Abstract
Cover cropping is a major challenge in the sustainable management of rainfed olive orchards. From a 5 year study (2009–2014), the effect of a mixture of early-maturing and self-reseeding annual legumes (Legs) grown as a cover crop in a rainfed olive orchard was compared with a cover of natural vegetation fertilized with 60 kg nitrogen (N) ha−1 year−1 (NV +N), and a cover of natural vegetation not fertilized (NV −N). The study took place in NE Portugal. The following were assessed: (1) the performance of the covers by measuring ground cover percentages, dry matter yields and N content in aboveground biomass; (2) the soil fertility through chemical and microbiological assays and by growing plants in pot experiments; and (3) the nutritional status of olive trees and olive yields. Legumes gave higher ground-cover percentages, produced more biomass and accumulated more N in shoots in comparison to natural vegetation, whether fertilized or not. The results showed intense biological activity [microbial carbon (C) and N, CO2–C evolved in a laboratory incubation, metabolic quotient, total culturable fungi and bacteria, and acid phosphatase activity] in the 0–10 cm soil layer of the treatments producing more biomass (Legs and NV +N). However, soil available N was greater in soil samples from the Legs plot. N recoveries by turnip (Brassica rapa var. rapa L.) and rye (Secale cereale L.) grown in pot experiments were 84.4 and 60.2 mg pot−1 in soil samples from the Legs treatment and 29.4 and 27.1 mg pot−1 and 14.2 and 13.6 mg pot−1, respectively in NV +N and NV −N plots. Sown legumes appeared less effective in increasing organic C than natural vegetation. Nevertheless, in the Legs plot the increase of easily mineralizable C was proportionally higher than the increase of total organic C, which may mean that a more reactive pool of organic C is created, which may reduce the turnover of organic C and N in the soil. In the 10–20 cm soil layer, total organic C was significantly lower in Legs (14.0 g kg−1) than in NV −N (22.1 g kg−1) and N +NV (25.2 g kg−1) treatments, likely due to a priming effect caused by mineral N coming from the surface layer. Two years after the trial started, the N nutritional status of the olive trees was significantly higher in Legs than in natural vegetation plots even when 60 kg N ha−1 year−1 was applied. The cumulative olive yields in NV −N and NV +N plots were only 58.6 and 77.7 % in comparison to those found in the Legs plot, if only the last four harvests were considered, which were those influenced by the ground-cover treatments (2010–2013).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Olive farming is the main economic activity in several areas of the region known locally as “the warm land” in NE Portugal. For most of the smallholdings that dominate the region, olives are the only cash crop. Olive farming is an important source of self-employment, and thus the main activity mitigating the depopulation of these lands. The sector also involves several ‘weekend’ part-time farmers growing small areas of olives, but which is nevertheless important to supplement their household income.
In NE Portugal, olive growing covers 75,000 ha and involves approximately 36,000 producers (INE 2013). Most of the olive plantations are currently rainfed, a situation that will certainly persist into the future (Monteiro 2007; Rodrigues 2009). It is therefore not expected that the sector will evolve into the high-density and irrigated orchards, as has occurred in the flat lands in the south of the country. Water resources are scarce, since the main watercourses are embedded in deep canyons which make it difficult to use water in agriculture. In addition, the landscape is dominated by a mosaic of small and widely scattered plots of olive trees which cannot be electrified, a requirement to pump the irrigation water.
In such marginal conditions for olive growing, mainly due to the lack of irrigation and low natural soil fertility, local experts and policymakers advocate less intensive agricultural systems, such as organic farming. In these agricultural systems, farmers’ income should be based on achieving quality products of high market value and less on higher yields (Monteiro 2007). However, in low-input production systems, such as organic farming where Haber–Bosch N is not allowed, it is very difficult to maintain the trees in a good N nutritional status. Regarding the warm land of NE Portugal, the region has suffered a hyper-specialization in olive cultivation, meaning that nowadays farms have no farmyard manure to fertilize their crops. Commercial organic amendments are also scarce, typically have low N use efficiency and are very expensive, particularly when allowed for organic farming (Rodrigues et al. 2006). Thus, to overcome these problems, a natural source of N must be sought. Cover cropping with legume species may provide the opportunity.
Nodulated legumes are able to access atmospheric di-nitrogen (N2), through the establishment of a symbiotic relationship with fixing bacteria (Cooper and Scherer 2012), allowing them to grow well even in low fertility soils (Rodrigues et al. 2013). Several studies have shown that the N fixed by legumes can be transferred to companion crops (Hardarson and Atkins 2003; Pirhofer-Walzl et al. 2012) or others that follow them in the rotation (Zotarelli et al. 2012; Monday et al. 2013). Perennial tree and shrub crops can also benefit from fixed N by legume cover crops, as demonstrated by Snoeck et al. (2000) from a study in a coffee tree plantation.
