Abstract
The objective of this study was to evaluate N2O fluxes from integrated crop-livestock (ICL) and integrated crop-livestock forest (ICLF) systems, continuous pasture and native Cerrado. The experiment was conducted at Embrapa Cerrados, Planaltina-DF, in a Red Oxisol, between February 2012 and April 2014, following the transition of crop to livestock, which began in March 2012, with the sowing of Brachiaria brizantha cv. Piatã, intercropped with sorghum. The experimental design was a randomized block with three replications. The treatments were: cultivated area intercropped with rows of Eucalyptus, spaced 2 × 2 m between plants and 22 m between rows (ICLF); and an area cultivated without tree species (ICL), and also two adjacent reference areas: native Cerrado and continuous pasture. N2O productions were characterized by fluxes below 20 μg N m−2 h−1. The ICL system had the highest cumulative flux with 2.84 kg N ha−1, while the ICLF system obtained cumulative fluxes of 2.05 kg N ha−1. The native Cerrado showed a negative balance, with –0.05 kg N ha−1. The dry season was mostly characterized by low N2O fluxes ranging between 10 μg N m−2 h−1 and negative values, whereas higher N2O fluxes were observed after precipitation events, especially those following a long drought period. The water filled pore space was the factor that best explained N2O fluxes, but higher fluxes were observed after the application of nitrogen fertilizer. There was a positive correlation between microbial biomass carbon and N2O fluxes in the ICL and ICLF systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the greenhouse gases (GHG), N2O is considered a very active gas in the process of global warming due to its high ability to absorb infrared radiation and because it is a stable gas in the atmosphere, contributing to approximately 6% of the radiative potential of GHG, and has a half-life of 120 years (WMO 2012). An increase of 0.2–0.3% in N2O atmospheric concentration would contribute to about 5% in global greenhouse warming (Mosier et al. 2004). The increase of N2O has been attributed to large amounts of nitrogen (N) fertilizers applied in agriculture, the conversion of forest areas to agriculture, use of fire and the intensification of livestock (Bustamante et al. 2012; Smith et al. 2010).
Currently in Brazil, 64% of the total N2O emissions come from the land use change and the agricultural sectors (MCTI 2014). However, the agricultural sector is responsible for about 87.2% of this total of N2O emissions, which is attributed mainly to animal excreta and management of agricultural soils (Signor and Cerri 2013). The Brazilian government has made considerable efforts to reduce the impact of the different sectors of its economy on climate change in the medium and long-term, through public policies such as the Low Carbon Emission Agriculture Program (ABC-Program) (Brasil 2015). The ABC-Program intends to adopt further measures that are consistent with the goal of holding the increase in global average temperature to less than 2 °C above pre-industrial levels in the agriculture sector, strengthen the efforts for sustainable agriculture development and sustainable intensification, including the restoration of an additional 15 million hectares of degraded pasturelands and the enhancement of 5 million hectares of integrated crop-livestock-forest systems (ICLF) by 2030.
The adoption of ICLF systems may be important to reduce GHG emissions and also provide environmental and economic benefits, such as soil and water conservation, carbon sequestration, wood production, and improved animal welfare, which can increase milk and beef production (Cordeiro et al. 2015). However, it is necessary to evaluate the effects of trees in ICLF on N2O emissions, since all the components of the ICLF compete for environmental resources i.e. water, light and nutrients (Franchini et al. 2014). According to Lorenz and Lal (2014), some agroforestry systems in particular, have received increased attention regarding their net carbon (C) sequestration effect by their ability to capture atmospheric CO2 and store carbon in plants and soil.
The best understanding of N2O fluxes in ICLF systems can help to predict the possible impact that the increasing ICLF area in Brazil may cause. The global N2O emission from crop residue has been estimated at 0.4 Tg N per year, using the IPCC default emission factor of 1.25% of applied residue N emitted as N2O (Mosier et al. 1998). However, this default emission factor is based on relatively few experimental studies (Novoa and Tejeda 2006). Thus, information is needed as to the likely impact of crop residues on soil N2O emission in ICLF systems. Some studies have been conducted in Cerrado soils to assess N2O fluxes under agricultural use (Martins et al. 2015: Metay et al. 2011; Lessa et al. 2014), but there are few that evaluate the emission of this gas in integrated crop-livestock-forest or agroforestry systems. Although agroforestry systems have shown to play an important role in the mitigation of GHG in the atmosphere (IPCC 2000; Verchot et al. 2008), there are no reports on the effects of using eucalyptus trees in ICLS systems on the N2O soil fluxes in the Cerrado region of Brazil.
The objective of this study was to evaluate the N2O fluxes from integrated crop-livestock (ICL) and integrated crop-livestock forest (ICLF) systems, continuous pasture and native Cerrado. It was hypothesized that the inclusion of tree component in crop-livestock systems can reduce N2O emissions by affecting soil attributes (co-variables) in comparison to the absence of trees, having as reference areas native Cerrado and continuous pasture.
Materials and methods
Experimental site and weather conditions
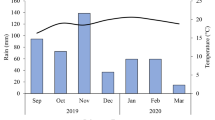
The study was conducted on the experimental station of Embrapa Cerrados, located in Planaltina, DF (15°35′30′′S, 14°42′30′′W and altitude of 1007 m) from February 2012 to April 2014. The climate is seasonal and corresponds to the rainy Aw-tropical type (Köppen), with an average annual rainfall of 1383 mm, concentrated in the months from October to March, and an average annual temperature of 27.7 °C (Silva et al. 2014). Figure 1 shows the average air temperature and daily precipitation during the period from February 2012 to April 2014 in the experimental area. The soil is characterized as an Oxisol, with clayey texture (Embrapa 2014).
Mean temperature and daily precipitation during the period from February 2012 to April 2014. The accumulated precipitation, number of rainy events and the average precipitation of single events during the rainy seasons were as follow: a (02/2012–05/2012)—355.60 mm, 35 rainy events, 10.16 mm rainy events−1; b (10/2012–05/2013)—1147.50 mm, 111 rainy events, 10.34 mm rainy events−1; c (10/2013–04/2014)—1037.70 mm, 100 rainy events, 10.38 mm rainy events−1, respectively
History, treatment and management of the experimental area
The treatments consisted of four areas with different land use: integrated crop-livestock-forest system (ICLF) with two rows of Eucalyptus urograndis (spaced 2 m between rows and trees) and spaces between the alleys of 22 m; a cultivated area at full sunlight in absence of tree species (ICL); and two adjacent areas used as references: native Cerrado and continuous pasture (CP). The ICL and ICLF areas consisted of experimental plots with 1.2 ha in a complete randomized block design with three replications. The reference sites consisted of homogeneous adjacent areas and therefore three sampling points were considered to determine N2O fluxes and soil co-variables.
