Abstract
Using pig slurry as starter fertilizer for maize (Zea mays L.), injected below the row prior to planting is a reasonable way to omit application of additional mineral fertilizer in areas with intensive animal farming. However, delayed early growth and a lack of knowledge on nutrient availability limit the interest of farmers. To extenuate farmers concerns a field trial was conducted in 2014 and 2015 to get detailed information on nitrogen (N) uptake, the subsequent influences on crop growth at different vegetative growth stages and final yield of silage maize. Besides an unfertilized control, two liquid manure injection treatments (without and with nitrification inhibitor [NI]) were compared to slurry broadcast application + mineral N and phosphorus (P) starter fertilizer at planting (MSF). In 2014, NI treatment yields increased (+16.5%) and N uptake increased (+9.6%) compared to broadcast treatment. In 2015, cold and dry conditions during early growth limited P plant availability and reduced crop growth in treatments without MSF. However, when a NI was added to the slurry prior to application, plants showed less P deficiency symptoms and better growth. At harvest no differences between the fertilized treatments were observed. In both years apparent N recovery was increased when manure was injected (48% without, and 56% with NI, respectively) compared to broadcast application of manure (43%) indicating that N losses were lower. However, further knowledge on soil N transformation and N loss pathways in systems with slurry injection is needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agriculture in northwestern Germany is traditionally dominated by intensive animal farming. Excessive use of organic manures causes phosphorus (P) accumulation in soils resulting in high soil test values for P in the region indicating only limited need for P fertilization on a large proportion of arable land (Leinweber et al. 1994). Furthermore nitrogen (N) and P from manures outbalance crop nutrient demand on many fields in the region and huge amounts of manure have to be exported (Warnecke et al. 2011). Maize (Zea mays L.), used as fodder and substrate for biogas plants is the dominating crop in the region (Keckl 2015).
Despite high soil test levels for P, plant availability of P typically is low in early growth stages of maize as low root zone temperatures reduce P diffusion speed (Imran et al. 2013) and restricted root growth restricts spatial nutrient acquisition (Mollier and Pellerin 1999). Limited P supply usually also impairs crop growth, as it reduces leaf appearance, leaf elongation and final leaf size (Plénet et al. 2000a) and thus, also aboveground biomass (Plénet et al. 2000b). In contrast, Muchow (1988) showed only minor effects of N deficiency on biomass accumulation at very early growth stages of maize. Although N concentrations in leaves can be reduced by 50%, leaf appearance, leaf elongation and final leaf area show only limited reactions to a wide range of N fertilization levels (Vos et al. 2005). Plénet and Lemaire (1999) also did not find major differences in early growth under N limitation, but N concentrations in maize should not fall below a critical value of 3.4% N, if aboveground biomass is below 1 Mg ha−1 to obtain maximum yields.
To enhance early growth development and ensure adequate yields by assuring optimum levels of N and P in plants, farmers commonly apply a mineral starter fertilizer (MSF) at planting (Withers et al. 2000). The combination of ammonium N and P proved most effective in several studies (e.g. Ma et al. 2013; Ohlrogge 1962) as both, lateral root proliferation and fine root proliferation are enhanced in zones where high concentrations of ammonium N and P occur (Ma et al. 2013). Additionally plant uptake of ammonium N induces lower rhizosphere pH, which can increase P availability (Neumann and Römheld 2012). Thus, farmers commonly use starters containing both ammonium N and P (e.g. diammonium phosphate, or blends of calcium ammonium nitrate with diammonium phosphate). On intensive animal farms, these starters are applied in addition to the broadcast application of manure, which usually already covers N and P demand of the crop. This practice results in accumulations of N and P in the soils increasing the risk of nutrient leaching and runoff (Touchton 1988). Especially nitrate leaching is a major problem on sandy soils (Cameron et al. 2013), which are common in northwestern Germany. Reducing reactive N emissions, such as nitrate leaching and N2O emissions, is a vital task for mankind (Sutton et al. 2011) and a goal of the European Union’s water framework directive (European Parliament 2000).
Obviating MSF by slurry injection in a band close to the maize rows was tested in several studies throughout North America (Bittman et al. 2012; Sawyer et al. 1991; Schmitt et al. 1995), Denmark (Petersen et al. 2010), and the Netherlands (Schröder et al. 1997, 2015). A series of trials in northwestern Germany showed the potential of manure injection to improve nutrient use efficiencies, when compared to broadcast application (Federolf et al. 2016). This might be due to higher proportions of plant available N when manure is injected. Compared to broadcast application of liquid manure, Sommer and Hutchings (2001) refer to reduced ammonia emissions, while Sørensen and Amato (2002) indicate reduced N immobilization as the interaction of soil and manure is lower. Higher soil mineral nitrogen (SMN) concentrations however, might increase denitrification losses (Cameron et al. 2013; Dosch and Gutser 1996). When a nitrification inhibitor (NI) is mixed into the manure prior to application, nitrification of the applied ammonium N from manure is retarded, and thus leads to lower leaching and denitrification losses (Ruser and Schulz 2015). While our previous study (Federolf et al. 2016), showed enhanced early growth when adding a NI, Sawyer et al. (1991) observed inconsistent increases in plant N content at V5 and V6 growth stages, when NIs were added to liquid beef manure. Along with these studies, Schmitt et al. (1995) reported varying effects on yields. Thus, further knowledge on N transformation in the soil after application of liquid manure (LM) with a high spatial resolution and the consequences for crop development is necessary (Westerschulte et al. 2015).