Although few farmers have yet adopted cover cropping strategies to manage their orchards, the issue has drawn the attention of the scientific community. In many countries of the Mediterranean basin a remarkable effort has been made to demonstrate the importance of reducing soil tillage in olive orchards, particularly in shallow and sloping soils. The management of natural or sown vegetation has been recommended since cover cropping is an effective way to reduce soil erosion and the loss of nutrients through runoff (Martínez et al. 2006; Gómez et al. 2009; Kairis et al. 2013), the main threat to the sustainability of production systems on steep slopes. Cover crops are also an important means of increasing soil organic matter (Mauromicale et al. 2010; Montanaro et al. 2010; Ferreira et al. 2013), also promoting many other physical, chemical and biological properties of soils (Moreno et al. 2009; Ramos et al. 2010, 2011). In the Mediterranean environment, self-reseeding annual legumes have been suggested as the most desirable species for cover cropping (Driouech et al. 2008; Mauromicale et al. 2010). They can fix N and persist over the years in the cover without mechanical reseeding, which may reduce costs (Arrobas et al. 2011).
This study consisted of the introduction of a mixture of several early-maturing and self-reseeding annual legumes as a cover crop in a rainfed olive orchard. The effect of the legume cover crop was compared to a cover of natural vegetation fertilized with 60 kg N ha−1 year−1 and an unfertilized cover of natural vegetation, in a study carried out from September 2009 to June 2014. The study had three main objectives: (1) to evaluate the performance of the legumes as a cover crop, in comparison to the other treatments, by measuring ground-cover percentages, dry matter yields and N recoveries in above-ground biomass; (2) to evaluate the effect of the cover crops on soil fertility, by determining soil organic C, total N, easily mineralizable N fractions, soil microbiology and microbial activity, and N recovery in pot grown plants; and (3) to assess the nutritional status of the olive trees and olive yields.
We hypothesized that: (1) the legume cover crop can produce more biomass and accumulate more N in the aboveground biomass than the covers of natural vegetation, even in the treatment fertilized with N, due to the access of atmospheric N2 by the legume species; (2) the covers of annual legumes can increase the soil microbial activity and the soil available N, by releasing a N-rich organic residue into the soil; and (3) the legume cover crops can replace the application of 60 kg N ha−1 year−1 as a fertilizer in maintaining the N nutritional status of the trees and the olive yields.
Materials and methods
Site and orchard characterization
The field trial was carried out in Mirandela (41.49N; 7.26W; 350 m asl), NE Portugal, from September 2009 to June 2014. The region benefits from a Mediterranean climate. Mean annual precipitation and temperature are respectively 508.6 mm and 14.3 °C. The plot where the orchard is planted has a slope of ~2 %. The soil is a Leptosol sandy loam textured originating from a bed-rock of schist. The soil analyses performed before the establishment of the trial revealed a soil with low organic matter (Walkley–Black) content (13.6 g kg−1), pH (soil:water, 1:2.5) 4.1, and with low and high levels of extractable (Egner–Riehm) phosphorus (21.0 mg kg−1) and potassium (96.3 mg kg−1), respectively.
The olive (Olea europaea L.) orchard is managed without irrigation and the trees of the cultivar Cobrançosa are planted 7 × 7 m apart. The trees were 20 years old when the trial started. Before the application of the groundcover treatments, 1500 kg/ha of lime (88 % CaCO3 and 5 % MgCO3) and 250 kg/ha of superphosphate (18 % P2O5) were applied, based on literature demonstrating the benefits of increasing soil pH and available P in terms of growth and N fixation by annual legumes (Cooper and Scherer 2012).
Ground management and experimental design
After a long period of soil management by tillage since the orchard was planted, the farmer changed the system to the use of herbicides. Since 2006, the farmer controlled the weeds by using glyphosate in a single application in mid-spring. In the early spring of 2009, the native vegetation was dominated by annual plants adapted to low fertility soils, which produce their seeds very early in the season, such as Mibora minima, Spergula arvensis and Rumex acetosella. Beneath the canopy, benefiting from a higher fertility soil, there were species of high nitrophily, such as Lolium rigidum and Stellaria media.
The orchard selected for this experiment is a homogeneous plot regarding slope and general soil characteristics. It was divided into nine experimental plots of 490 m2 each to receive in three replications the following groundcover treatments: natural vegetation fertilized with 60 kg N ha−1 year−1 (NV +N), the fertilizer being broadcasted over the entire area of the plot; natural vegetation not fertilized (NV −N); and a cover crop consisting of a mixture of eleven self-reseeding annual legume species and cultivars (Legs). The mixture was composed of 11 annual pasture legumes (Ornithopus compressus L. cv. Charano; Ornithopus sativus Brot. cvs. Erica and Margurita; Trifolium subterraneum L. ssp subterraneum Katzn. and Morley cvs. Dalkeith, Seaton Park, Denmark and Nungarin; Trifolium resupinatum L. ssp resupinatum Gib and Belli cv. Prolific; Trifolium incarnatum L. cv. Contea; Trifolium michelianum Savi cv. Frontier; and Biserrula pelecinus L. cv. Mauro). The mixture of the legume species was sown at a rate of one 11th of that recommended for each individual species if it had been seeded alone in pure culture. In each plot three trees with similar canopy sizes were selected and marked for assessing plant nutritional status and olive yields. After the trial had started the vegetation of all the plots was mechanically managed. When most seeds of legumes reached physiological maturity the vegetation was destroyed with a rotary slasher and left on the ground as a mulch.
Cover crops performance
The degree of ground cover by the vegetation was assessed by the pin-point method during four growing seasons from February 2010 to May 2014. In a pin-point analysis, a frame with a fixed grid pattern is placed randomly above the vegetation and a pin is inserted vertically through the grid points into the vegetation as described by Damgaard et al. (2011). The types of hits recorded were sown legumes, natural vegetation and bare soil.