The ICL and ICLF areas were implemented in January 2009 with the planting of an annual crop with no-tillage. The chemical characteristics of the soil, prior to the setup of the experimental plots are shown in Table 1. The cropping history from the ICL and ICLF from 2009 to 2014 is presented in Table 2. Previously, between 2007 and 2009, the area was planted with Brachiaria brizantha cv. Marandu; Brachiaria brizantha cv. Marandu intercropped with Stylosanthes guianensis; and B. brizantha intercropped with Leucaena leucocephala (Lam.) de Wit (Fabaceae). In March 2012, after the soybean harvest, seeds of B. brizantha cv. BRS Piatã were broadcast (8 kg ha−1 of viable seeds) immediately before sowing the sorghum to establish a pasture in the off-season period. After harvesting sorghum (July 2012), the pasture of B. brizantha was left to establish until January 2013, when the livestock (cattle) were introduced. The pasture was left to establish from July 2012 to January 2013 because of its intercropping period with sorghum that retarded its development due to competition for nutrients and light. Also, the dry season of approximately six months (April to September—Fig. 1), did not help to produce enough quality forage for the animals. Therefore, a rainy period is recommended to establish enough and good quality forage for the animals (Cordeiro et al. 2015). The ICL and ICLF pastures were managed under continuous grazing with variable stocking rates. The pastures were grazed according to forage availability (7–10 kg of forage per 100 kg of animal weight) and the mean carrying capacities from April 2013 to April 2014 were 2.5 and 1.44 AU (=450 kg live weight) for ICL and ICLF, respectively. The quantity of forage biomass removed from grazing in the period from January 2013 to March 2014 (420 days) was estimated based on dry matter intake (DMI) with the grazing pressure set at 10% (Mandarino et al. 2015) and corresponded to 10,836 and 6086 kg ha−1 in ICL and ICLF, respectively. Nitrogen fertilizer was applied to ICL and ICLF pastures in March 12, 2013 (200 kg ha−1 of urea) and in February 24, 2014 (130 kg ha−1 of urea).
The CP was established in November 2007 with Brachiaria brizantha cv. Piatã (6 kg ha−1 of viable seeds). The CP was also grazed according to forage availability (7–10 kg of forage per 100 kg of animal weight) and the mean carrying capacity from April 2013 to April 2014 was 2.1 AU per ha. The quantity of forage biomass removed by grazing (January 2013 to March 2014) corresponded to 6749 kg ha−1.
The native Cerrado was described as a xeromorphic forest type known as cerradão and typically dominated by woody species (Ribeiro and Walter 1998). The cerradão annual litterfall production has been reported to range from 5.4 to 7.8 t ha−1 and to be highly seasonal and negatively correlated with relative humidity and air temperature (Cianciaruso et al. 2006; Peres et al. 1983).
N2O fluxes
Soil N2O fluxes were measured using the technique of static chambers (Gomes et al. 2009; Mosier et al. 2006). The sampling period was from February 2012 to April 2014, representing the period of harvesting of soybean and the intercropping of sorghum with Brachiaria. Three static chambers were placed in each plot, totaling 18 chambers in the ICL and ICLF. For each reference area (native Cerrado and continuous pasture) three chambers were installed. Each chamber consisted of a rectangular hollow metal frame (38 cm wide, 58 cm long, 6 cm in height) that was inserted 5 cm into the soil and a top polyethylene tray that was coupled to the base during gas sampling. The chambers were equipped with a layer of sponge and of aluminium foil to avoid heat insulation. In order to ensure that the system was sealed during sampling, the metal base contained a trough that was filled with soft rubber and the tray was coupled with rubber bands stretched over the top and clipped with both ends to the metal base. The top of the tray contained a triple Luer valve for fastening the sampling syringes, thus allowing the removal of the gases at the time of sampling. The samples were collected in 60 mL polypropylene syringes and immediately transferred to 20 mL glass pre-evacuated vials (−80 kPa). The glass vials were closed with 2 mm butyl rubber septa, sealed with aluminum tops.
At the ICLF plots, the chambers were placed parallel to the rows of sorghum, two of which remained at a distance of three meters from the rows of E. urograndis and the other one in the middle of the plot. At the ICL plots, the chambers were placed according to the same alignment of the chambers placed in the ICLF plots. The chambers were frequently moved (every three months) to avoid soil compaction around them, keeping the same distance between the chambers.
Air samples from the chambers were collected at time 0, immediately after the closure of the chambers, and after 15 and 30 min of closure, always in the morning, between 08:00 and 11:00 h (Alves et al. 2012). Gas sampling frequency was carried out, on average, three to four consecutive days a week during the rainy season, weekly during the short period of drought, and biweekly during the dry season. In the rainy season, between October and April, samples were collected on the three following days after raining events, and on the five days following N fertilizer applications. In addition to the gas sampling, air temperatures were also measured inside the chambers, as well as soil temperatures using portable digital thermometers (Incoterms, 9791.16.1.00).
The analysis of N2O concentrations were performed at the Laboratory of Gas Chromatography of the Embrapa Cerrados, using a gas chromatograph (ThermoTraceGC) equipped with a Porapak Q packed column and an electron capture detector. The result of the analysis of each sample was obtained by integrating the area under the curve representing the variation of the gas concentration. The standards used were 200, 600, 1000 and 1500 ppb of N2O. The N2O fluxes (FN2O) consisted of the difference between the concentrations of each sample of treatment and the concentration of air samples. Initially, the linear regression was made between the concentrations of the standards and their respective areas, finding a factor of transformation of the reading of the samples. After conversion, the fluxes were calculated by the equation: FN2O = δC/δt (V/A) M/Vm; where δC/δt is the change of N2O concentration in the chamber during the incubation period; V and A are respectively the volume of the chamber and the ground area covered by the camera; M is the molecular weight of N2O and Vm is a molecular volume in the sample temperature. The N2O flux was calculated from the slope of the curve obtained with N2O concentrations at 0, 15 and 30 min (Livingston and Hutchinson 1995), being expressed as μg N-N2O m−2 h−1. The correction proposed by Conen and Smith (2000) to compensate the effect of the chamber on the N2O concentration in the soil was used.
Soil sampling and analysis
Soil samples were also collected at each gas sampling to determine the gravimetric water content and the concentration of mineral forms of N in the soil (N–NO −3 and N–NH +4 ). Soil samples, composed of three sub-samples were collected at each plot at depths of 0–5 and 5–10 cm, with the use of a Dutch auger. The samples were subsequently placed in metal cans, for the determination of soil moisture, and another portion was kept wrapped in a Styrofoam box with ice and kept in the freezer until the moment of analysis. The gravimetric soil water content was determined after drying soil samples at 105 °C for 48 h. Based on these results and the bulk density, the percentage of WFPS was calculated, using the following formula: WFPS (%) = (gravimetric moisture (%) × bulk density)/total soil porosity × 100; where: total soil porosity = [1− (bulk density/2.65)], with 2.65 [g cm−3] the assumed density of the soil particles. Nitrate (N–NO −3 ) and ammonium (N–NH +4 ) were analyzed following Embrapa (1997).