Consequently, a field trial was established to monitor SMN dynamics following liquid manure injection, as well as plant growth during maize vegetation in 2014 and 2015. Besides manure injection treatments with and without NI, an unfertilized control, and a local standard treatment where liquid manure was surface banded and incorporated was tested. While plots in the local standard treatment received MSF at planting, no further fertilization was applied to injection treatment plots.
The objective of our study was to compare liquid pig manure injection versus broadcast application in terms of the consequences on plant nutrient acquisition, focusing on obviating the addition of mineral starter fertilizer by slurry injection. Our study is based on the hypotheses that after injection of liquid manure, compared to broadcast application plus MSF, plant availability of nutrients is higher, leading to comparable early growth and equal yields with reduced nutrient input. High manure N concentrations in the injection zones delay turnover of the applied ammonium due to reduced soil–manure interaction (Dosch and Gutser 1996) and thus, reduce the risk of N translocation out of the root zone. We assume that the addition of a nitrification inhibitor to the slurry delays nitrification thereby enhancing ammonium–phosphate interactions, comparable to mineral starter fertilizer. In a corresponding article, Westerschulte et al. (2016) focus on spatial and temporal soil mineral N dynamics.
Material and methods
Experimental sites, soil conditions and weather conditions
In 2014 and 2015, field trials were conducted in Hollage, Lower Saxony, Germany (52°20′N, 07°58′E) at two adjacent fields. On both fields soil type can be categorized as plaggic podzol (IUSS Working Group WRB 2014) with sandy soil texture (>87% sand). Organic matter content was 2.0% in 2014 and 2.9% in 2015, respectively (for details see Table 1).
Maritime climate is dominating in northwest Germany. Mean annual air temperature at the study site is 10.0 °C and mean annual precipitation 799 mm. On average, monthly precipitation increases from 41 mm in April to 79 mm in August (Table 2). However, in 2014 a mild winter and above average temperatures in March and April led to higher soil temperatures. Higher air temperature throughout July, along with 129 mm of precipitation enabled very high growth rates for the plants. Thus, thermal time from planting to the end of June in 2014 were above long-term average (Fig. 1). By contrast, in 2015 May and June (i.e. during the early growth period of maize) were cold and dry. High temperatures in August 2015 however, led to high crop growth rates. Finally thermal time duration from planting to harvest was 1272 °Cd, being close to the 2014 observation (1450 °Cd).
Thermal time according to McMaster and Wilhelm (1997) from April 01 to June 30 at the study site. Comparing long-term average (1994–2014) with 2014, and 2015 growing season
Experimental design and treatments
In both years, the trial was set up in a randomized complete block design with four treatments and four replicates. Each plot was 3 m wide and 25 m long covering four rows (75 cm row spacing) and. The following treatments were compared:
-
1.
Control (C) without any fertilization to monitor N mineralization from the SMN pool
-
2.
Surface banding (B) of liquid manure with immediate incorporation (disc harrow 0–10 cm in <5 min after manure application) plus MSF at planting
-
3.
LM injection treatment (I)
-
4.
LM injection treatment with a nitrification inhibitor (ENTEC® FL, active ingredient: 3,4-dimethylpyrazol phosphate (DMPP), EuroChem Agro GmbH, Mannheim, Germany) added to the slurry at a rate of 10 l ha−1 (1.21 kg DMPP ha−1) prior to application [I(N)].
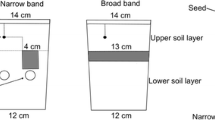
For both injection treatments, the upper rim of the liquid manure band was 12 cm (2014) and 10 cm (2015) below soil surface. In both years the slurry injector X-Till (Hugo Vogelsang Maschinenbau GmbH, Essen (Oldb.), Germany) adjusted for plot trial operations was used for slurry application. A rotary piston pump and a precision dispenser provided proper longitudinal and lateral distribution of the slurry. The injector had four tines (75 cm apart) followed by injection hoses, allowing the liquid manure to flow into the opened slot. A mineral NP fertilizer was used as MSF in B treatment plots at a rate of 115 kg ha−1 to apply 23 kg N ha−1 (9.4 kg NO3–N ha−1, 13.6 kg NH4–N ha−1) and 10 kg P ha−1 (5.6 kg ha−1 water-soluble P ha−1). MSF was applied 5 cm beside, and 5 cm below the seeds via separate special shares at planting. The N fertilization rate was calculated according to regional advisory standards (Baumgärtel et al. 2010). The recommended fertilization rate is 180 kg N ha−1 reduced by preplant SMN, N applied via MSF and site-specific conditions like recent organic fertilization and catch cropping. Liquid manure application rate was equal for the treatments B, I and I(N). As there was no compensation for MSF in the I and I(N) treatments, total N and P rates were lower.