In mid-spring, close to the maximum growth of the vegetation, the above-ground biomass was cut for dry mater yield and elemental composition evaluation. Random samples of vegetation defined by a square grid of 0.25 m2 were collected in four replications per treatment. Tissue analysis was performed by Kjeldahl (N), colorimetry (boron and phosphorus), flame emission spectrometry (potassium) and atomic absorption spectrophotometry (calcium, magnesium, copper, iron, zinc and manganese) methods (Walinga et al. 1989).
Soil sampling and laboratory analyses
Six composite soil samples of ~10 kg per treatment and depth (0–10 and 10–20 cm) were collected in October 2013, 4 years after the beginning of the field experiment to reflect the cumulative effect of the ground cover treatments on soil fertility. The soil was collected from a vertical wall of holes of 20 cm opened in the ground with a hoe. Each composite sample was prepared from soil of five holes. Approximately 90 % of each sample was air dried after it had been collected and thereafter sieved to pass through a grid of 2 mm. Dried and sieved soil was used in soil organic carbon and soil available nitrogen determinations and in pot experiments. The remaining ~10 % of the sample was frozen until used in the soil microbiology and microbial activity essays.
Several laboratory analyses were performed to assess the soil C and the potentially available N. Briefly, total organic carbon (TOC) was determined by dry combustion which consisted of the mass difference of 1 g oven-dried sample at 100 °C and heated in a furnace at 400 °C for 6 h. Walkley–Black Carbon (WBC), an easily oxidizable fraction of TOC, was obtained after a wet digest in an acid dichromate solution followed by back titration of the remaining dichromate with ferrous ammonium-sulfate and phenanthroline as the color indicator. Kjeldahl-N was determined after the digestion of 1.0 g of soil with H2SO4 and selenium as the catalytic agent in a heated (400 °C) aluminum digestion block. After cooling, the suspension was distilled with alkali, and the NH4-N in the digest was titrated with hydrochloric acid in a Kjeldahl Auto 1030 Analyzer.
Indices of easily mineralizable-N were also determined. The methodologies followed were essentially the same as those reported by Rodrigues et al. (2010b). Hot KCl extractable NH4-N (HotKCl) was determined by heating (100 °C) 5.0 g of soil and 200 mL of 2M KCl for 4 h in a digestion block. After cooling, the extract was filtered through Whatman No 42. This procedure was repeated cold (ColdKCl). Nitrate and ammonium concentrations in the extracts were analyzed in an UV–VIS spectrophotometer Varian Cary 50. The UV absorbance of NaHCO3 extracts (NaHCO3) were prepared by shaking 2.5 g soil with 0.01 mol L−1 NaHCO3 for 15 min in 125-mL Erlenmeyer flasks. The suspension was filtered through a Whatman No 42 paper. The UV absorbance of the extracts was measured at 205 and 260 nm wavelengths.
Essays of soil microbiology and microbial activity were also performed. Microbial analyses were performed in six replications for each ground-cover treatment and soil depth. Soil microbial biomass C (Mic-C) and N (Mic-N) were determined on fresh soil samples, using the chloroform fumigation–extraction method (Vance et al. 1987), after 24 h of conditioning at 25 °C and 60 % water holding capacity (WHC). Organic C and total soluble N were determined by near infrared detection (NIRD) and by chemiluminescence detection after combustion at 850 °C in an elemental analyzer (Formac, Skalar). The Mic-C and Mic-N were calculated using a KEC factor of 0.33 (Vance et al. 1987) and KEN factor of 0.54 (Brookes et al. 1985), respectively. All results are expressed on an oven-dry (105 °C) weight basis.
Soil basal respiration was measured during 7 days laboratory incubation, as described by Pereira et al. (2011). Briefly, moist sample (50 g) adjusted to 60 % WHC was placed in a 1.5 L glass jar with a plastic vial containing 40 mL NaOH (1 M) solution. After incubation at 25 °C for 1, 3 and 7 days, BaCl2 (1.5 M) solution was added into plastic vial, and then titrated with 0.24 M HCl. The jars without soil served as the controls. Soil respiration was calculated as the accumulative CO2-C released from the soil. The metabolic quotient (qCO2) was calculated as the ratio of respiration (mg C-CO2 mg−1 d−1) to Mic-C.
Total counts of heterotrophic bacteria and total fungi in soil samples were determined by plating serial dilutions on agar media. Total heterotrophic bacteria were enumerated on the Plate count agar (PCA, Liofilchem, Italy) supplemented with cycloheximide (1 mg/mL, Sigma-Aldrich, Milan, Italy) to suppress fungal growth and incubated at 30 °C for 2 days. Fungal counts were determined using Rose Bengal Agar medium (RBA, Liofilchem, Italy) and the plates incubated at 25 °C for 5 days. After incubation the colonies were counted as colony forming unit (cfu). Results were expressed as log cfu g−1 of dry soil.
The acid phosphatase activity was determined by measuring p-nitrophenol released from 1 g of soil after incubation with 0.25 ml toluene, 4 ml MVB (Modified Universal Buffer) adjusted at pH 6.5 and 1 ml p-nitrophenylphosphate at 37 °C for 1 h (Tabatabai and Bremner 1969).