In order to evaluate carbon (C) and N in the microbial biomass, soil samples were collected in triplicate at 0–10 cm depth in two periods: September/2013 and February/2014 in each land use. Microbial biomass N (MBN) was determined with the method of chloroform fumigation-extraction, described by Brookes et al. (1985) and Vance et al. (1987), using a correction factor of 0.54 (Wardle 1994). Microbial biomass carbon (MBC) was determined according to Vance et al. (1987), using a correction factor of 0.38 (Wardle 1994). Total organic carbon (TOC) and total N (TN) were analyzed according to Embrapa (1997). Basal respiration (BR) was also estimated by measuring CO2 released from pre-incubated soil samples for a period of seven days (Alef and Nannipieri 1995). The carbon microbial quotient (qMic) was calculated by dividing the MBC by TOC and was expressed in percentage (%).
Statistical analysis
A descriptive analysis of N2O fluxes and of soil and climate covariables was performed. Emissions were estimated by plotting the average values of N2O fluxes and the time scale on a chart and calculating the resulting area under the curve by integration, using the Sigmaplot® Version 10 software (Systat Software Inc., Chicago, USA, 2007). Emissions of N2O were divided into 3 seasons, each one comprising different month intervals of the sampled years: dry season, which considered the intervals from May to September 2012 and May to September 2013; rainy season, when there was no application of N fertilizer, comprising the periods of October 2012–February 2013 and October 2013–February 2014; and the rainy season, in which there was fertilizer application, corresponding to the periods of February–May 2012, February–May 2013, and February–April 2014.
The resulting data were subjected to analysis of variance and averages were compared using the nonparametric Kruskal–Wallis test (p < 0.05) that allowed the comparison of the treatments distributed in randomized blocks (ICL and ICLF) and references (CP and native Cerrado) that have no experimental design. Pearson’s correlation and multiple linear regressions to relate emissions of N2O and microbiological soil properties were used. Aiming at an exploratory analysis of all soil variables and possible relations with soil nitrous oxide fluxes, a principal component analysis (PCA) was performed in a matrix composed of 24 lines (four land use, two sampling periods and three replications) and 11 columns (soil attributes: nitrous oxide fluxes—N2O; microbial biomass carbon—MBC; microbial biomass N—MBN; basal respiration—BR; organic carbon—OC; total N—TN; carbon microbial quotient—qMic; soil temperature—Tsoil; nitrate—NO −3 and ammonium—NH +4 contents; water filled pore space—WFPS) to identify the variables with greater weight on the linear combination of the first two principal components. To facilitate the interpretation of results, in addition to the correlation circle between the eigenvectors of the variables, a discriminant analysis, based on the Mahalanobis distance or dissimilarity was applied to compare the mathematical distances between samples from land use. This type of analysis uses a permutation test, which calculates the total inertia interclass for each random distribution of individuals and, by association with a statistical probability, maximizes the discriminating power of the analysis. This step was performed using ADE-4 software (Thioulouse et al. 1997).
Results and discussion
Temporal variation of N2O fluxes in the soil
The temporal variation of N2O fluxes in the study areas during the two years of monitoring was mostly characterized by values below 20 µg N m−2 h−1, combined with periods of higher fluxes during the rainy season (Figs. 2, 3). Also, the temporal variation of N2O fluxes in the ICL and ICLF varied from the first and the second year of evaluation; the highest N2O flux observed in the first year (March/2012) was approximately 50 μg N m−2 h−1 (Fig. 2), whereas the highest flux in the second year (March/2013) was higher than 150 μg N m−2 h−1 (Fig. 3). These peaks were observed after the application of N fertilizer and in the rainy season. In the first year, the amount applied was 30 kg N ha−1 compared to 90 kg N ha−1 applied in the second year, which may explain the higher peak in the second year. However, during the dry season (from June to October) and in both years of evaluation, fluxes of N2O from ICL, ICLF and native Cerrado were mostly less than 10 μg N m−2 h−1 and often near zero.
Average daily fluxes of N2O, water filled pore space (WFPS), soil nitrate (N–NO −3 ) and ammonium (N–NH +4 ) during the period from February 2013 to April 2014. The first arrow from left to right indicate the application of 90 kg N ha−1 in March/2013, and the second arrow the application of 60 kg N ha−1 in February/2014
Due to low natural fertility of the Cerrado Oxisols, the N application resulted in high response in N2O fluxes, with over 50% of fluxes observed during the experiment being obtained in the period of application of fertilizers. Hickman et al. (2014) in an experiment testing different doses of N fertilizers in an African savanna, noted that over 60% of fluxes occured in the three weeks following the application of fertilizer. However, such fluxes were only noticed after a rainfall event, when soil moisture increased. Generally, the response to N occurs from the first to the second week after its application and generally disappears within two months (Signor et al. 2013).
The areas under native Cerrado and continuous pasture (CP) showed lower N2O fluxes, with 51 and 35% of these fluxes below 1 μg N m−2 h−1, respectively. In the ICL and ICLF, these lower N2O fluxes represented only 13 and 15%, respectively. These results were also observed in other studies in the Cerrado region with values between −0.6 and 0.5 μg N m−2 h−1 (Carvalho et al. 2006; Cruvinel et al. 2011; Metay et al. 2007).
These lower values can be attributed to the physical and chemical characteristics of the Cerrado Oxisols (Carvalho et al. 2006; Cruvinel et al. 2011; Metay et al. 2007), which are generally well-drained and with relative low production of N–NO −3 that rarely exceeds the amount demanded by microorganisms and plant roots (Nardoto and Bustamante 2003). Well-drained soils (with high drainable porosity) tend to have few anaerobic sites, limiting the N2O production from denitrification (Baggs and Philippot 2010).
In our study, N2O influx was mainly observed in the native Cerrado during the dry season (soil WFPS < 40%) associated with nitrate levels below 10 mg kg−1 (Figs. 2, 3). The influx of N2O under native vegetation may be related to low soil pH found under Cerrado vegetation in Brazil (Lopes and Cox 1977), since nitrification tends to decrease with increasing soil acidity (Hickman et al. 2014). Cuhel et al. (2010) observed that the activity of denitrification (with N2O production) at acid pH was three times lower than that observed at alkaline pH.
Grover et al. (2012), in an experiment in the Australian savanna, obtained negative N2O fluxes in a native forest and a low productive pasture during the dry season. These negative values were associated with the low N content (Chapuis-Lardy et al. 2007) and humidity of the soils, when sufficient air can diffuse through soil macro and micropores, allowing the microorganisms to use N2O as a source of N (Rosenkranz et al. 2006). Carvalho et al. (2014) mention that the low fluxes of N2O soil under the Cerrado region are associated with low mineral N levels and rapid drainage of water in the soil, not offering favorable conditions for high N2O fluxes. Therefore, the low N2O fluxes observed in our study are likely due to high soil pososity, dry condition and low N content.
