pore fluids can be simulated in laboratory testing regime for collapsible soils instead of standard laboratory fluids.
In order to avoid air entrapment in the centre of specimens during wetting, care was taken to ensure that wetting was done in one-dimension. That is, wetting gradually from bottom to top according to the recommendation of Alan and Robert (1988). CP was defined in terms of the percentage decrease in height of specimens resulting from wetting, as shown in Eq. 5 (ASTM, D 5333 - 03).
where CP is collapse potential, ΔH is change in specimen height resulting from wetting, H0 is specimen height before wetting
A system traditionally adopted by many authors for classifying the degree or severity of collapse was proposed in 1975 by Jennings and Knight. Table 2 was modified by Bell and Culshaw (2001) in line with British perspective of collapse, and was adapted here to classify sample collapsibility. There, soils exhibiting CP less than 1% are not regarded as collapsible or metastable.
In the SOT, specimens are loaded incrementally in their ‘as prepared dry state’ up to a predetermined stress level, and allowed to equilibrate, then wetted progressively until completely inundated to induce collapse. Wetting was performed at 300 kPa overburden stress. Though the literature appear to favour 200 kPa as a general wetting stress, 300 kPa was chosen here because of the high dry stiffness of samples caused by CaCO3 content (Langroudi (2014) recognizes high dry stiffness as common with calcareous soils). At pre-wetting stage, each loading was maintained until compression was completed or no more than 0.05 mm/h compression was recorded (Alan and Robert 1988) while at wetting stage, the 300 kPa load was maintained for 24 h before further load increments. While collapse was defined to be the settlement occurring within 10 min of wetting (Fookes and Best 1969), longer periods were necessary to allow for sufficient bond dissolution in accordance with ASTM (D 5333 - 03). At the end of the 24-h soaking, further increments to reach 500 kPa were carried out before decrements in the reverse order down to 200 kPa.
The DOT involved testing two nominally identical specimens, one as in the SOT and the second differing as follows. The specimen placed under the oedometer loading frame was inundated simultaneously with the initial 100 kPa loading. This is here referred to as ‘presoaked’. Incremental loading and unloading procedures are identical to the SOT.
The DOT is used to estimate the amount of deformation or collapse that would occur if a soil sample was wetted at different stages of its stress history, a process well presented by Fredlund and Gan (1995); Rogers (1995); Alan and Robert (1988). The curves of overburden stresses against axial deformations for both presoaked and SOT are compared. The amount of deformation at each stress level on the presoaked curve relative to identical stress level on the SOT curve represent an estimate of the degree of collapse that could occur if the soil was wetted at such stress level during its loading history (Alan and Robert 1988).
3 Results and Discussions
3.1 Collapsibility of Non-Calcareous Silty Clays
Low dry density unbonded, non-calcareous (i.e., 0% CaCO3 content) samples were initially tested as control in order to enhance the understanding of the role of CaCO3 in the collapse behaviour of calcareous soils. The impact of the wetting fluid type (in the case of collapse upon wetting) on these soils was also examined. These samples labelled A0, B0, and C0 have been described in Table 1 as well as in Opukumo et al. (2022b). At least three specimens were tested in each and mean final compressions at each loading stage and CP taken. Standard deviations between 0.1 and 1.0 were estimated for the CP values.
Oedometric specimens with dry density approximately 1.01 Mg/m3 were wetted with distilled water or 5% acetic acid solution, at 300 kPa overburden stress in the SOT while only the acidic solution was used to pre-soak specimens in the DOT. Figures 2 and 3 present deformation curves obtained from both the single and double oedometer collapse testing. The axial deformations of the samples due to loading before and after wetting, as well as CP and degree of severity, for the different wetting fluids, are summarized in Table 3.
In the SOT (Fig. 2), curves for each pair of specimens (distilled water and acid solution wetted) were expected to show uniform deformations up until the wetting stage (300 kPa) thereafter, they could differ depending on the effect of the wetting fluid type. However, minor variations have been recorded in some cases. These are not thought to be significant and can be attributed to experimental errors arising from the setting up of the apparatus and small operational differences.