Nitrogen recovery in pot grown plants
Nitrogen recovery in plants grown in pots is usually referred to as a good indicator of soil available N. In these essays there were used dried soil samples from six sources: three ground-cover treatments (Legs, NV +N, NV −N); and two soil depths (0–10 and 10–20 cm). The experimental design also included six replicates of each soil. Turnip (Brassica rapa var. rapa L.) and rye (Secale cereale L.), two species with high nitrophily, were used in this experiment. Turnip, with a short growing cycle, gave an indication of N release in the short-term. Rye, with a longer growing cycle, provided information on N release in the long-term. The pots were filled with 2 kg dry soil. Ten seeds of each species were sown on October 22nd 2013, being thinned to up to five plants per pot after emergence. The pots were placed in a greenhouse, regularly irrigated with distilled water and plants cut when growth ceased due to exhaustion of the available nutrients. Turnip was cut on January 20th 2014, and rye on March 12th. The biomass was oven-dried at 70 °C, weighed and ground before elemental analysis.
Tree nutritional status and olive yield
Twice a year, during the winter resting period and in summer during July at endocarp sclerification, leaf samples were taken to assess the nutritional status of the olive trees. Young mature leaves were collected from the middle of nonbearing, current season, shoots of the four quadrants around the tree canopy. The samples were then oven-dried at 70 °C and ground. Elemental analyses for several macro and micronutrients were performed using appropriate laboratory methods (see “Cover crops performance” section).
The olive yields were recorded in five consecutive harvests from November 2009 to November 2013. The trees were harvested by a trunk shaker head which detaches the olives which are collected by an associated inverted umbrella system. The crops were thereafter weighed per individual tree.
After fruit harvesting, random samples of 100 fruits per tree were weighed. Thereafter, random subsamples of 20 fruits were ginned and oven-dried to determine the pulp-to-pit ratio. Thereafter, these subsamples were ground and subjected to elemental analyses.
Data analysis
Data analysis was carried out using JMP statistical software. Comparisons among ground-cover treatments were provided by ANOVA. Means with significant differences (α < 0.05) were separated by Tukey HSD test (α = 0.05). A correlation analysis was established between the soil organic matter and extractable P with acid phosphate activity. Mean confidence limits were also applied to the data presented graphically to allow easy viewing and comparison among treatments.
Results
Performance of the ground covers
Figure 1 was prepared from data collected in the winter/spring season of 2014. Data from the previous growing seasons, 2010–2013, showed similar patterns (data not shown). In the sown legumes plots, the legume component showed dominance in the cover from the winter, reaching more than 90 % of ground cover in mid-spring (Fig. 1a). Apart from this, the non-legume component declined during the growing season as the legume component increased, and bare soil hardly existed. In the natural vegetation plots, fertilized or not, the legume component was residual (Fig. 1b, c). In NV +N plots, the ground cover percentage increased over the winter/spring season reaching values always higher than 90 % and bare soil decreased proportionally (Fig. 1b). The pattern observed in the NV −N plots was similar to that of NV +N plots but the ground cover percentage in the middle of spring was usually below 80 % and uncovered soil usually above 20 % (Fig. 1c).
In the Legs plots the dry matter (DM) yield reached its maximum earlier than in the NV +N plots, due to the species dominating the cover (Fig. 2a). DM yield in the Legs plots was higher than in the NV +N plots, reaching a value close to 4 t ha−1. Maximum DM yield in the NV −N plots was very low and did not increase from mid-winter. As the growing season progresses, N concentration in plant tissues usually decreases, with the ageing process of plant tissues. This was found in Legs and NV −N plots. However, maximum N concentration in above-ground biomass in the NV +N plot was found in early April as a response of the vegetation to previous fertilizer-N applications. N content in above-ground biomass reached values close to 90, 40 and 10 kg N ha−1, respectively in Legs, NV +N and NV −N plots. The peak of N content in biomass in the Legs plot was found early in the growing season in comparison to the NV +N plots, due to the early-maturing nature of the species and cultivars used in the seed mixture. Data from the growing seasons of 2010–2013 showed similar patterns (data not shown) compared to those presented in Fig. 2.
Chemical indices of soil fertility
Total organic carbon (TOC), Walkley–Black carbon (WBC), total (Kjeldahl) N (Kjeldahl-N) and C/N ratio are usually seen as major predictors of soil available N. Four years of different ground management systems produced significant differences in the levels of C and N in the soil (Table 1). In the 0–10 cm soil layer, TOC was significantly higher in NV +N plots (31.0 g kg−1) in comparison to NV −N plots (25.9 g kg−1). WBC was significantly lower in NV −N plots than in NV +N and Legs plots. Kjeldahl-N was significantly higher in Legs than in NV +N and NV −N plots in the 0–10 cm soil layer. The lowest TOC/N ratio was found in the 0–10 cm soil layer of the legs plots, while the highest value was found in the 10–20 cm layer of the NV-N plot. The 0–10 cm soil layer showed lower TOC/N and higher WBC/N ratios in comparison to the 10–20 cm soil layer.
The UV absorbance of NaHCO3 extracts was not statistically different among soil samples from the different groundcover treatments and soil depths (Table 2). The samples subjected to hot and cold KCl extractions released significantly different amounts of inorganic N according to the treatment and depth. The samples from the 0–10 cm soil layer subjected to hot KCl extraction, for instance, released 31.5, 22.7 and 20.2 mg kg−1 respectively if they came from the Legs, NV +N and NV −N plots.