Cumulative flux of N2O in the soil
Soil cumulative N2O fluxes from the native Cerrado and CP did not change among the evaluated periods (Table 3). However, ICL and ICLF cumulative fluxes varied between the two years of evaluation. The highest cumulative fluxes were found during the rainy season and with the application of N fertilizer in 2013 (02–05/2013) in the ICL and ICLF, representing more than 50% of the total N2O cumulative flux from these systems. Hickman et al. (2014) also observed that 60% of the total N2O fluxes occurred up to three weeks after the application of N fertilizer and may last up to two months (Signor et al. 2013). There was no variation in the cumulative fluxes between the areas of native Cerrado and CP throughout the study period (Table 3). Such systems did not receive N fertilizer or any other fertilizer during our study.
Considering the sum of the cumulative flux of N2O during the two years of evaluation, the cumulative flux of soil N2O was higher (p < 0.05) for ICL (2.84 kg N ha−1) compared to ICLF (2.05 kg N ha−1) and those systems presented higher N2O fluxes compared to native Cerrado (−0.05 kg N ha−1). The lower N2O fluxes in ICLF are probably due to changes in phenolic contents added by eucalyptus litter (Soumare et al. 2015), to lower soil microbial community size and enzyme activities and increased microbial physiological stress (Chen et al. 2013). In this same experimental area and period, the forage dry matter production of the Piatã grass decreased under ICLF (Santos et al. 2016).
There are few studies in Brazil that have evaluated soil N2O fluxes for a period of two years under ICL and ICLF systems. Salton et al. (2014), during a soybean cycle (December–April) in Dourados—MS found under ICL a cumulative flux of 0.486 kg N ha−1, whereas under continuous crop and no-tillage system found 0.523 kg N ha−1. Soil management, edaphoclimatic conditions and also the type of culture being cultivated can influence fluxes of N2O. Jantalia et al. (2008) found cumulative flux ranging from 0.596 to 0.984 kg N ha−1 in the period of one year, in the corn-soybean-wheat rotation, in Southern Brazil.
Allen et al. (2009), in an experiment with eucalyptus reforestation with various ages and pasture grown under intensive grazing in Australia, observed that, generally, N2O fluxes are lower under the trees in relation to pasture. However, during the establishment of plants to canopy closure phase (2–3 years), the soil under the trees presented higher N2O fluxes than the adjacent pasture areas. This trend was reversed in the average rotation cycles and late (5–23 years), where the N2O fluxes under the trees were lower than the adjacent pastures. The authors associated the lower values under the trees to the plant community composition and its interaction with the soil, as some plants can have suppressive effects on the microbial communities of the soil, and related compounds released by plants which inhibit nitrification have been identified (Niklaus et al. 2006).
In order to estimate the system productivity during the sorghum cycle and relate it to the cumulative N2O fluxes, the ratio of mg N2O m−2 to grain productivity (kg m−2) was calculated (Martins et al. 2015). Although the cumulative N2O fluxes did not differ significantly between ICLF (31.33 mg N2O m−2) and ICL (29.73 mg N2O m−2) during the sorghum cycle (February–July, 2012), the cumulative N2O fluxes per unit of grain production varied significantly (p < 0.05), with ICLF showing three times greater value (224 mg N2O m−2 kg−1 grains) than ICL (76 mg N2O m−2 kg−1 grains). This result is due to the lower sorghum yield in ICLF (1416.11 kg ha−1) in comparison to ICL (3927.5 kg ha−1), which may be explained, mainly, by the effect of the shade of the Eucalyptus urograndis trees (Santos et al. 2016) that suppressed nearly half of the sorghum yield. Thus, the ICL presented higher productivity, suggesting lower impact in the conversion of emission/product, around 66% less when compared to ICLF (p < 0.05). Therefore, considering the sorghum cycle, the ICL system stands out as more efficient in the ratio of N2O fluxes/grain productivity, providing more food with less N2O fluxes.
Influence of covariables on N2O fluxes in the soil
The dynamics of N2O fluxes can be attributed to differences among the integrated systems, continuous pasture and native Cerrado, due to their environmental conditions. Most of the covariables (NO −3 , NH +4 , WFPS and rainfall) demonstrated to be highly correlated with the N2O fluxes, although the Pearson coefficients were low (Table 4). In addition, all correlations were positive, reinforcing the relationship and direct influence that these covariables present with N2O fluxes. Nitrification and denitrification processes are dynamic and dependent on various edaphoclimatic factors, mainly the availability of N in the soil and soil moisture (Ussiri and Lal 2013).
Water filled pore space and precipitation
The WFPS was the variable that best explained N2O fluxes, mainly at the 0–5 cm soil layer (Table 4). Generally, high fluxes coincide with periods after precipitation which caused an increase in WFPS (Figs. 2, 3), which was also observed by Dick et al. (2001) and Ussiri and Lal (2013). The highest N2O emission peaks occurred in periods after rainfall and consequent elevation of the WFPS, and in periods associated with the application of N fertilizer as topdressing.
Rainfall influences N2O fluxes by changing the soil water content. Several studies show the increase of N2O fluxes after rainfall events (Dick et al. 2001; Grover et al. 2012; Jantalia et al. 2008; Parkin and Kaspar 2006; Ruser et al. 2006), resulting in an increased amount of WFPS. High WFPS increases denitrifying activity induced by the reduction of O2 diffusion in the soil (Dobbie et al. 1999; Ussiri and Lal 2013).
A homogeneous rainfall distribution during the rainy period was observed (Fig. 1). The accumulated precipitation, number of rainy events and the average precipitation of single events during the rainy seasons were as follows: (02/2012–05/2012)—355.60 mm, 35 rainfall events with a mean of 10.16 per event; (10/2012–05/2013)—1147.50 mm, 111 rainy events, with a mean of 10.34 per event; (10/2013–04/2014)—1037.70 mm, 100 rainy events, with a mean of 10.38 per event. Although the average precipitation of single events was approximately 10 mm, precipitation reached 80 mm and soil WFPS 61%.
There was positive correlation between N2O fluxes and precipitation. The diffusivity of N2O is lower in soils that micropores predominate, such as those containing a high percentage of clay (Eickenscheidt and Brumme 2013). Therefore, higher fluxes are only registered for a few hours or days after precipitation (Dick et al. 2001). In dry conditions, the soil serves as a sink of N2O (Goldberg and Gebauer 2009; Grover et al. 2012; Stewart et al. 2012). Although the underlying mechanisms are not clear, one possible explanation is that N2O diffusion from the atmosphere into the soil is favored under dry soil conditions and limited availability of inorganic N, and atmospheric N2O is reduced to N2 by denitrifying bacteria (Dijkstra et al. 2013).