Generally, clays are less easily densified compared to silts due to their diffuse double layer, flocculated structure and high water-retention capacity. This was demonstrated by the amount of compressions obtained during pre-wetting loading. Both the early (pre-wetting stages) and the late (post-wetting) halves of the compression curves of single oedometer tests appear to indicate a higher deformation with lower clay/silt ratios in these unbonded non-calcareous samples (Fig. 2). Similarly, presoaked curves of the DOT (Fig. 3) show greater wetted deformations with decreasing clay/silt ratios at every stage of loading. Conversely, greater CPs (see Table 3 above) were recorded for higher clay/silt ratios in both wetting fluid types of the single oedometers. While CPs estimated from the double oedometers at 300 kPa overburden stress may closely relate (by a maximum difference of ~ 1 (Table 3)) to those of the single oedometers, at lower overburden stresses CPs have not followed a pattern but appear in many cases to show higher values compared to the single oedometers.
Following results in Table 3, especially from the single oedometers, there seems to be an indication of a textural (clay/silt ratio) control on both deformability and collapsibility. Of course, clays contribute to in situ strength of collapsible soils (Osipov and Sokolov (1995); El Howayek et al. (2011); Jefferson and Rogers (2012)).
As noted earlier, clay resistance to deformation in a dry state leaves more room for samples containing higher clay content to settle or collapse when wetted. Again, clay minerals are generally known for their inter-particle attraction which in principle, contributes to bond strength in their unsaturated state. The type of bond strength or particle affinity, however, depends on the distance between the particles. Nevertheless, this bond strength resulting from particle attraction can easily be broken by factors such as, increased moisture content above a certain threshold. Increased water content in the diffused double layers of a clay structure increases distance between the layers, and the force of attraction holding layers together is broken, making the clay structure weaker (Mitchell and Soga 2005). On the other hand, quartz silt particles possess no affinity for each other and only derive strength from frictional interlock. However, this depends on grain size.
Sample C0 contains 80/20% kaolin clay/quartz silt which explains why it exhibits lower pre-wetting deformations in its unsaturated state relative to samples B0 and A0 having 65/35 and 50/50% clay/silt proportions, respectively. Therefore, in the same way, B0 deforms less than A0 at pre-wetting stages and collapsed more post-wetting. However, it is important to note that the amount of structural collapse upon wetting may depend on the pore spaces available to accommodate such collapse as well as the amount of overburden load to enhance collapsing.
In addition, the initial void ratio and porosity, which are factors that determine how much a fine-grained soil may compress under loading, have shown decreasing values with increasing clay contents in samples before testing (Table 3). However, at the end of early loading (i.e. pre-wetting) the void ratio trend was reversed, increasing with higher clay content, confirming higher clay content leads to a higher resistance to densification. Since samples with higher silt contents compressed significantly at pre-wetting stages, they might have had their structure broken and are more packed with less void space available for any further densification.
Considering curves in Fig. 2 and CP values in Table 3, it is evident that wetting fluid type (distilled water or 5% acid solution) did not influence the collapsibility of these non-calcareous samples indicating that collapse was therefore merely mechanically driven. Identical specimens wetted with either distilled water or the 5% acidic solution collapsed to approximately the same degree and compression trends after the collapse region were also similar to those in the pre-wetting region. This demonstrates the geochemical inertness of the samples to both the 5% acidic solution and distilled water. The mechanism of collapse in these samples was possibly a result of broken physical or physicochemical force such as suction and molecular attraction, which provided the initial strength.
As is expected of non-expansive soils, the kaolin clay (with quartz silt) having relatively low water absorption capacity, exhibited irrecoverable behaviour after loading and unloading. This has been illustrated by relatively flat curves with close to zero or marginally negative slopes in all the rebound curves. In other words, no significant recovery or swelling pressures can be expected from these kinds of samples after densification. Beyond the collapse region, further settlements occurred indicating that samples still exhibited a loose state relative to the prevailing overburden stresses.
3.2 Collapsibility of Calcite Bonded (Calcareous) Samples
The key feature of samples here is that CaCO3 crystalizes in situ and acts as the inter-particle bonding material. Samples basically possess the main criteria (low dry density, high void ratio and porosity) of a typical collapsing soil. These specimens were manufactured as would be tested, i.e., directly into oedometer rings with no further preparations needed.
Samples contained calcite contents of varying degrees characterising them into moderately (~ 12%), strongly (~ 25%) and very strongly (~ 40%) calcareous samples. They were all oven-dried under temperatures of 45 – 50 0C to produce nearly completely dry or unsaturated state (residual moisture content of 0.12 to 0.45%) before testing.