Soil microbiology and microbial activity
The numbers of culturable heterotrophic bacteria and fungi significantly differed among ground-cover treatments (Table 3). Average soil bacteria population was significantly higher in Legs (7.20 log cfu g−1) and NV +N (7.05 log cfu g−1) plots in comparison to NV −N plots (6.74 log cfu g−1). Average soil fungi population was also significantly lower in NV −N plots in comparison to Legs and NV +N plots.
Microbial-C was significantly higher in Legs plots (569.9 mg kg−1) in comparison to NV +N (494.8 mg kg−1) and NV −N (421.3 mg kg−1) plots in the 0–10 cm soil layer (Table 3). The CO2-C evolved during 7 days incubation followed the pattern of Mic-C. In the 10–20 cm layer the highest Mic-N values were found in NV +N plots, whereas the highest values of the cumulative CO2-C evolved were found in Legs plots. Mic-N differed greatly between the two layers of soil, but within each layer no significant differences were found among treatments. The metabolic quotient (qCO2), expresses the CO2-C evolved per unit of microbial biomass and time increased significantly with depth in all plots.
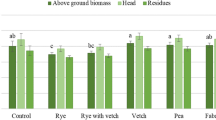
The acid phosphatase activity was higher in the top soil (0–10 cm) than in the deeper layer (10–20 cm) (Fig. 3a). In the NV −N treatment the acid phosphatase activity was lower at both depths in comparison with Legs and NV +N treatments. There was observed a positive correlation between acid phosphatase activity and soil organic matter (Fig. 3b). There were also found other positive correlations between acid phosphatase activity and soil extracted P by Olsen (Fig. 3c) and Egner–Riehm (Fig. 3d) methods, but with lower coefficients of correlation.
Acid phosphatase activity (a) and relationships between soil organic matter (b), Olsen-P (c) and Egner–Riehm-P (d) and acid phosphate activity from soil samples collected in October 2013 4 years after the establishment of the ground-cover treatments (Legs, NV +N, NV −N). Error bars are the mean confidence limits (α = 0.05)
Nitrogen recovery in pot grown plants
Turnip and rye grew better in soil sampled from Legs plots than from NV +N and particularly from NV −N plots (Table 4). Mean turnip DM yields were 3.44, 1.37 and 0.64 g/pot, respectively in soil sampled from Legs, NV +N and NV −N treatments. Mean rye DM yields were respectively for the same treatments 4.46, 2.14 and 1.27 g/pot. Significant differences were also found in N concentration in plant tissues. The highest values were found in the soil samples taken from the Legs plots. Mean N recoveries by turnip grown in soils from 0 to 10 cm layer were 84.4, 29.4 and 14.7 mg/pot, respectively in Legs, NV +N and NV −N plots. The respective values of rye were 60.2, 27.1 and 13.6 g/pot. The performance of plant growth in soil sampled from the 10–20 cm soil layer was worse in comparison to that observed in soil sampled from the 0–10 cm layer. However, the best results regarding DM yield, tissue N concentration and N recovery were found in the Legs plots.
Tree nutritional status and olive yield
From January 2010 to July 2011 no significant differences in leaf N concentrations were found in the olive trees of the different treatments (Fig. 4). From January 2012, the trees of the Legs plots usually presented higher leaf N concentrations than the trees of the NV +N plots and these trees presented significantly higher Leaf N concentrations than those of the NV −N plots. Regarding the other macro- and also micro-nutrients, no consistent significant differences in leaf nutrient concentrations were found among the three groundcover treatments (data not shown).
The mass of the pulp of 100 olives was significantly higher in fruits from the NV −N plots than from those of the Legs plots (Table 5). Similarly, the pulp/pit ratio was also higher in olives from NV −N plots than from those from Legs plots. However, N concentration in pulp and pit followed the trend observed in olive leaves, i.e., the higher values were found in olives from the Legs plots. The concentration of the other nutrients in pulp and pit was not statistically different among the groundcover treatments (data not shown).
The cumulative olive yields in the five harvests of the experiment were 85.1, 68.6 and 54.6 kg/tree, respectively in Legs, NV +N and NV −N treatments (Fig. 5). In the second harvest (2010), little over a year after the establishment of the groundcover treatments, olive yields became significantly higher in Legs plots in comparison to NV −N plots. From the fourth harvest, the olive yields were significant higher in Legs plots in comparison to NV +N plots. At the end of the experiment, the trees subjected to the Legs treatment produced significantly more than the trees subjected to NV +N treatment and these produced significantly more than those of the NV −N treatment.