Nevertheless, the highest correlation of precipitation with N2O fluxes in the soil occurred during the dry period, and this result may be a consequence of the “Birch effect” (Jarvis et al. 2007). This effect occurs in the drying/rewetting events, in which a long drought period is followed by rainfall events, resulting in a rapid organic matter decomposition and mineralization in soil (Birch 1964). The “Birch effect” was observed on October 18 (2012) and November 1 (2012) after rainy events of 30 and 18 mm, respectively; N2O fluxes increased from 2 μg N m−2 h− to 20 μg N m−2 h− after 25 days without rain and from 13 μg N m−2 h−1 to 42 μg N m−2 h−1 after 13 days without rain.
In the central region of Brazil, the occurrence of rain over a long period of drought is common, which can reach up to 5–6 months, promoting an intense response in microbial activity. The addition of water quickly increases the population of microorganisms in the soil, and reactivates their metabolism (Jarvis et al. 2007) and may explain the relationship between rainfall and N2O fluxes in the soil during the dry season. The increase of microbial activity and size can explain the N2O peaks observed at the beginning of the rainy season in our study. The “Birch effect” promotes a peak of soil respiration, stimulating the inactive microrganisms, thus accelerating the biochemical processes in the soil (Dick et al. 2001). This process increases the available C concentration and stimulates denitrifying bacteria to use nitrate as an electron acceptor for the oxidation of C and generation of energy (Ussiri and Lal 2013). Other authors have also obtained high N2O fluxes after soil rewetting, reaching, at certain times, higher fluxes than those obtained in response to the application of N fertilizer (Pelster et al. 2011; Ruser et al. 2006).
In this study, the WFPS showed the best correlation with the N2O fluxes, with values ranging from 19 to 51% in the dry season and 29 to 55% in the rainy season. Several authors reported that WFPS is a limiting factor in N2O fluxes and below 60% the activity of denitrifying bacteria in the soil is decreased (Denmead et al. 2010; Liu et al. 2007; Pimentel et al. 2015). However, Zhang and Han (2008) observed fluxes of N2O when WFPS was below 60% in native pasture and fallow management. These authors associated these fluxes to be mainly performed by nitrifying bacteria. In our study, the WFPS was mostly below 60% and N2O fluxes and peaks were observed, thus indicating that these fluxes could be associated with nitrifying bacteria and/or to a few anaraerobic sites that allow the denitrification process to happen.
Nitrate and ammonium in the soil
N2O fluxes can be positively correlated with the availability of inorganic N in the soil (Holtgrieve et al. 2006), as observed in this study (Table 4). The average content of N–NH +4 (0–93 mg N kg−1) was generally higher than the concentration N–NO −3 (0–25 mg N kg−1). The overall average of the N–NH +4 was 73% larger compared to the N–NO −3 and showed more variability throughout the study (standard deviation of 12.5 mg N kg−1 for N–NH +4 and 4.25 mg N kg−1 for N–NO −3 ). At very low levels of soil moisture, the dissolution rate of N sources becomes slower, reducing the availability of NH +4 solution (Cameron et al. 2013). This reduction will reflect the activity of nitrifying bacteria, especially Nitrobacter, resulting in the decrease of available NO −3 concentration (Baggs and Philippot 2010).
After the application of N as topdressing, increases in N2O fluxes in the soil of the ICL and ICLF systems were observed. The first application (90 kg N ha−1) was made on March 2013 and the second (60 kg N ha−1) on February 2014 and both resulted in increases in N2O fluxes (Fig. 2). The application also resulted in increases in soil N–NO −3 and N–NH +4 contents in both systems. Furthermore, N–NO −3 content and N–NH +4 were positively correlated with the fluxes of N2O in soil (Table 4).
The application of N fertilizers to soil can stimulate the production of N2O through biochemical processes of nitrification and denitrification under field and laboratory conditions (Baggs and Philippot 2010; Davidson et al. 2000; Fuß et al. 2011; Liu et al. 2007; Stehfest and Bouwman 2006).
Correlation of soil attributes with N2O fluxes
The Pearson correlation between the cumulative fluxes of N2O and microbiological soil properties (MBC, MBN, MBC, TOC, TN and QMic) are presented in Table 5 for the ICL and ICLF treatments and the multiple linear regression between these factors is found in Table 6. Only MBC and BR were significantly correlated with the fluxes of N2O. MBC showed a positive correlation, whereas BR showed a negative correlation. In the evaluation of multiple linear regression, only BR resulted in a significant relationship with the N2O fluxes.
The significant correlation between MBC and N2O can be associated with the quantity and quality of plant residues added to the soil (Carvalho et al. 2014). With the increase of the soil microbial biomass, the biological activity may stimulate biochemical processes such as denitrification, which is responsible for the production of N2O. Participation of microbial biomass may be relevant to the N2O fluxes. Stieven et al. (2014) observed lower values of soil microbial biomass in eucalypt plantations during the rainy season. The same authors describe that the shading of eucalyptus can cause these changes in ICLF system, which results in reduced plant growth of crops planted and consequently less deposition of crop residues and biological activity in the soil. Because N2O fluxes are the result of the activity of nitrifying and denitrifying microorganisms in the soil, the reduction in biological activity will result in a decrease in the intensity of biochemical processes, reflecting in N2O fluxes, which will be smaller (Signor and Cerri 2013).
According to the PCA analysis of soil attributes (Fig. 4), the first two principal components explained approximately 56% of the total variance—PC1 (35.69%) and PC2 (20.6%). PC1 distinguished land uses with a gradient of MBN, WFPS, N2O and soil temperature, with negative eigenvalues and BR, qMic and MBC, with positive eigenvalues. PC2 is related to a gradient of nitrate—NO −3 and ammonium—NH +4 contents and OC, with negative eigenvalues. Permutation tests of site coordinates, for all extracted axes, showed a significant difference (p < 0.001) between land use systems (Fig. 4). Axis 1 separated the Cerrado with higher values of BR, qMic and MBC from the agricultural systems, which are related to the other variables. Axis 2 distinguished integrated systems from pasture with a tendency to high levels of C, N and N2O fluxes in integrated systems in contrast with a higher soil temperature in continuous pasture. This result may be related to a higher N supply in integrated systems in relation to other land uses (Cardoso et al. 2016).