The physical properties of the test specimens are presented in Table 1. Results obtained after testing are summarized in Table 4 with deformation collapse curves for the moderately calcareous samples presented in Fig. 4 and Fig. 5 as representative examples. The CP from single oedometer tests are those achieved at 300 kPa overburden stress while those from the double oedometer (acidic solution presoaked) were estimated at 100, 200 and 300 kPa overburden stresses.
As can be seen in Table 1, these specimens are highly porous and could only have exhibited structural stability through the CaCO3 inter-particle cementation. This was intended to enhance understanding of the impact of calcite content (or degree of calcareousness) and cement dissolution on both compressibility and the collapse phenomenon (sudden volume decrease due to wetting). The Figures above show relationships between different samples with similar calcite cementation levels. The impact of the degree of calcareousness is discussed later.
Generally, different samples with comparable degrees of calcareousness maintained similar physical properties, such as initial dry density, void ratio and porosity with very minimal differences following both calcite and clay contents. A small increase in dry density can be observed with increasing calcite content while initial void ratio and porosity have reduced with increasing clay and calcite contents. From a soil mechanics viewpoint, these parameters indicate the capacity for smaller compressions and CPs with increasing clay and calcite contents. This is evident in the Table 4.
Furthermore, considering collapse phenomenon as an aftermath of calcite-bond dissolution, it is reasonable to anticipate the degree of collapse following the amount of dissolution. That has been demonstrated by comparing the respective CP values obtained from distilled water wetting and acidic solution wetting. The results are as expected because acidic solutions (pH ≤ 5) readily dissolve CaCO3 in soils as reported in Al-Kaysi (1983), increasing their pore volume and weakening inter-particle bonding and giving room for higher wetted deformations under loading. In the same vein, it is also expected to have lower CPs where distilled water applied as wetting fluid since calcite remains insoluble or extremely weakly soluble in distilled water (Smalley (1971); Mansour et al. (2008); Li et al. (2016)).
Additionally, it is also logical to expect that higher CaCO3 contents could lead to higher pore volumes after dissolution occurred, with a consequent higher CP. Unfortunately, the present results have not corroborated this.
The pre-wetting compressions have also appeared to decrease with increasing CaCO3 content (calcareousness). That is, stiffness increased with CaCO3 content. For instance, pre-wetting compression (at 300 kPa) values approximating 1.2, 0.15 and 0.12 mm were recorded for the moderately, strongly, and very strongly calcareous samples, respectively but there was no identifiable trend relating to their clay/silt ratios. In some cases, higher clay contents yielded higher pre-wetting compressions, a situation in contrast with the non-calcareous samples. It is not clear if this is a consequence of other factors, which may include inter-particle bonding patterns. However, their CP values both for the distilled water wetted and acidic solution wetted tend to show similar influence of clay/silt ratios as in the non-calcareous samples. CPs (at 300 kPa overburden stress) from single oedometers ranged between “severe trouble to very severe trouble” (16 to 26%), “no trouble to trouble” (0.4 to 10%), and “no trouble to moderate trouble” (0.09 to 3.6%) for moderately, strongly and very strongly calcareous samples, respectively with higher values resulting from acidic solution wetting. Even though comparable classes of collapse severity were also estimated from the double oedometers at the same 300 kPa overburden stress, discrepancies existed in terms of actual CP values. Double oedometer revealed overestimated CP values in some cases and underestimated values in others. At lower overburden stresses CPs are obviously lower.
On the contrary, beyond the collapse region, amount of compression for both distilled water and acidic solution wetted conditions, trends appeared to align with clay/silt ratios. It is interesting to note that, irrespective of the degree of calcareousness, samples having lower clay/silt ratios exhibited higher settlements beyond the collapse region. A similar trend was displayed by the pre-soaked curves at all loading stages of the double oedometer testing with both pre-soaked curves and wetted-after-loading curves nearly converging from the collapsed stage onwards. This clearly demonstrates a lesser impact of wetting on the compression of more clayey soils compared to lesser clayey ones (Fig. 5).
3.3 Effect of CaCO 3 Content on Collapse Potential
In the literature, there is the absence of quantifying impact of CaCO3 on the CP of collapsible soils despite arguments in favour of the role of CaCO3 on collapsibility.