Discussion
The sown legumes had a good establishment and achieved dominance in the cover from autumn to mid-spring in every year. Bare soil was minimal in the Legs plots. This aspect has great ecological significance since living mulches are the best way to reduce soil erosion (Martínez et al. 2006; Gómez et al. 2009; Kairis et al. 2013). On the other hand, the peak of growth in the vegetation occurred earlier in spring in the Legs plots in comparison to the NV +N plots. In the NV +N plot, the maximum ground-cover percentage was observed later in spring as a response to the N applied as fertilizer. This may mean that in the Legs plots there was a lesser overlap between the root activities of the herbaceous and tree strata in the spring, since the sown legumes were ending their life cycle and the olive trees were returning to biological activity after the resting period of winter. This aspect should not be overlooked because of the possibility of competition for water and nutrients between cover crops and olive trees. The DM yield in the different plots showed a similar pattern to that observed with ground-cover percentage. In Legs plots, DM yield reached the highest values among the ground-cover treatments but the peaks occurred at mid-spring. In NV +N plots the peaks of biomass occurred later in spring at the expense of transpiration of water that would otherwise have been taken up by the olive trees. Also N concentration and N content in above-ground biomass were higher in Legs plots than in NV +N plots and the maximum values occurred earlier in spring. The legumes mixture was mostly composed of early-maturing species and cultivars finishing their growing cycles by mid-spring. The early senescence and death of the legumes residues allow the beginning of the decay process, favoured by the rains that often fall in late spring, giving the opportunity for N uptake by the olive trees.
TOC in the 0–10 cm soil layer was greater in the NV +N plots than in the Legs plots even if more biomass had been produced in the latter. Contrarily, easily mineralizable C (WBC) and Kjeldahl-N were higher in the Legs plots. This may mean that in the 0–10 cm soil layer of the Legs plots more native organic matter is being mineralized and more fresh organic substrates are being deposited. Consequently, it seems that a more reactive pool of organic matter is being created, which in practice accelerates the turnovers of soil organic C and N. Inorganic N extracted by salt solutions of hot and cold KCl supports this thesis, i.e., in the upper soil layer of the Legs plot the labile C fraction hydrolyzed by the salt solution was higher in comparison to the other treatments. However, the most surprising and unexpected result occurred in the 10–20 cm soil layer. The Legs plots recorded the lowest TOC, WBC and Kjeldahl-N values among all the ground-cover treatments, although without significant differences for the NV −N plots but with significant differences for NV +N plots. It seems that few legume residues are reaching the 10–20 cm soil layer, despite the higher amounts of biomass produced in these plots. Or alternatively, it even seems that organic C is disappearing from the 10–20 cm layer likely due to the increase of net mineralization enhanced by mineral N coming from the soil surface. Phenomena like this, where a stimulus of the mineralization of soil native organic matter occurs due to the increased availability of mineral N in soil, are known as priming or added N interaction (Jenkinson et al. 1985; Schnier 1994).
There were found to exist high microbial populations (bacteria and fungi) in the plots producing more above-ground biomass (Legs and NV +N), as had been found in previous studies (Moreno et al. 2009; Sackett et al. 2010). However, no significant differences were found according to the nature of the substrate (legumes or non-legumes). Varennes et al. (2007) found significant differences in the number of fungi developed in lupine residues in comparison with oat residues, but not in the number of culturable bacteria. The difference in culturable fungi was found in the treatments where straw (both from lupine and oats) was incorporated into the soil, presumably because lupine tissues had more N and less lignin and decomposed faster. When the residues were not incorporated into the soil, no significant differences in culturable fungi were found. According to Kumar and Goh (2002), surface-applied residues decompose more slowly due to smaller water content and decreased access to soil microbes. In the present study, the plant residues (legumes and natural vegetation) were left on the ground, and the main available substrate for microbial activity would be the biomass of roots, which might differ less than the aerial plant parts in their C/N ratio and lignin content. Microbial-C and CO2-C evolved during 7 days incubation were significantly higher in the Legs than in the other plots in the 0–10 cm soil layer. These results seem to support the thesis of the increased turnover of organic C and N in Legs plots in the upper soil layer. Although there is little organic-C, including Mic-C, in the 10–20 cm soil layer of the Legs plots, it seems that a great amount of C is lost, judging by the high values of CO2-C evolved and high values of qCO2, which may also explain the low values of TOC found in this layer. Indeed, higher qCO2 levels in the 10–20 cm soil layer indicated decreased substrate use efficiency and lower rates of conversion of total C into microbial C, as mentioned by Agnelli et al. (2004) and Dinesh et al. (2012). Anderson and Domsch (2010) suggested that a high value of qCO2 reflects a high maintenance C demand and, if the soil system cannot replenish the C which is lost through respiration, microbial biomass must decline.
The acid phosphatase activity was positively and significantly related to soil organic matter. Soil samples from the Legs and NV +N plots showed higher acid phosphatase activity than those from NV −N plots. Some legume species are able to acclimate to phosphorus (P) deficiency by forming proteoid roots and other mechanisms, resulting in the mobilization of sparingly soluble soil phosphate in the rhizosphere (Uhde-Stone et al. 2003; Zhu et al. 2005; Tomasi et al. 2008; Wang et al. 2010), and, consequently, higher acid phosphatase activity can be observed in the soil (Le Bayon et al. 2006). Legumes with such mechanisms of P mobilization can also have a positive effect on P nutrition of a subsequent crop (Nuruzzaman et al. 2005, 2006; Eichler-Löbermann et al. 2009). In this study, the presence of legume species in the cover did not significantly increase the acid phosphatase activity over the natural vegetation fertilized with N.