Principal component analysis of all soil attributes in different land use systems. a Correlation circle of soil attributes with principal components PC1 and PC2 projections; b ordination diagram in the PC1/PC2 plane, according to land use systems; CLFS integrated crop-livestock-forest system, CLS integrated crop-livestock system, p grouping probability by permutation test. N2O nitrous oxide fluxes, MBC microbial biomass carbon, MBN microbial biomass nitrogen, BR basal respiration, OC total organic carbon, TN total nitrogen, qMic carbon microbial quotient, Tsoil soil temperature, NO −3 nitrate and NH +4 ammonium contents, WFPS water filled pore space
Conclusions
N2O is one of the main greenhouse gases that is related to agricultural activities and integrated production systems with the use of annual crops, pasture and trees. Woody plants of economic interest, especially eucalyptus, are an alternative for diversification in agriculture and for the mitigation of greenhouse gases. Among the integrated systems evaluated during a period of two years with crop and cattle grazing phases, soil N2O fluxes were lower under ICLF system compared to ICL. Such decrease in N2O fluxes could have been related to eucalyptus litter that is rich in phenolic compounds leading to lower microbial biomass carbon. It should be highlighted that under ICLF the forage dry biomass and sorghum yield were lower than ICL due to the shade of the trees, thus compromising food production. The ICLF system is one of the alternatives of interest for agricultural production for the consolidation of an Economy of Low-Carbon Emission in Agriculture (Brazilian ABC Program) and can be considered an agricultural system that offers possibilities for mitigation of GHG.
References
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press, London
Allen DE, Mendham DS, Bhupinderpal-Singh Cowie A, Wang W, Dalal RC, Raison RJ (2009) Nitrous oxide and methane emissions from soil are reduced following afforestation of pasture lands in three contrasting climatic zones. Aust J Soil Res 47:443–445. doi:10.1071/SR08151
Alves BJ, Smith KA, Flores RA, Cardoso AS, Oliveira WRD, Jantalia CP, Boddey RM (2012) Selection of the most suitable sampling time for static chambers for the estimation of daily mean N2O flux from soils. Soil Biol Biochem 46:129–135. doi:10.1016/j.soilbio.2011.11.022
Baggs EM, Philippot L (2010) Microbial terrestrial pathways to nitrous oxide. In: Smith K (ed) Nitrous oxide and climate change. Earthscan, London, pp 4–36
Birch HF (1964) Mineralisation of plant nitrogen following alternate wet and dry conditions. Plant Soil 20:43–49
Brasil (2015) Federative Republic of Brazil—Intended Nationally Determined Contribution Towards Achieving the Objective of the United Nations Framework Convention on Climate Change. Brasilia Ministry of Foreign Affairs. http://www.itamaraty.gov.br/images/ed_desenvsust/BRAZIL-iNDC-english.pdf. Accessed 26 August 2016
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Bustamante MMC, Nobre CA, Smeraldi R, Aguiar APD, Barioni LG, Ferreira LG, Longo K, May P, Pinto AS, Ometto JPHB (2012) Estimating greenhouse gas emissions from cattle raising in Brazil. Clim Change 115:559–577. doi:10.1007/s10584-012-0443-3
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Ann Appl Biol 162:145–173. doi:10.1111/aab.12014
Cardoso AS, Berndt A, Leytem A, Alves BJR, Carvalho INO, Soares LHB, Urquiaga S, Boddey RM (2016) Impact of the intensification of beef production in Brazil on greenhouse gas emissions and land use. Agric Syst 143:86–96. doi:10.1016/j.agsy.2015.12.007
Carvalho AM, Bustamante MMC, Kozovits AR, Miranda LN, Vivaldi LJ, Sousa DM (2006) Emissão de óxidos de nitrogênio associada à aplicação de uréia sob plantio convencional e direto. Pesqui Agropecu Bras 41(4):679–685. doi:10.1590/S0100-204X2006000400020
Carvalho JLN, Raucci GS, Frazao LA, Cerri CEP, Bernoux M, Cerri CC (2014) Crop-pasture rotation: a strategy to reduce soil greenhouse gas emissions in the Brazilian Cerrado. Agric Ecosyst Environ 183:167–175. doi:10.1016/j.agee.2013.11.014
Chapuis-Lardy L, Wrage N, Metay A, Chotte J-C, Bernoux M (2007) Soil a sink for N2O? A review. Glob Change Biol 13:1–17. doi:10.1111/j.1365-2486.2006.01280.x
Chen F, Zheng K, Ouyang Z, Li H, Wu B, Shi Q (2013) Soil microbial community structure and function responses to successive planting of Eucalyptus. J Environ Sci 25(10):2102–2111. doi:10.1016/S1001-0742(12)60319-2
Cianciaruso MV, Pires JSR, Delitti WBC, Silva EFLP (2006) Produção de serapilheira e decomposição do material foliar em um cerradão na Estação Ecológica de Jataí, município de Luiz Antônio, SP, Brasil. Acta Bot Bras 20:49–59. doi:10.1590/S0102-33062006000100006
Conen F, Smith KA (2000) An explanation of linear increases in gas concentration under closed chambers used to measure gas exchange between soil and the atmosphere. Eur J Soil Sci 51:111–117. doi:10.1046/j.1365-2389.2000.00292.x
Cordeiro LAM, Vilela L, Marchao RL, Klutujcouski J, Martha Junior GB (2015) Integração lavoura-pecuária e integração lavoura-pecuária-floresta: estratégias para intensificação sustentável do uso do solo. Cad Ciência Tecnologia 32:15–43
Cruvinel EBF, Bustamante MMC, Kozovits AR, Zepp RG (2011) Soil emissions of NO, N2O and CO2 from croplands in the savanna region of central Brazil. Agric Ecosyst Environ 144:29–40. doi:10.1016/j.agee.2011.07.016
Cuhel J, Simek M, Laughlin RJ, Bru D, Cheneby D, Watson CJ, Philippot L (2010) Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl Environ Microbiol 76(6):1870–1878. doi:10.1128/AEM.02484-09
Davidson EA, Kelles M, Erickson HE, Verchot LV, Veldkamp E (2000) Testing a conceptual model of soil emissions of nitrous and nitric oxides. Bioscience 50(8):667–680
Denmead OT, MacDonald BCT, Bryant G, Naylor T, Wilson S, Griffith DWT, Wang WJ, Salter B, White I, Moody PW (2010) Emissions of methane and nitrous oxide from Australian sugarcane soils. Agric For Meteorol 150(6):748–756. doi:10.1016/j.agrformet.2009.06.018
Dick J, Skiba U, Wilson J (2001) The effect of rainfall on NO and N2O emissions from Ugandan agroforest soils. Phyton-Ann Rei Bot A 41:73–80