Crop management practices
For the 2014 trial, after harvest of the previous crop maize no tillage operation was done until residue incorporation and seedbed preparation, using a disk harrow twice on March 05 and March 27. Previous crop for the 2015 trial was spring barley (Hordeum vulgare L.), followed by a frost-sensitive catch crop blend consisting of mustard (Sinapis alba L.) and oil radish (Raphanus sativus L.). A disc harrow was used to incorporate the catch crop on March 04, and for seedbed preparation on April 14, 2015. 23 m3 ha−1 of manure from a nearby pig fattening farm were applied at April 11 in 2014, containing 166 kg ha−1 of total N, and 42 kg ha−1 of P. In 2015, on April 14, 24 m3 ha−1 of manure from the same farm were applied, containing 130 kg ha−1 of total N, and 34 kg ha−1 of P (Table 3). Maize (Zea mays L. cv. Ricardinio, KWS SAAT AG, Einbeck, Germany) was planted at April 25 in 2014 and April 22 in 2015 4.5 cm below soil surface at a rate of 9.2 seeds m−2. Two herbicide applications according to local recommendations were done each year.
Measurements and samplings
Aboveground biomass was sampled at several vegetative (Vn stage when collar of nth leaf in B treatment was visible) and generative growth stages to monitor plant development (Table 4). Sixteen plants (20 plants at V3 and V4 to ensure that sufficient material for the lab analysis) per plot were cut at stem base in the middle rows and dried at 80 °C to a constant weight. At silage maturity (R5 stage) 7 m in the two center rows of each plot were harvested with a plot sized field chopper and fresh weight was measured gravimetrically. A representative sample was taken to determine dry matter content (drying to constant weight at 80 °C) and for lab analysis. Nitrogen concentrations in all samples were determined using the Kjeldahl method (DIN 2005). All samples were also analyzed for P concentrations after microwave assisted pressure digestion to dissolve P from organic compounds by ICP-AES (DIN 2012).
Calculations
Based on dry matter accumulation and N concentrations, N uptake, and the N balance (N applied minus N uptake) was calculated for each treatment. Apparent nitrogen recovery efficiency (NRE) was assessed according to Ciampitti and Vyn (2011). These calculations are based on total N applied. Thermal time was calculated according to McMaster and Wilhelm (1997): \(TT = \left[ {\frac{{\left( {T_{max} + T_{min} } \right)}}{2}} \right] - T_{base}\) where if \(\left[ {\frac{{T_{max} + T_{min} }}{2}} \right] < T_{base}\), then \(\left[ {\frac{{T_{max} + T_{min} }}{2}} \right] = T_{base}\). T min and T max were the observed daily minimum and maximum temperatures, respectively. T base was set to 8 °C.
Statistical analysis
Dry matter above ground biomass, N concentration and N uptake were statistically analyzed using the PROC MIXED procedure (SAS Institute Inc. 2011). For both seasons, treatments and replications were tested as fixed effects, the latter to check for heterogeneity of replications (model: treatment replication treatment*replication). Means of treatments were compared using the Tukey procedure for treatments when significant differences at P < 0.05 occurred. For all sampling occasions that were similar in both years (V6, V10, VT (tasseling) and harvest), a mixed model was performed including years, also as fixed effect to check for year*treatment interactions (model: treatment year treatment*year with replication(year) as random effect). The Pearson’s correlation between biomass accumulation (means of treatments) of all samplings and the respective thermal time durations was calculated with EXCEL .
Results
Biomass accumulation
Maize aboveground biomass accumulation was affected by treatment, season and the interaction of both (see Table 5). From emergence to VT biomass in 2015 was lower than in 2014. At V6 in 2015 mean biomass of all treatments was only 20% of the respective 2014 value, while at harvest it was 24% higher (Table 6).
In 2014 at V6, significant differences in above ground biomass production for the treatments were observed. Biomass in I(N) treatments was higher than in treatment B (569 vs 466 kg ha−1, respectively), with I treatment in between (523 kg ha−1). For all following growth stages, plants in B treatment showed reduced growth, compared to I and I(N) treatments growth, resulting in 12 and 16% higher yields at harvest for I and I(N), respectively. Treatment C showed significantly lower values than the other treatments for all sampling dates in 2014 (Table 6).
At V3 stage in 2015, aboveground biomass was not significantly influenced by fertilization. Values for B and I(N) treatments were slightly above C and I treatment (Table 6). All following sampling dates in 2015 showed significant differences in plant growth between treatments. Until VT, B treatment showed the highest dry matter accumulation followed by I(N) treatment. Differences between these treatments were significant at V6, V10 and VT samplings, whereas at V3, V4, V8, and harvest samplings they were not. The I and C treatments showed significantly reduced growth from V4 to VT. Throughout the vegetation period biomass for I treatment compared to C treatment was inconsistently higher (no significant differences at V3, V4, V6, and VT samplings and significant differences at V8, V10 and harvest samplings, respectively). The maximum difference between treatments was found at V10 sampling (B treatments aboveground biomass was 129% larger than C treatment). At harvest, biomass in the three fertilized treatments was 23% higher compared to control.
Nitrogen concentrations
N concentrations were affected by year and treatment for all growth stages (Table 5). The treatment*year interaction was also significant except at harvest. Mean N concentrations for both seasons were significantly enhanced for fertilized plots, when compared to non-fertilized plots at all samplings. The differences between fertilized treatments decreased with proceeding plant development.