According to Langroudi (2014), the crystal shape and structure of CaCO3 in soils differ following the process of formation, and these differences influence the wetted behaviour of such soils. In the present samples, SEM images [Figure S5 (a and b) of Opukumo et al. (2022b)] roughly reveal calcite occurring as inter-particle meniscus cement supporting silt grains and clay clods in some samples. In others with larger amounts of clay content, calcite occur within clay matrix that overwhelmed the few silt grains, thus, reinforcing the clay structure. However, these microstructures were not clearly studied due to poor SEM images, and it was difficult to relate them to the behaviour of samples.
While it is debatable, increased CaCO3 content relates to increased bonding. Expectedly, higher bonding should lead to greater stability, thus, a reduced collapsibility under similar stress conditions. Keykha et al. (2014) demonstrated that increased CaCO3 precipitate improved shear strength of soil. Milodowski et al. (2015) also identified calcite bond occurring as reinforcement in meniscus clay bridges to reduce both the dispersion of the clay and collapse upon wetting. However, the relationship between strength and CP identified in the present study appear to depend more on two variables. As illustrated in Fig. 6, CaCO3 content and its degree of dissolution are critical in controlling the magnitude of collapse. Wetting with distilled water and a 5% acidic solution reveals that fluid pH impacts CP.
Regardless of a supposed higher stiffness of the moderately calcareous (~ 12% CaCO3 content) samples compared to the non-calcareous (0% CaCO3) ones, they still yielded greater CPs in all acid solution wetted conditions and in one case of the distilled water wetted condition. While this occurrence could be explained by the different hypothesized collapse mechanisms of the samples, it is worth noting that at certain construction loads a more stable ground could rather present greater trouble if in the long-term wetting fluids cause chemical dissolution of components of such a ground.
Firstly, the non-calcareous samples had undergone higher pre-wetting compressions due to loading because of their unbonded nature, reducing their capacity for further densification upon wetting. Secondly, while non-calcareous specimens seemed to undergo only a mechanical phase (particles packing) upon wetting, the moderately calcareous specimens were additionally subjected to a chemical bond dissolution phase, especially in the acidic solution wetting condition. In soil collapse, disaggregated clay bonds/bridges usually migrate into pore spaces during repacking (Klukanova and Frankovska (1995); Mellors (1995)). While this causes total soil volume reduction, it will not decrease the volume of solids. In the case of CaCO3 bonds, dissolution can result also in solid lose, thus, a greater total soil volume reduction. This could mean that in a typical field condition, soils containing higher CaCO3 content relative to others would yield higher voids upon CaCO3 dissolution, thus exposing such soils to greater collapses.
Nevertheless, the oedometer experimental data here suggested otherwise. Increasing the CaCO3 content of the samples from ~ 12 to ~ 25% and to ~ 40% has rather significantly reduced the propensity of dissolution (even in acidic solution) and consequent collapse. For instance, increasing CaCO3 content from ~ 12 to ~ 25% reduced collapsibility by about 64 – 75% and further increase of CaCO3 content to ~ 40%, further reduced CP by 86 – 91%. Extrapolations in Fig. 6 suggest that for all samples tested, despite their very low dry density (1 ± 0.1 Mg/m3), CaCO3 contents reaching about 44% could reduce CP to about 0%.
The high degrees of calcareousness have rather strengthened (or stabilized) the samples, which though maintained a metastable structure, are not supportive of the sudden densification upon wetting phenomenon. That is, while those specimens still possess an open structure their inter-particle (and perhaps, inter-clods) bonding became too strong to be easily eliminated, particularly by distilled water, thus, yielding nearly negligible CP values. This is consistent with the collapse behaviour of the calcareous soils of Arizona and New Mexico cited in Demars and Chaney (1982). Those soils were grouped into five stages on their magnitude of collapsibility consequent of their levels of cementation. From weakly bonded (collapsible) to very strongly bonded (not influenced by moisture), and further to moderate hard rocks (requiring rock mechanics analysis).