Dijkstra FA, Morgan JA, Follett RF, Lecain DR (2013) Climate change reduces the net sink of CH4 and N2O in a semiarid grassland. Glob Change Biol 19(6):1816–1826. doi:10.1111/gcb.12182
Dobbie KE, McTaggart IP, Smith KA (1999) Nitrous oxide emissions from intensive agricultural systems: variations between crops and seasons, key driving variables, and mean emission factors. J Geophys Res 104(D21):26891–26899. doi:10.1029/1999JD900378
Eickenscheidt N, Brumme R (2013) Regulation of N2O and NOx emission patterns in six acid temperate beech forest soils by soil gas diffusivity, N turnover, and atmospheric NOx concentrations. Plant Soil 369:515–529. doi:10.1007/s11104-013-1602-7
Embrapa (1997) Manual de métodos de análise de solos. Rio de Janeiro
Embrapa (2014) Sistema brasileiro de classificação de solos. Brasília, Distrito Federal
Franchini JC, Balbinot Junior AA, Sichieri FR, Debiasi H, Conte O (2014) Yield of soybean, pasture and wood in integrated crop-livestock-forest system in Northwestern Parana state, Brazil. Rev Cienc Agron 46(5):1006–1013. doi:10.1590/S1806-66902014000500016
Fuß R, Ruth B, Schilling R, Scherb H, Munch JC (2011) Pulse emissions of N2O and CO2 from an arable field depending on fertilization and tillage practice. Agric Ecosyst Environ 144:61–68. doi:10.1016/j.agee.2011.07.020
Goldberg SD, Gebauer G (2009) Drought turns a Central European Norway spruce forest soil from an N2O source to a transient N2O sink. Glob Change Biol 15(4):850–860. doi:10.1111/j.1365-2486.2008.01752.x
Gomes J, Bayer C, Costa FS, Piccolo MC, Zanatta JA, Vieira FCB, Six J (2009) Soil nitrous oxide emissions in long-term cover crops-based rotations under subtropical climate. Soil Tillage Res 106:36–44. doi:10.1016/j.still.2009.10.001
Grover SPP, Livesley SJ, Hutley LB, Jamali H, Fest B, Beringer J, Butterbach-Bahl K, Arndt SK (2012) Land use change and the impact on greenhouse gas exchange in north Australian savanna soils. Biogeosciences 9:423–437. doi:10.5194/bg-9-423-2012
Hickman JE, Palm CA, Mutuo P, Melillo JM, Tang J (2014) Nitrous oxide (N2O) emissions in response to increasing fertilizer addition in maize (Zea mays L.) agriculture in western Kenya. Nutr Cycl Agroecosyst 100(2):177–187. doi:10.1007/s10705-014-9636-7
Holtgrieve GW, Jewett PK, Matson PA (2006) Variations in soil N cycling and trace gas emissions in wet tropical forests. Oecologia 146:584–594. doi:10.1007/s00442-005-0222-1
IPCC (2000) Intergovernmental Panel on Climate Change. Land use, land use change and forestry. A special report for IPCC. Cambridge University Press, UK
Jantalia CP, Santos HP, Urquiaga S, Boddey RM, Alves BJR (2008) Fluxes of nitrous oxide from soil under different crop rotations and tillage systems in the South of Brazil. Nutr Cycl Agroecosyst 82:161–173. doi:10.1007/s10705-008-9178-y
Jarvis P, Rey A, Petsikos C, Wingate L, Rayment M, Pereira J, Banza J, David J, Miglietta F, Borghetti M, Manca G, Valentini R (2007) Drying and wetting of Mediterranean soils stimulates decomposition and carbon dioxide emission: the “Birch effect”. Tree Physiol 27:929–940. doi:10.1093/treephys/27.7.929
Lessa ACR, Madari BE, Paredes DS, Boddey RM, Urquiaga S, Jantalia CP, Alves BJR (2014) Bovine urine and dung deposited on Brazilian savannah pastures contribute differently to direct and indirect soil nitrous oxide emissions. Agric Ecosyst Environ 190:104–111. doi:10.1016/j.agee.2014.01.010
Liu XJ, Mosier AR, Halvorson AD, Reule CA, Zhang FS (2007) Dinitrogen and N2O emissions in arable soils: effect of tillage, N source and soil moisture. Soil Biol Biochem 39:2362–2370. doi:10.1016/j.soilbio.2007.04.008
Livingston GP, Hutchinson GL (1995) Enclosure-based measurement of trace gas exchange: applications and sources of error. Biogenic trace gases: measuring emissions from soil and water. Blackwell, Oxford, pp 14–51
Lopes AS, Cox FR (1977) A survey of the fertility status of surface soils under cerrado vegetation in Brazil. Soil Sci Soc Am J 41:742–747. doi:10.2136/sssaj1977.03615995004100040026x
Lorenz K, Lal R (2014) Soil organic carbon sequestration in agroforestry systems. A review. Agron Sustain Dev 34(2):443–454. doi:10.1007/s13593-014-0212-y
Mandarino RA, Pereira LGR, Barbosa FA, Vilela L, Maciel GA, Guimaraes Junior R (2015) Methane emissions of Nellore heifers on integrated crop-livestock-forest systems in the Brazilian Cerrado. In: World congress on integrated-crop-livestock-forest systems, 3rd international symposium on integrated crop-livestock systems towards sustainable intensification. Embrapa, Brasília, Brazil
Martins MR, Jantalia CP, Polidoro JC, Batista JN, Alves BJR, Boddey RM, Urquiaga S (2015) Nitrous oxide and ammonia emissions from N fertilization of maize crop under no-till in a Cerrado soil. Soil Tillage Res 151:75–81. doi:10.1016/j.still.2015.03.004
MCTI (2014). Estimativas anuais de emissões de gases de efeito estufa no Brasil. Segunda Edição. Ministério da Ciência, Tecnologia e Inovação, Brasília, Brazil. http://www.mct.gov.br/upd_blob/0235/235580.pdf. Accessed 20 March 2016
Metay A, Oliver R, Scopel E, Douzet JM, Moreira JAA, Maraux F, Feigl BJ, Feller C (2007) N2O and CH4 emissions from soils under conventional and no-till management practices in Goiânia (Cerrados, Brazil). Geoderma 141:78–88. doi:10.1016/j.geoderma.2007.05.010
Metay A, Chapuis-Lardy L, Findeling A, Oliverd R, Alves JA, Moreira C (2011) Simulating N2O fluxes from a Brazilian cropped soil with contrasted tillage practices. Agric Ecosyst Environ 140:255–263. doi:10.1016/j.agee.2010.12.012
Mosier AR, Kroeze C, Nevison C, Oenema O, Seitzinger SP, Van Cleemput O (1998) Closing the global N2O budget: nitrous oxide emission through the agricultural nitrogen cycle. OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology. Nutr Cycl Agroecosyst 52:225–248. doi:10.1023/A:1009740530221
Mosier A, Wassmann R, Verchot L, King J, Palm C (2004) Methane and nitrogen oxide fluxes in tropical agricultural soils: sources, sinks and mechanisms. Environ Dev Sustain 6(1):11–49. doi:10.2134/jeq2005.0232