Pot experiments with growing plants are reliable methods of assessing soil available nutrients (Eichler-Löbermann et al. 2009; Rodrigues et al. 2010a; Arrobas and Rodrigues 2013). The plants grown in pots (turnip and rye) from soil samples of the 0–10 cm layer of the Legs plots produced significantly more biomass and recovered more N than those grown in soil samples from the other ground-cover treatments. The cover of the legumes mixture had created an easily mineralizable organic pool releasing more N than the other treatments. The soil samples from the 10–20 cm depth of the Legs plots also produced, in a general way, higher DM yields and N recoveries than the soil samples from the other treatments. Thus, in spite of the loss of organic matter from this layer, the amount of N released was currently higher than that recorded from the other treatments, likely due to the maintenance of a higher net mineralization rate.
The olive trees grown in the Legs plots showed significantly higher leaf N concentrations than the other treatments by January 2012, 2 years after the establishment of the ground-cover treatments. It was clear that N fixed by the annual legumes was transferred to the olive trees. Although it was not possible to quantify the amount annually transferred, the effect on the N nutritional status of trees was higher than the application of 60 kg N ha−1 in the NV +N plots. In a previous study, Snoeck et al. (2000) have shown isotopic evidence of the transfer of N fixed by legumes to coffee trees. From a study of Ovalle et al. (2010) the amount of legume N estimated to be recovered by cover cropped vine plants was similar to the calculated contribution of 40 kg of fertilizer N applied to vines. Abundant literature has also shown the transfer of N from legumes to non-legume intercropped species (Hardarson and Atkins 2003; Pirhofer-Walzl et al. 2012) or to others following in the crop rotation (Zotarelli et al. 2012; Monday et al. 2013). In this particular study, it seems that from mid-spring, after the senescence of legumes, to the autumn, until the establishment of the covers, the trees may benefit from the N mineralized from legume residues. Fruits also showed higher tissue N concentration in the legume treatment. However, the pulp-to-pit ratio decreased in these plots, likely due to the increase in fruit number, which reduced the size of the individual fruits.
The accumulated olive yields reached the highest mean value in the Legs treatment due to the better N nutritional status of olive trees. Cover cropping can reduce olive yields due to competition for water (Rodrigues et al. 2011; Gucci et al. 2012; Ferreira et al. 2013). However, in this experiment, the increase in soil available N had the greatest value in olive yield, since the cover was composed of early-maturing species and cultivars with reduced competition for water.
Conclusions
The Legs plots showed high ground cover percentages from very early in winter which increases soil protection from erosion in the rainy season. In Legs plots were found higher biomass yields and N contents in shoots in comparison with NV +N plots, but the peaks were found much earlier in spring, which may mean a reduced competition for resources with trees late in spring when soil available water is scarcer.
In Legs plots, the increased biomass yield gave no more organic C in the 0–10 cm soil layer in comparison with the NV +N plots. Thus, legumes appear to be less effective in increasing organic C than non-legume species. Nevertheless, in Legs plots the increase of WBC was proportionally higher than the increase of TOC in comparison to the other treatments. It seems that a more reactive pool of organic C is created, which may reduce the turnover of Organic C and N in the soil. In both Legs and NV +N treatments there was observed high soil biological activity although soil available N was higher in the Legs treatment which ultimately benefited the olive trees. In Legs treatments there was observed a loss in total organic C in the 10–20 cm soil layer, likely due to a priming effect caused by mineral N coming from the soil surface which contributed to the mineralization of the native organic matter. This aspect must be carefully analyzed in further studies, due to its negative ecological implications.
By January 2012, 2 years after the start of the experiment, N concentration in olive leaves was significantly higher in Legs plots than in the other treatments including in NV +N. Thus, it can be assumed that the effect of the annual legumes in the olive trees’ nutrition was higher than the application of 60 kg N ha−1 year−1. The competition between the annual legumes and the olive trees seems to have been reduced due to the asynchrony between the biological activities of herbaceous and tree strata. The olive trees could access N fixed by legumes from mid-spring, when legumes initiated senescence and the decay process of their residues started, until late autumn before the full establishment of legume covers. It was the asynchrony in the biological cycles of olive trees and legumes and the increase in soil available N that gave the higher olive yields found in the Legs plots.