As early as V6 stage in 2014, significant differences in nitrogen concentrations within treatments occurred (Table 6). Highest concentrations were found in I(N) treated plots, followed by I treatment (−6.7%, compared to I(N) treatment). Values for B and C treatments were significantly lower than I (−26.5%) and I(N) treatments (−24.6%). In the following weeks, N concentrations and differences between treatments decreased. At V10 stage I(N) still showed highest N concentrations, followed by I treatment (−10.7%, compared to I(N) treatment). C treatment concentration was significantly lower than I treatment, with B treatment in between. At tasseling there were no significant differences between treatments. At harvest, the highest concentrations were found in B treatment plots. I and I(N) treatments showed significantly lower values than B treatment (−8.1 and −5.9%, respectively). C treatment showed the lowest N concentrations (−13.6%, compared to B treatment).
In 2015 the measured N concentrations were more inconsistent. At V3 sampling, B treatment showed highest values and C treatment lowest, with I(N) and I treatments in between. At V8 and V10 stage however, B treatments N concentration was lower than in C treatment. While at V8 the highest values were found in I and I(N) treatments (49.5 and 50.2 g kg−1, respectively), at V10 N concentration in I(N) treatment was significantly lower than in I treatment. At harvest, N concentrations of the fertilized treatments were significantly higher than in C treatment.
Nitrogen uptake
Mean N uptake of plants in C treatment, was consistently lower than N uptake of the fertilized treatments for all sampling occasions (Table 6). These differences were significant, except for I treatment at early samplings in 2015 (V3–V6).
In 2014, compared to I(N) treatment, N uptake in C treatment was 77.7% (V6 stage) to 49.8% (harvest stage) lower. N uptake in B treatment was also consistently lower compared to I(N), but the largest difference was found at V10 stage (−50.9%). At harvest, the difference between B, and I(N) treatments was just −8.7%. Closest to I(N) treatment was I treatment, with significantly lower values at V6 and V10 (−14.2 and −17.9%, respectively) and only marginal differences at later samplings (−7.6% at VT and −6% at harvest, respectively).
In 2015, differences in N uptake occurred as early as V3 stage. Significantly higher values were found in B and I(N) plots (0.57 and 0.58 kg ha−1, respectively) compared to I and C treated plots (0.49 and 0.47 kg ha−1, respectively). The same order was observed for the following samplings. At V8 treatment B showed significantly reduced values (−16.6%) compared to I(N) treatment, while I treatment showed 55% higher values than C treatment. These differences between I and C treatments then slowly decreased (+43% at V10, +39% at VT and +35% at harvest, respectively). At V10 B treatment showed the same values as I(N), whereas at VT it was significantly higher (+12.7%). At the final harvest only C showed lower N uptake (−29%, compared to B) whereas N uptake in I and I(N) was at the same level as B.
Nitrogen balance and apparent nitrogen recovery efficiency
Nitrogen balances were mainly negative, except for B treatment in 2014, where a positive balance (+15 kg N ha−1) occurred (Fig. 2). Most noticeable are the major differences for the two seasons. In 2014 the balances for C and B treatments were −91 and +15 kg ha−1, while in 2015 they were −167 and −83 kg ha−1, respectively. Differences between the treatments were similar in both seasons. Mean apparent nitrogen recovery efficiency (NRE) for fertilized treatments was 49% in both seasons, with only minor differences within the seasons (Fig. 2). Lowest values for NRE were calculated for B treatment, and highest for I(N) treatment.
Nitrogen balance (left) and nitrogen recovery efficiency (right) for 2014 and 2015. Treatments fertilized with manure and mineral starter fertilizer (MSF) [C, no manure [ ], no MSF; B, manure broadcast with MSF [
], no MSF; B, manure broadcast with MSF [ ]; I, manure injection without MSF [
]; I, manure injection without MSF [ ]; I(N), manure injection with nitrification inhibitor without MSF [
]; I(N), manure injection with nitrification inhibitor without MSF [ ]
]
Discussion
Major differences concerning temperature and precipitation between the two seasons led to differences in crop development during early growth. For example in 2014, thermal time from emergence to V6 was 262 °Cd, while in 2015 it was only 172 °Cd. While Birch et al. (2003) refer to a constant thermal interval between initiation of successive leafs, the present results show a correlation (r2 = 0.92) between biomass accumulation and thermal time duration from emergence. We determined sampling occasions based on growth stages, but growth stages proved rather variable as biomass and thermal time duration to V6 in 2014 were quite similar to the respective V8 data of 2015 (Fig. 3).