However, wetting the strongly calcareous (~ 25% CaCO3) samples with the acidic solution results in quite substantial CP values but a further increase in calcite content to about ~ 40% drastically reduced CP. It is important to note here that, it is common knowledge that the dissolution of increased carbonate contents would certainly require greater amounts of solvent. Unfortunately, the amount of dissolving fluid in the standard oedometer cell used cannot be varied. Perhaps, a leaching process could be used in future to investigate if higher calcite contents could lead to higher CPs. However, Smalley (1971); Mansour et al. (2008); Li et al. (2016) have noted that a carbonate soil requires several cycles of wetting and drying to dissolve CaCO3 before collapsing. On the contrary, authors in Mansour et al. (2008) have described leaching process as differing from the principle of sudden collapse upon wetting. They termed such a process to result in what they referred to as chemical piping or leaching-and-collapse.
It is clearly demonstrated here in the \* MERGEFORMAT Fig. 6 that for CaCO3 at certain contents the collapsibility of calcareous silty-clay soil increases. On the other hand, increasing CaCO3 content above certain levels becomes beneficial for the stability of the soil. However, this appears to be a function of the ratio of CaCO3 content to dissolving fluid. Nonetheless, other factors, such as density, porosity, void ratio, etc. capable of affecting CP need to be considered in conjunction with CaCO3 content.
3.4 Effect of Wetting Fluid Type and Rate of Collapse
The phenomenon of sudden volume decrease upon wetting commonly referred to as hydrocollapse, hydroconsolidation or hydrocompaction is greatly a function of time rate of inter-particle bond elimination and ease of particles repacking (or mechanism of collapse) under a constant load. Plots of axial deformation against time during the collapsing stage (i.e., wetted stage) of single oedometer tests are presented in Figs. 7, 8, 9, 10. The shapes of curves are comparable to that achieved from standard consolidation tests on fine-grained soils and evidently are three distinct regions of collapse. The first region is a time-lapse stage indicating the initial period when wetting fluid flows through bottom porous disc and enters the specimen in one-dimension. The slope of the curves at this stage depended on the nature and initial strength of specimen where in some cases (especially in non-calcareous sample owing to near absence of cement bonding) a clear distinction from the second region appearing difficult.
The second region represents period when the wetting fluid completely saturated the specimen and it is believed that at this stage inter-particle, inter-macropeds (or aggregates) and intra-macropeds bonding and bridges are weakened, reduced and/or eliminated. The bulk of collapse takes place at this stage. In cases of low initial bonding and where distilled water applied as wetting fluid, nearly complete collapse occurred here but in high calcite bonding collapse gradually continues in the acidic solution wetted condition.
Region 3 can be interpreted differently at certain degrees of calcareousness and differences in wetting fluid type. For a distilled water wetting, this region represented the end of a complete collapse and the beginning of some sort of minor post-collapse deformation. On the other hand, where an acidic solution applied as wetting fluid, this region witnesses either some minor or major post-collapse deformation depending on specimen degree of calcareousness. This is therefore, indicative that although sudden large settlement may occur very quickly, deformation may continue for longer periods perhaps until bond material dissolution is fully achieved.
There are reports in the literature arguing that collapse is only achieved quickly in laboratory flooded oedometer specimens, usually within several hours or less. Vandanapu et al., (2017) recorded collapse occurring 6 – 9 h at a rate of 0.2 mm/min. Booth (1977) report three sands (soils A, B, and D) tested under a range of moisture, density, and stress conditions to have achieved 90% collapse in not more than 25 min. Huang, 1989 investigated a lean clay (Lawton et al. 1992) and stated a variation in time required to achieve complete collapse depending on overburden stress at wetting. About 4 and 0.5 h were noted for overburden stresses of 96 kPa and 383 kPa, respectively for a soil compacted to Rs = 80% and Si = 36%. Lawton et al. (1992) investigated soil samples with different proportions of kaolin clay and Ottawa sand under marginally similar conditions with a 400 kPa stress at wetting and reported time to achieve complete collapse ranging between 15 and 100 min. They found that the rate of collapse was increasing with increasing clay proportion up to around 40% kaolin, above which the rate of collapse was nearly constant. Additionally, that collapse occurred rapidly (not more than 2 h) even for higher clay proportions. Fookes and Best (1969) said a 95% collapse settlement upon wetting is achievable within 10 min while anything above 10 min is referred to as subsidence settlement.
In the present study, four key factors have been identified that control the time rate of collapse in the silty-clay samples (both calcareous and non-calcareous). They are calcite content, density, overburden stress at wetting, and wetting fluid type. Even though clay content did not appear to delay complete collapse as is illustrative of the second region, it is seen to limit deformation in terms of total settlement experienced by sample. The higher the clay content the less total deformation a specimen undergoes.