Mosier AR, Halvorson AD, Reule CA, Liu XJ (2006) Net global warming potential and greenhouse gas intensity in irrigated cropping systems in Northeastern Colorado. J Environ Qual 35:1584–1598. doi:10.2134/jeq2005.0232
Nardoto GB, Bustamante MMC (2003) Effects of fire on soil nitrogen dynamics and microbial biomass in savannas of Central Brazil. Pesqui Agropecu Bras 38:955–962. doi:10.1590/S0100-204X2003000800008
Niklaus PA, Wardle DA, Tate KR (2006) Effects of plant species diversity and composition on nitrogen cycling and the trace gas balance of soils. Plant Soil 282:83–98. doi:10.1007/s11104-005-5230-8
Novoa R, Tejeda HR (2006) Evaluation of the N2O emissions from N in plant residues as affected by environmental and management factors. Nutr Cycl Agroecosyst 75:29–46. doi:10.1007/s10705-006-9009-y
Parkin TB, Kaspar TC (2006) Nitrous oxide emissions from corn-soybean systems in the Midwest. J Environ Qual 35:1496–1506. doi:10.2134/jeq2005.0183
Pelster DE, Larouche F, Rochette P, Chantigny MH, Allaire S, Angers DA (2011) Nitrogen fertilization but not soil tillage affects nitrous oxide emissions from a clay loam soil under a maize–soybean rotation. Soil Tillage Res 115–116:16–26. doi:10.1016/j.still.2011.06.001
Peres JRR, Suhet AR, Vargas MAT, Drozdowicz A (1983) Litter production in areas of Brazilian ‘Cerrados’. Pesq Agropecu Bras 18:1037–1043
Pimentel LG, Weiler DA, Pedroso GM, Bayer C (2015) Soil N2O emissions following cover-crop residues application under two soil moisture conditions. J Plant Nutr Soil Sci 178(4):631–640. doi:10.1002/jpln.201400392
Ribeiro JF, Walter BMT (1998) Fitofisionomias do bioma Cerrado. In: Sano SM, Almeida SP (eds) Cerrado: ambiente e flora. Embrapa Cerrados, Planaltina, pp 89–166
Rosenkranz P, Bruggemann N, Papen H, Xu Z, Seufert G, Butterbach-Bahl K (2006) N2O, NO and CH4 exchange, and microbial N turnover over a Mediterranean pine forest soil. Biogeosciences 3:121–133. doi:10.5194/bg-3-121-2006
Ruser R, Flessa H, Russow R, Schmidt G, Buegger F, Munch JC (2006) Emission of N2O, N2 and CO2 from soil fertilized with nitrate: effect of compaction, soil moisture and rewetting. Soil Biol Biochem 38:263–274. doi:10.1016/j.soilbio.2005.05.005
Salton JC, Mercante FM, Tomazi M, Zanatta JA, Concenço G, Silva WM, Retore M (2014) Integrated crop-livestock system in tropical Brazil: toward a sustainable production system. Agric Ecosyst Environ 190:70–79. doi:10.1016/j.agee.2013.09.023
Santos DC, Guimarães Júnior R, Vilela L, Pulrolnik K, Bufon VB, França AFS (2016) Forage dry mass accumulation and structural characteristics of Piatã grass in silvopastoral systems in the Brazilian savannah. Agric Ecosyst Environ 233(3):16–24. doi:10.1016/j.agee.2016.08.026
Signor D, Cerri CEP (2013) Nitrous oxide emissions in agricultural soils: a review. Pesqui Agropecu Trop 43(3):322–338. doi:10.1590/S1983-40632013000300014
Signor D, Cerri CEP, Conant R (2013) N2O emissions due to nitrogen fertilizer applications in two regions of sugarcane cultivation in Brazil. Environ Res Lett 8(1):1–9. doi:10.1088/1748-9326/8/1/015013
Silva FAM, Evangelista BA, Malaquias JV (2014) Normal Climatológica de 1974 a 2003 da Estação Principal da Embrapa Cerrados. Embrapa Cerrados, Planaltina
Smith K, Crutzen P, Mosier A, Winiwarter W (2010) The global nitrous oxide budget: a reassessment. In: Smith K (ed) Nitrous oxide and climate change. Earthscan, London, pp 63–84
Soumare A, Manga A, Fall S, Hafidi M, Ndoye I, Duponnois R (2015) Effect of eucalyptus camaldulensis amendment on soil chemical properties, enzymatic activity, Acacia species growth and roots symbioses. Agrofor Syst 89:97–106. doi:10.1007/s10457-014-9744-z
Stehfest E, Bouwman L (2006) N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agroecosyst 74:207–228. doi:10.1007/s10705-006-9000-7
Stewart KJ, Brummell ME, Farrell RE, Siciliano SD (2012) N2O flux from plant-soil systems in polar deserts switch between sources and sinks under different light conditions. Soil Biol Biochem 48:69–77. doi:10.1016/j.soilbio.2012.01.016
Stieven AC, Oliveira DA, Santos JO, Wruck FJ, Campos DTS (2014) Impacts of integrated crop-livestock-forest on microbiological indicators of soil. Rev Bras Cienc Agrar 9(1):53–58. doi:10.5039/agraria.v9i1a3525
Thioulouse J, Chessel D, Dolédec S, Olivier JM (1997) ADE-4: a multivariate analysis and graphical display software. Stat Comput 7(1):75–83
Ussiri DAN, Lal R (2013) Soil emission of nitrous oxide and its mitigation. Springer, Dordrecht
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(6):703–707. doi:10.1016/0038-0717(87)90052-6
Verchot LV, Junior SB, de Oliveira C, Mutegi JK, Cattanio H, Davidson EA (2008) Fluxes of CH4, CO2, NO and N2O in an improved fallow agroforestry system in eastern Amazonia. Agric Ecosyst Environ 126:113–121. doi:10.1016/j.agee.2008.01.012
Wardle DA (1994) Metodologia para a quantificação da biomassa microbiana do solo. In: Hungria M, Araújo RS (eds) Manual de métodos empregados em estudos de microbiologia agrícola. Embrapa, Brasília
WMO—World Meteorological Organization (2012) Greenhouse Gas Bulletin http://www.wmo.int/pages/prog/arep/gaw/ghg/documents/GHG_Bulletin_No.8_en.pdf. Acessed 16 November 2014
Zhang J, Han X (2008) N2O emission from the semiarid ecosystem under mineral fertilizer (urea and superphosphate) and increased precipitation in northern China. Atmos Environ 42(2):291–302. doi:10.1016/j.atmosenv.2007.09.036
Acknowledgements
To Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for the scientific productitvity fellowships granted to the first and the third author. Also, to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior for the Post-Doctorate fellowship granted to the fourth author. Project funding was provided by Empresa Brasileira de Pesquisa Agropecuária, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Carvalho, A.M., de Oliveira, W.R.D., Ramos, M.L.G. et al. Soil N2O fluxes in integrated production systems, continuous pasture and Cerrado. Nutr Cycl Agroecosyst 108, 69–83 (2017). https://doi.org/10.1007/s10705-017-9823-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-017-9823-4