Dry matter related to thermal time of all sampling occasions in the 2014 (filled) and 2015 (no fill) seasons. Means of treatments fertilized with manure and mineral starter fertilizer (MSF) [C, no manure ( /
/ ), no MSF; B, manure broadcast with MSF (
), no MSF; B, manure broadcast with MSF ( /
/ ); I, manure injection without MSF (
); I, manure injection without MSF ( /
/ ); I(N), manure injection with nitrification inhibitor without MSF (
); I(N), manure injection with nitrification inhibitor without MSF ( /
/ )]
)]
According to Muchow (1988) and Vos et al. (2005), low N concentrations in plants do not interfere leaf area expansion until V6 stage, as maize allows a wide variation of leaf N concentrations. Although leaf area expansion is not impaired, photosynthetic capacity might be reduced (Vos et al. 2005). Thus, low N concentrations in plants 2014 might have only minor impact on biomass accumulation at the first sampling, at least in fertilized treatments. Applying the critical N approach of Plénet and Lemaire (1999) to our dataset, B and C treatments in 2014 show limiting N concentrations throughout all sampling occasions (Fig. 4). These low to very low plant N concentrations are following major nitrate leaching events in May and June 2014, which lead to very low plant available N in the root zone (for more details on soil mineral N dynamics see Westerschulte et al. 2016), and consequently to low plant N concentrations. At V10 sampling, SMN in the topsoil layer (0–30 cm) of I and B treatments were nearly at level with C treatment (12.3 kg ha−1 for I and 12.8 kg ha−1 for B vs 11.4 kg ha−1 for C treatment). Only I(N) showed higher values (15.9 kg ha−1), resulting in higher plant N concentration at this growth stage. At VT, highest SMN values were found in B treatment (47.7 kg ha−1 in 0–90 cm), although mainly in the layer 60–90 cm below surface. Wiesler and Horst (1993) found a high proportion of pre-silking N uptake from soil layers up to 45 cm depth, whereas post-silking N was mainly taken up from below 60 cm. Thus, the higher SMN concentrations found in B treatment are most likely not plant available. A reasonable amount of broadcast incorporated slurry N is immobilized shortly after application (Kirchmann and Lundvall 1993), but can be remineralized later (Sørensen and Amato 2002). Thus, B treatment recovered to a certain extent from severe N limitation between tasseling and harvest. As the N concentrations in the I and I(N) treatments also drop below critical values for later sampling dates due to nitrate displacement out of the rooting zone, final yield reductions due to N limitation seem plausible for the 2014 season.
Critical N concentration according to Plénet and Lemaire (1999) (black line) DM versus N concentrations of the samplings in 2014 (filled) and 2015 (no fill). Means of treatments fertilized with manure and mineral starter fertilizer (MSF) [C, no manure ( /
/ ), no MSF; B, manure broadcast with MSF (
), no MSF; B, manure broadcast with MSF ( /
/ ); I, manure injection without MSF (
); I, manure injection without MSF ( /
/ ); I(N), manure injection with nitrification inhibitor without MSF (
); I(N), manure injection with nitrification inhibitor without MSF ( /
/ )]
)]
In 2015, no nitrate displacement was found during the vegetation period until harvest sampling (Westerschulte et al. 2016). Thus, N concentrations were always above critical values, even in C treatment (Fig. 4). Nevertheless, significant differences of N availability at early samplings might somehow be correlated to differences in biomass accumulation as early as V4 stage. The abundance of ammonium N in the injected slurry band is higher when a NI is added (Westerschulte et al. 2016), and plants under low root-zone temperatures prefer ammonium uptake for energetic reasons (Macduff and Jackson 1991; Subbarao et al. 2006). Thus, probably more assimilates are available for biomass production, resulting in significantly higher biomass in I(N) treatment, when compared to I treatment. At later samplings B treatments biomass was always the largest, whereas at V8 and V10, N concentration in B treatment was significantly lower than in all other treatments. Only minor precipitation events prior to V8 sampling (8.4 mm within 18 days), might have reduced nitrate availability to a certain extent, as SMN analysis did not show nitrate displacement from the soil zone where the slurry was applied (0–10 cm) to deeper zones (21–30 cm) until tasseling (Westerschulte et al. 2016). However, 30 mm rainfall between V8 and V10 samplings also did not result in higher plant N concentrations in B treatment, compared to the other treatments. At V8, plant N uptake in B treatment (~19 kg ha−1) was more or less at level with the applied N via MSF. Thus, the reduced N concentrations might follow a depletion of MSF N and a sharp decline in SMN below the maize plants (from 58 mg kg−1 at V6 to 23 mg kg−1 at V10; Westerschulte et al. 2016). Maybe plants needed to adapt to this change in N supply from localized MSF placement to the broadcast slurry N. At tasseling, only C treatment showed N concentrations close to critical values and below critical at harvest. The higher N uptake for C treatment in 2015, compared to 2014, might be due to different weather conditions, or due to differences in soil organic matter content (1.66% in 2015, and 1.14% in 2014, respectively).
Along with the critical N approach according to Plénet and Lemaire (1999) N availability cannot be the sole reason for differences of crop growth between fertilized treatments in this trial. As plants in C, I and to a lesser extent also in I(N) treatments showed visible P deficiency symptoms (purpling of leaves), biomass samples were analyzed for P (Fig. 5). Significant differences in P concentrations within treatments were found (B > I(N) > I ≥ C), ranging from 5.77 to 2.78 g kg−1 at V4 and from 4.21 to 2.17 g kg−1 at V6 stage, respectively. Jones (1983) described a function for optimum shoot P concentrations in relation to growth stages, which results in optimum shoot P concentration of 5.76 g kg−1 at V4. So, as only B treatment showed optimum P concentrations, differences in biomass can be related to P limitation. We found the greatest differences in P concentrations between treatments at V10 sampling. In a field trial with maize under P deficiency, Plénet et al. (2000b) reported the greatest differences from 8 to 15 visible leaves.