Even though in many cases, soaking of specimen in the oedometer test was sustained for about 24 h, majority of collapse in all the samples occurred merely in not more than 1500 s (25 min). However, samples with high degrees of calcareousness demonstrated huge potential for further, but gentler continuous collapsing where they are wetted with acidic solution (see Figs. 9 and 10 in particular). This potentially demonstrated both collapse settlement (achieved in less than 10 min) and subsidence settlement (collapse achieved in more than 10 min) according to Fookes and Best (1969).
Surprisingly, non-calcareous samples wetted with distilled water achieved complete collapse at about 1000 s as against calcareous samples wetted under similar conditions achieving complete collapse in merely about 300 s. This therefore, alludes to the fact that while ordinary water may go through a process of gradual weakening of clay macropeds and clay bridges between macropeds and silt particles, it is nearly nonreactive with calcite cementation. Smalley (1971); Mansour et al. (2008); Li et al. (2016) have argued that only a prolonged period of sustained or several cycles of wetting and drying may relatively dissolve calcite.
Non-calcareous samples also appear to collapse quicker under acidic solution wetting than in distilled water wetting. Perhaps, this is due to the effect of acid on clay mineral, such as, clay particles disaggregation, dissolution of alumina layers, reduction of clay Al2O3, MgO, CaO and K2O contents (Panda et al. (2010); Komadel (2016)). Nevertheless, a microscopic study was not carried out to confirm this in the present research.
4 Conclusions
From the experimental study carried out, the following conclusions are reached.
In non-calcareous (0% calcite) samples, collapse is practically entirely mechanically controlled, and the time lag between wetting and complete collapse is instantaneous. The mechanics of collapse been controlled mainly by broken clay bonds. The more clayey the sample the less the pre-wetting compression, which availed greater void space for consequent settlement upon wetting. However, where calcite was present, its content controls CP, and high calcite contents up to about 40% have been found to eradicate collapse in the silty-clay soils irrespective of their physical properties. For instance, notwithstanding the relatively low dry densities (1 ± 0.1 Mg/m3) and high void ratios of samples, insignificant collapses were recorded in the high CaCO3 samples.
CP results have demonstrated that both the SOT and DOT can produce comparable results under certain conditions. However, outside those conditions, variances occur. Thus, they can be used as index tests as recommended by Fredlund and Gan (1995); Jefferson et al., (2005).
Generally, acidic solution wetting produces higher collapse potentials in calcareous soils than distilled water wetting because of the effect of calcite dissolution. Where distilled water applies as wetting fluid, complete collapse is achieved faster in calcareous soils than in non-calcareous ones because of calcite support and its inertness to water dissolution.
Additionally, wetting of non-calcareous samples with low acidic solution results in quicker collapses compared to wetting with distilled water. This perhaps is due to the ability of acids to destroy the chemistry and structure of clay (Panda et al. (2010); Komadel (2016)).
Results revealed that depending on certain conditions (e.g., density and calcite content) acidic solution wetting will produce higher CP in a calcareous soil compared to a non-calcareous one of similar physical properties. For example, the three samples with ~ 12% calcite content yielded CPs higher than their counterparts having 0% calcite by a range of approximately 10 to 30%.
Calcite content and wetting fluid type defined collapses as either rapid (< 10 min) (“collapse settlement”) or slow (> 10 min) (“subsidence settlement”) according to Fookes and Best (1969). The findings here showed that in high calcite contents (≥ 25%) subsidence settlement will continue for a long time where soil was soaked with a low pH solution.
Availability of Data
All data generated or analysed during this study are available in the first author’s PhD thesis (Opukumo 2020).
Abbreviations
- AS:
-
Acid solution
- CP:
-
Collapse potential
- DOT:
-
Double oedometer test
- MT:
-
Moderate trouble
- NT:
-
No trouble
- SOT:
-
Single oedometer test
- ST:
-
Severe trouble
- T:
-
Trouble
- VST:
-
Very severe trouble
References
Alan JL, Robert TS (1988) Determination of collapse potential of soils. Geotech Test J 11(3):173–178
Al-Kaysi SC (1983) Physical and chemical characterization of carbonate minerals in Iraqi soils. University of Newcastle upon Tyne, Newcastle upon Tyne
Assallay AM (1998) Structure and hydrocollapse behaviour of loess. Doctoral dissertation, Loughborough University, Loughborough
ASTM (D 5333 - 03) 'Standard Test Method for Measurement of Collapse Potential of Soils'.