Phosphorus concentrations related to dry matter of the samplings in 2014 (filled) and 2015 (no fill). Means of treatments fertilized with manure and mineral starter fertilizer (MSF) [C, no manure ( /
/ ), no MSF; B, manure broadcast with MSF (
), no MSF; B, manure broadcast with MSF ( /
/ ); I, manure injection without MSF (
); I, manure injection without MSF ( /
/ ); I(N), manure injection with nitrification inhibitor without MSF (
); I(N), manure injection with nitrification inhibitor without MSF ( /
/ )]
)]
Compared to the 2014 season, the period mid-May to late June 2015 was extraordinary cold and dry. Both, soil temperature (Imran et al. 2013), and soil water content (Bhadoria et al. 1991) affect P diffusion speed. Thus, P limitation in C treatment is not surprising. But the application of 34 kg ha−1 P via liquid manure in B, I, and I(N) treatments and another 10 kg ha−1 P via MSF in B treatment should lead to sufficient plant available P close to the seedlings. As at V3 only B treatment showed P concentrations superior to C treatment, either spatial or chemical nutrient availability of the injected liquid manure must have been lower compared to MSF. The differences (Fig. 5) between I and I(N) treatments for P concentration at V3, V4 and V6 samplings can only be due to the NI, as all other factors (liquid manure, injector, injection depth, application rate) were equal. As shown by Westerschulte et al. (2016), nitrification of NH4–N in the slurry band was delayed in I(N) treatment, when compared to I treatment. Subbarao et al. (2006) and Withers et al. (2000) refer to better growth, when ammonium and P are applied together, versus each nutrient alone. Application of an ammonium-based fertilizer with NI lowered rhizosphere pH and increased P uptake in a pot experiment with common bean (Phaseolus vulgaris L., Thomson et al. 1993). However, the differences in ammonium N found in our experiment are probably not comparable to a pot experiment, where ammonium and nitrate fertilizers were tested. Thomson et al. (1993) furthermore referred to an inhibitory effect on root growth of the used NI (Nitrapyrin). Possibly the compound used in our experiment (DMPP) also influences crop growth.
In two contrasting seasons, despite some restrictions in crop development, the treatments with liquid manure injection showed consistent yields, compared to the manure broadcast treatment. Major SMN displacement in 2014 reduced yields in all treatments, but to a lesser extent when slurry was injected. In 2015, a cold and dry period during early growth lowered P availability resulting in major growth differences. At harvest, however no significant differences between fertilized treatments were found. In both years, the addition of a NI to manure did not lead to significantly increased yields and N uptake, but it increased early growth by assuring higher P concentrations in the plant. Compared to B treatment, I(N) treatment showed equal N uptake and significantly higher yields, despite a noteworthy reduction in N (−23 kg ha−1) and P (−10 kg ha−1) fertilization. Thus, the lower N balances and higher nutrient recovery efficiencies for slurry injection treatments found in this experiment are comprehensible, and go along with the findings of other studies (Federolf et al. 2016; Schröder et al. 1997). Negative N balances indicate a reduced potential for nitrogen losses to the environment, thus, major sustainability targets (European Parliament 2000; Sutton et al. 2011) are met to a greater extent. Although Westerschulte et al. (2016) showed a significant delay in nitrification of the applied ammonium via liquid manure, the inconsistent and insignificant effects on yields and N uptake were also found by others (Federolf et al. 2016; Sawyer et al. 1991; Schmitt et al. 1995).
Thus, for a final evaluation of the agronomic effects of nitrification inhibitors further studies, and a more detailed knowledge on the mode of action of DMPP, as well as the interactions on soil microbiology and plant nutrition are necessary. Even if the agronomic value of nitrification inhibitors is variable, the environmental impact needs to be regarded as well. As nitrification inhibitors are able to reduce leaching and denitrification (Barneze et al. 2015; Ruser and Schulz 2015; Subbarao et al. 2006), they might be able to meet the target of reducing reactive nitrogen emissions into the environment (Sutton et al. 2011). On the other hand possible discharge of nitrification inhibitor compounds and their metabolites into aquatic environments needs to be taken into account (Scheurer et al. 2016).
Five sinks for fertilizer nitrogen are known, (1) plant uptake, (2) ammonia emissions, (3) trace gas emissions, (4) nitrate leaching, and (5) the soil nitrogen pool (organic and inorganic). When cumulating the data for SMN of Westerschulte et al. (2016) with our observations, among all tested treatments the highest proportions of applied N were found in I(N) treatment throughout all sampling occasions. Yet, still major pathways for N losses and N contents of plant roots were not quantified. Thus, further research on this topic measuring all possible N sinks ideally using labelled N is necessary.
Conclusion
Injection of liquid manure close to maize seedling ensured optimal nutrients supply for the crop. Under cold conditions, the addition of a nitrification inhibitor seems to promote phosphorus availability in early growth stages. The impact of nitrification inhibitors on soils under field conditions however, needs further studies.
Manure injection showed a huge potential to reduce nitrogen and phosphor fertilization rates, without impairing maize yields on sandy soils in northwestern Germany. Thus, farmers can use this technology to decrease nutrient surpluses and benefit the environment.
References
Barneze AS, Minet EP, Cerri CC, Misselbrook T (2015) The effect of nitrification inhibitors on nitrous oxide emissions from cattle urine depositions to grassland under summer conditions in the UK. Chemosphere 119:122–129. doi:10.1016/j.chemosphere.2014.06.002
Baumgärtel G, Benke M, Eiler T (2010) Düngeempfehlungen Stickstoff: Getreide, Raps, Hackfrüchte, Mais. Landwirtschaftskammer Niedersachsen, Hannover
Bhadoria PBS, Kaselowsky J, Claassen N, Jungk A (1991) Phosphate diffusion coefficients in soil as affected by bulk density and water content. Z Pflanzenernaehr Bodenkd 154:53–57. doi:10.1002/jpln.19911540111
Birch CJ, Vos J, van der Putten PE (2003) Plant development and leaf area production in contrasting cultivars of maize grown in a cool temperate environment in the field. Eur J Agron 19:173–188. doi:10.1016/S1161-0301(02)00034-5
Bittman S, Liu A, Hunt DE, Forge TA, Kowalenko CG, Chantigny MH, Buckley K (2012) Precision placement of separated dairy sludge improves early phosphorus nutrition and growth in corn (L.). J Environ Qual 41:582–591. doi:10.2134/jeq2011.0284
Cameron KC, Di HJ, Moir JL (2013) Nitrogen losses from the soil/plant system: a review. Ann Appl Biol 162:145–173. doi:10.1111/aab.12014
Ciampitti IA, Vyn TJ (2011) A comprehensive study of plant density consequences on nitrogen uptake dynamics of maize plants from vegetative to reproductive stages. Field Crops Res 121:2–18. doi:10.1016/j.fcr.2010.10.009
DIN (2005) Futtermittel—Bestimmung des Stickstoffgehaltes und Berechnung des Rohproteingehaltes—Teil 1: Kjeldahl-Verfahren. DIN EN ISO 5983-1:2005, Beuth Verlag GmbH, Berlin
DIN (2012) Futtermittel—Bestimmung von Calcium, Natrium, Phosphor, Magnesium, Kalium, Schwefel, Eisen, Zink, Kupfer, Mangan und Kobalt nach Druckaufschluss mittels ICP-AES. DIN EN 15621:2012-04, Beuth Verlag GmbH, Berlin
Dosch P, Gutser R (1996) Reducing N losses (NH3, N2O, N2) and immobilization from slurry through optimized application techniques. Fertil Res 43:165–171. doi:10.1007/BF00747697
European Parliament (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy: Directive 2000/60/EC
Federolf C-P, Westerschulte M, Olfs H-W, Broll G, Trautz D (2016) Enhanced nutrient use efficiencies from liquid manure by positioned injection in maize cropping in northwest Germany. Eur J Agron 75:130–138. doi:10.1016/j.eja.2016.01.016
Imran M, Mahmood A, Römheld V, Neumann G (2013) Nutrient seed priming improves seedling development of maize exposed to low root zone temperatures during early growth. Eur J Agron 49:141–148. doi:10.1016/j.eja.2013.04.001
IUSS Working Group WRB (2014) World reference base for soil resources 2014: international soil classification system for naming soils and creating legends for soil maps. FAO, Rome, p 203
Jones CA (1983) A survey of the variability in tissue nitrogen and phosphorus concentrations in maize and grain sorghum. Field Crops Res 6:133–147. doi:10.1016/0378-4290(83)90053-9
Keckl G (2015) Bodennutzung und Ernte 2014. Landesamt für Statistik, Hannover
Kirchmann H, Lundvall A (1993) Relationship between N immobilization and volatile fatty acids in soil after application of pig and cattle slurry. Biol Fertil Soils 15:161–164. doi:10.1007/BF00361605
Leinweber P, Geyer-Wedell K, Jordan E (1994) Phosphorgehalte von Böden in einem Landkreis mit hoher Konzentration des Viehbestandes. Z Pflanzenernaehr Bodenkd 157:383–385. doi:10.1002/jpln.19941570510
Ma Q, Zhang F, Rengel Z, Shen J (2013) Localized application of NH4 +–N plus P at the seedling and later growth stages enhances nutrient uptake and maize yield by inducing lateral root proliferation. Plant Soil 372:65–80. doi:10.1007/s11104-013-1735-8
Macduff JH, Jackson SB (1991) Growth and preferences for ammonium or nitrate uptake by barley in relation to root temperature. J Exp Bot 42:521–530. doi:10.1093/jxb/42.4.521
McMaster GS, Wilhelm WW (1997) Growing degree-days: one equation, two interpretations. Agric For Meteorol 87:291–300. doi:10.1016/S0168-1923(97)00027-0
Mollier A, Pellerin S (1999) Maize root system growth and development as influenced by phosphorus deficiency. J Exp Bot 50(333):487–497. doi:10.1093/jxb/50.333.487
Muchow RC (1988) Effect of nitrogen supply on the comparative productivity of maize and sorghum in a semi-arid tropical environment I. Leaf growth and leaf nitrogen. Field Crops Res 18:1–16
Neumann G, Römheld V (2012) Rhizosphere chemistry in relation to plant nutrition. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants 2012. Academic Press, San Diego, pp 347–368
Ohlrogge AJ (1962) Some soil-root-plant relationships. Soil Sci 93:30–38
Petersen J, Jensen HH, Rubæk GH (2010) Phosphorus fertilization of maize seedlings by side-band injection of animal slurry. In: Proceedings 15th RAMIRAN conference, Lisboa, Portugal, 12 Sept 2010
Plénet D, Lemaire G (1999) Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil 216:65–82. doi:10.1023/A:1004783431055