Ayeldeen M, Negm A, El-Sawwaf M, Kitazume M (2017) Enhancing mechanical behaviors of collapsible soil using two biopolymers. J Rock Mech Geotech Eng 9(2):329–339
Bell FG, Culshaw MG (2001) Problem soils: a review from a British perspective. In: Jefferson I, Murray EJ, Farangher E, Fleming PR (eds) Problematic soils. Thomas Telford, Nottingham
Booth AR (1977) Collapse settlement in compacted soils. CSIR Research report 324. Natl Inst Transp Road Res 13:1–34
BSI 1377, (1990) Methods of test for soils for civil engineering purposes. British Standard Institution, London
Culshaw MG, Northmore KJ, Jefferson I, Assadi-Langroudi A, Bell FG (2020) Chapter 6 collapsible soils in the UK. Geological Society, London, Engineering Geology Special Publications, 29(1):187–203
Delage P, Cui Y-J, Antoine P (2008) Geotechnical problems related with loess deposits in Northern France. arXiv preprint arXiv:0803.1435
Demars KR, Chaney RC (1982) Geotechnical properties, behavior, and performance of calcareous soils. In: A symposium sponsored by ASTM Committee D-18 on soil and rock, Ft. Lauderdale, Fla., 20 Jan 1981. ASTM International
El Howayek A, Huang P-T, Bisnett R, Santagata MC (2011) Identification and behavior of collapsible soils. Joint Transp Res Prog Tech Rep Ser Rep SPR-3109, Indiana Department of Transportation and Purdue University, West Lafayette, IN
Fattah MY, Al-Musawi HH, Salman FA (2012) Treatment of collapsibility of gypseous soils by dynamic compaction. Geotech Geol Eng 30:1369–1387
Feda J (1995) Mechanisms of collapse of soil structure. In: Genesis and properties of collapsible soils, pp 149–172
Fookes PG, Best R (1969) Consolidation characteristics of some kate Pleistocene periglacial metastable soils of East Kent. Q J Eng GeolHydrogeol 2(2):103–128
Fredlund DG, Gan JKM (1995) The collapse mechanism of a soil subjected to one-dimensional loading and wetting. NATO ASI Ser C Math Phys Sci Adv Study Inst 468:173–206
Grennfelt P, Engleryd A, Forsius M, Hov Ø, Rodhe H, Cowling E (2020) Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 49:849–864
Houston S, Houston W, Zapata C, Lawrence C (2001) Geotechnical engineering practice for collapsible soils. Geotech Geol Eng 19(3–4):333–355
Jefferson I, Rogers C (2012) Collapsible soils. In: Proceedings of ICE manual of geotechnical engineering, ICE Publishing, London, pp 391–411.
Jefferson I, Rogers C, Evstatiev D, Karastanev D (2005) Treatment of metastable loess soils: lessons from Eastern Europe. Elsevier Geo Eng Book Ser 3:723–762
Kadry LT (1973) Distribution of calcareous soils in the Near East Region, their reclamation and land use measures and achievements. FAO Soils Bulletin (FAO)
Keykha HA, Huat BB, Asadi A (2014) Electrokinetic stabilization of soft soil using carbonate-producing bacteria. Geotech Geol Eng 32:739–747
Kishchuk BE (2000) Calcareous soils, their properties and potential limitations to conifer growth in southeastern British Columbia and western Alberta: a literature review. Northern Forestry Centre.