Plénet D, Etchebest S, Mollier A, Pellerin S (2000a) Growth analysis of maize field crops under phosphorus deficiency. I. Leaf growth. Plant Soil 223:119–132. doi:10.1023/A:1004877111238
Plénet D, Mollier A, Pellerin S (2000b) Growth analysis of maize field crops under phosphorus deficiency. II. Radiation-use efficiency, biomass accumulation and yield components. Plant Soil 224:259–272. doi:10.1023/A:1004835621371
Ruser R, Schulz R (2015) The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils—a review. J Plant Nutr Soil Sci 178:171–188. doi:10.1002/jpln.201400251
SAS Institute Inc (2011) SAS/STAT® 9.3 user’s guide. SAS Institute Inc., Cary
Sawyer JE, Schmitt MA, Hoeft RG, Siemens JC, Vanderholm DH (1991) Corn production associated with liquid beef manure application methods. J Prod Agric 4:335–344
Scheurer M, Brauch HJ, Schmidt CK, Sacher F (2016) Occurrence and fate of nitrification and urease inhibitors in the aquatic environment. Environ Sci Process Impacts 18:999–1010. doi:10.1039/c6em00014b
Schmitt MA, Evans SD, Randall GW (1995) Effect of liquid manure application methods on soil nitrogen and corn grain yields. J Prod Agric 8:186–189. doi:10.2134/jpa1995.0186
Schröder JJ, Ten Holte L, Brouwer G (1997) Response of silage maize to placement of cattle slurry. Neth J Agric Sci 45:249–261
Schröder JJ, Vermeulen GD, van der Schoot JR, van Dijk W, Huijsmans J, Meuffels G, van der Schans DA (2015) Maize yields benefit from injected manure positioned in bands. Eur J Agron 64:29–36. doi:10.1016/j.eja.2014.12.011
Sommer SG, Hutchings NJ (2001) Ammonia emission from field applied manure and its reduction—invited paper. Eur J Agron 15:1–15. doi:10.1016/S1161-0301(01)00112-5
Sørensen P, Amato M (2002) Remineralisation and residual effects of N after application of pig slurry to soil. Eur J Agron 16:81–95. doi:10.1016/S1161-0301(01)00119-8
Subbarao GV, Ito O, Sahrawat KL, Berry WL, Nakahara K, Ishikawa T, Watanabe T, Suenaga K, Rondon M, Rao IM (2006) Scope and strategies for regulation of nitrification in agricultural systems—challenges and opportunities. Crit Rev Plant Sci 25:303–335
Sutton MA, Oenema O, Erisman JW, Leip A, van Grinsven H, Winiwarter W (2011) Too much of a good thing. Nature 472:159–161. doi:10.1038/472159a
Thomson CJ, Marschner H, Römheld V (1993) Effect of nitrogen fertilizer form on pH of the bulk soil and rhizosphere, and on the growth, phosphorus, and micronutrient uptake of bean. J Plant Nutr 16:493–506. doi:10.1080/01904169309364548
Touchton JT (1988) Starter fertilizer combinations for corn grown on soils high in residual P. J Fertil Issues 5:126–130
Vos J, van der Putten P, Birch CJ (2005) Effect of nitrogen supply on leaf appearance, leaf growth, leaf nitrogen economy and photosynthetic capacity in maize (Zea mays L.). Field Crops Res 93:64–73. doi:10.1016/j.fcr.2004.09.013
Warnecke S, Biberacher M, Brauckmann HJ, Broll G (2011) Nachhaltige Verwertung von Nebenprodukten aus der Erzeugung tierischer Nahrungsmittel durch Initiierung eines regionalen Stoffstrommanagements. In: Windhorst H-W, Veauthier A (eds) Nachhaltige Tierproduktion in agrarischen Intensivgebieten Niedersachsens. Weiße Reihe 35. Vechtaer Druckerei und Verlag, Vechta, pp 107–126
Westerschulte M, Federolf C-P, Pralle H, Trautz D, Broll G, Olfs H-W (2015) Soil nitrogen dynamics after slurry injection in field trials: evaluation of a soil sampling strategy. J Plant Nutr Soil Sci 178:923–934. doi:10.1002/jpln.201500249
Westerschulte M, Federolf C-P, Broll G, Trautz D, Olfs H-W (2016) Nitrogen dynamics following slurry injection in maize: soil mineral nitrogen. Nutr Cycl Agroecosyst. doi:10.1007/s10705-016-9799-5
Wiesler F, Horst WJ (1993) Differences among maize cultivars in the utilization of soil nitrate and the related losses of nitrate through leaching. Plant Soil 151:193–203. doi:10.1007/BF00016284
Withers PJ, Peel S, Chalmers AG, Lane SJ, Kane R (2000) The response of manured forage maize to starter phosphorus fertilizer on chalkland soils in southern England. Grass Forage Sci 55:105–113. doi:10.1046/j.1365-2494.2000.00204.x
Acknowledgements
We thank the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt DBU) for financing this research within the project “Optimizing nitrogen and phosphorus use efficiencies from liquid manure by injection to reduce environmental pollution” (Grant 30364/01). EuroChem Agro GmbH, Mannheim, thankfully granted additional support. We are grateful to the field technicians and students who did magnificent work, Elke Nagel, Maren Johannes and Yvonne Garlich in the laboratory, as well as Hans-Georg Schön and Herbert Pralle for their assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Federolf, CP., Westerschulte, M., Olfs, HW. et al. Nitrogen dynamics following slurry injection in maize: crop development. Nutr Cycl Agroecosyst 107, 19–31 (2017). https://doi.org/10.1007/s10705-016-9813-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-016-9813-y