Klukanova A, Frankovska J (1995) The Slovak Carpathians loess sediments, their fabric and properties. In: Genesis and properties of collapsible soils. Springer, pp 129–147
Kokusho T, Yoshida Y, Esashi Y (1982) Dynamic properties of soft clay for wide strain range. Soils Found 22(4):1–18
Komadel P (2016) Acid activated clays: materials in continuous demand. Appl Clay Sci 131:84–99
Lamas F, Irigaray C, Chacón J (2002) Geotechnical characterization of carbonate marls for the construction of impermeable dam cores. Eng Geol 66(3–4):283–294
Langroudi AA (2014) Micromechanics of collapse in loess. University of Birmingham, Birmingham
Lawton EC, Fragaszy RJ, Hardcastle JH (1989) Collapse of compacted clayey sand. J Geotech Eng 115(9):1252–1267
Lawton EC, Fragaszy RJ, Hetherington MD (1992) Review of wetting-induced collapse in compacted soil. J Geotech Eng 118(9):1376–1394
Li P, Vanapalli S, Li T (2016) Review of collapse triggering mechanism of collapsible soils due to wetting. J Rock Mech Geotech Eng 8(2):256–274
Mansour ZM, Chik Z, Taha MR (2008) On the procedures of soil collapse potential evaluation. J Appl Sci 8(23):4434Y – 4439
Mellors TW (1995) The influence of the clay component in loess on collapse of the soil structure. In: Genesis and properties of collapsible soils. Springer, pp 207–216
Milodowski AE, Northmore KJ, Kemp SJ, Entwisle DC, Gunn DA, Jackson PD, Boardman DI, Zoumpakis A, Rogers CDF, Dixon N (2015) The mineralogy and fabric of ‘Brickearths’ in Kent, UK and their relationship to engineering behaviour. Bull Eng Geol Environ, pp 1–25
Mitchell JK, Soga K (2005) Fundamentals of soil behavior. Fundamentals of soil behavior, (Ed. 3)
Opukumo AW (2020). Sodium silicate stabilization of collapsible clayey calcareous soils (Doctoral dissertation, Newcastle University)
Opukumo AW, Davie CT, Glendinning S, Oborie E (2022a) A review of the identification methods and types of collapsible soils. J Eng Appl Sci 69(1):1–21
Opukumo AW, Davie CT, Glendinning S (2022b) A simple laboratory method to simulate calcite bonded loose-structured soil samples for collapsibility study. J Eng Appl Sci 69(1):1–20
Osipov VI, Sokolov VN (1995) Factors and mechanism of loess collapsibility. In: Derbyshire E, Dijkstra T, Smalley IJ (eds) Genesis and properties of collapsible soils. Springer Netherlands, Dordrecht, pp 49–63
Panda AK, Mishra BG, Mishra DK, Singh RK (2010) Effect of sulphuric acid treatment on the physico-chemical characteristics of kaolin clay. Colloids Surf A 363(1–3):98–104
Rogers CDF (1995) Types and distribution of collapsible soils. In: Genesis and Properties of Collapsible Soils. Springer, pp 1–17
Rogers CDF, Dijkstra TA, Smalley IJ (1994) Hydroconsolidation and subsidence of loess: studies from China, Russia, North America and Europe: in memory of Jan Sajgalik. Eng Geol 37(2):83–113
Seiphoori A, Zamanian M (2022) Improving mechanical behaviour of collapsible soils by grouting clay nanoparticles. Eng Geol 298:106538
Shibuya S, Wang S, Mitachi T (1998) Elastic shear modulus of soft clays from shear wave velocity. Pre-failure deformation behaviour of geomaterials, p 207
Smalley IJ (1971) “In-situ” theories of loess formation and the significance of the calcium-carbonate content of loess. Earth Sci Rev 7(2):67–85
Thompson D (2007) The National Soil Map and Soil Classification. National Soil Resources Institute: Information Paper
Vandanapu R, Omer JR, Attom MF (2017) Laboratory simulation of irrigation-induced settlement of collapsible desert soils under constant surcharge. Geotech Geol Eng 35:2827–2840
Yong RN, Ouhadi VR (2007) Experimental study on instability of bases on natural and lime/cement-stabilized clayey soils. Appl Clay Sci 35(3):238–249
Acknowledgements
The authors wish to acknowledge the Petroleum Technology Development Fund (PTDF) Nigeria for their financial sponsorship of the first author, to carry out this study as part of a PhD programme at the Newcastle University, United Kingdom.
Funding
This work was part of a PhD thesis funded by the Petroleum Technology Development Fund, Nigeria.
Author information
Authors and Affiliations
Contributions
This article is part of the PhD work of the first author (AWO). The PhD work was jointly supervised by the second (CTD) and third (SG) authors at the School of Engineering, Newcastle University, UK. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Opukumo, A.W., Glendinning, S. & Davie, C.T. Collapse of Calcareous Silty Clay: Implication of Calcite Content and Wetting Fluid Type. Geotech Geol Eng 42, 165–184 (2024). https://doi.org/10.1007/s10706-023-02563-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-023-02563-w