References
Agnelli A, Ascher JC, Ceccherini MT, Nannipieri P, Pietramellara G (2004) Distribution of microbial communities in a forest soil profile investigated by microbial biomass, soil respiration and DGGE of total and extracellular DNA. Soil Biol Biochem 36:859–868
Anderson T-H, Domsch KH (2010) Soil microbial biomass: the eco-physiological approach. Soil Biol Biochem 42:2039–2043
Arrobas M, Rodrigues MA (2013) Agronomic evaluation of a fertiliser with D-CODER technology, a new mechanism for the slow release of nutrients. J Agr Sci Tech 15:409–419
Arrobas M, Ferreira IQ, Claro M, Correia CM, Moutinho-Pereira J, Rodrigues MA (2011) Introdução de cobertos de leguminosas anuais em olival. Vida Rural, pp 32–34
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Cooper J, Scherer H (2012) Nitrogen fixation. In: Marschner P (ed) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, London, pp 389–408
Damgaard C, Merlin A, Mesléard F, Bonis A (2011) The demography of space occupancy: measuring plant colonization and survival probabilities using repeated pin-point measurements. Methods Ecol Evol 2:110–115
Dinesh R, Srinivasan V, Hamza S, Manjusha A, Kumar PS (2012) Short-term effects of nutrient management regimes on biochemical and microbial properties in soils under rainfed ginger (Zingiber officinale Rosc.). Geoderma 173(174):192–198
Driouech N, Abou Fayad A, Ghanem A, Al-Bitar L (2008) Agronomic performance of annual self-reseeding legumes and their self-establishment potential in the Apulia region of Italy. In: 16th IFOAM Organic World Congress, Modena, Italy, June pp 16–20
Eichler-Löbermann B, Gaj R, Schnug E (2009) Improvement of soil phosphorus availability by green fertilization with catch crops. Commun Soil Sci Plant Anal 40:70–81
Ferreira IQ, Arrobas M, Claro AM, Rodrigues MA (2013) Soil management in rainfed olive orchards may result in conflicting effects on olive production and soil fertility. Span J Agric Res 11(2):472–480
Gómez JA, Guzmán MG, Giráldez JV, Ferreres E (2009) The influence of cover crops and tillage on water and sediment yield, and on nutrient, and organic matter losses in an olive orchard on a sandy loam soil. Soil Till Res 106:137–144
Gucci R, Caruso G, Bertolla C, Urbani S, Tatichi A, Esposto S, Servili M, Sifola MI, Pellegrini S, Pagliai M, Vignozzi N (2012) Changes of soil properties and tree performances induced by soil management in a high-density olive orchard. Eur J Agron 41:18–27
Hardarson G, Atkins C (2003) Optimising biological N2 fixation by legumes in farming systems. Plant Soil 252:41–54
Instituto National de Estatísticas (INE) (2013) Estatísticas Agrícolas. Instituto Nacional de Estatísticas, I.P., Lisbon
Jenkinson DS, Fox RH, Rayner JH (1985) Interactions between fertilizer nitrogen and soil nitrogen—the so-called “priming effect”. J Soil Sci 36:425–444
Kairis O, Karavitis C, Kounalaki A, Salvati L, Kosmas C (2013) The effect of land management practices on soil erosion and land desertification in an olive grove. Soil Use Manag 29:597–606
Kumar K, Goh KM (2002) Recovery of 15N-labelled fertilizer applied to winter wheat and perennial ryegrass crops and residual 15N recovery by succeeding wheat crops under different crop residue management practices. Nutr Cycl Agroecosys 62:123–130
Le Bayon RC, Weisskopf L, Martinoia E, Jansa J, Frossard E, Keller F, Föllmi KB, Gobat J-M (2006) Soil phosphorus uptake by continuously cropped Lupinus albus: a new microcosm design. Plant Soil 283:309–321
Martínez JRF, Zuazo VHD, Raya AM (2006) Environmental impact from mountainous olive orchard under different soil-management systems (SE Spain). Sci Total Environ 358:46–60
Mauromicale G, Occhipinti A, Mauro RP (2010) Selection of shade-adapted subterranean clover species for cover cropping in orchards. Agron Sustain Dev 30:473–480
Monday TA, Foshee WG, Blythe EK, Dozier WA, Wells LW, Sibley JL, Brown JE (2013) Nitrogen requirements for sweetpotato following a crimson clover cover crop. Int J Veg Sci 19:78–82
Montanaro G, Celano G, Dichio B, Xiloyannis C (2010) Effect of soil-protecting agricultural practices on soil organic carbon and productivity in fruit tree orchards. Land Degrad Dev 21:132–138
Monteiro AM (2007) Olivicultura: uma estratégia possível para Trás-os-Montes e Alto Douro, região de oliveiras, olivais, azeites e azeitonas. Ouro Virgem 3:94–115
Moreno B, Garcia-Rodriguez S, Cañizares R, Castro J, Benítez E (2009) Rainfed olive farming in South-eastern Spain: long-term effect of soil management on biological indicators of soil quality. Agric Ecosyst Environ 131:333–339
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2005) Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant Soil 271:175–187
Nuruzzaman M, Lambers H, Bolland MDA, Veneklaas EJ (2006) Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 281:109–120
Ovalle C, Pozo A, Peoples MB, Lavín A (2010) Estimating the contribution of nitrogen from legume cover crops to the nitrogen nutrition of grapevines using a 15N dilution technique. Plant Soil 334:247–259
Pereira EL, Santos SAP, Arrobas M, Patrício MS (2011) Microbial biomass and N mineralization in mixed plantations of broadleaves and nitrogen-fixing species. For Syst 20(3):516–524
Pirhofer-Walzl K, Rasmussen J, Høgh-Jensen H, Eriksen J, Søegaard K, Rasmussen J (2012) Nitrogen transfer from forage legumes to nine neighboring plants in a multi-species grassland. Plant Soil 350:71–84
Ramos ME, Benítez E, García P, Robles AB (2010) Cover crops under different managements vs. frequent tillage in almond orchards in semiarid conditions: effects on soil quality. Appl Soil Ecol 44:6–14
Ramos ME, Robles AB, Sánchez-Navarro A, González-Rebollar JL (2011) Soil responses to different management practices in rainfed orchards in semiarid environments. Soil Till Res 112:85–91
Rodrigues MA (2009) Nota introdutória. In: Rodrigues MA, Correia C (eds) Manual da Safra e Contra Safra do Olival. Instituto Politécnico de Bragança, Portugal, pp 7–8
Rodrigues MA, Pereira A, Cabanas E, Dias L, Pires J, Arrobas M (2006) Crops use-efficiency of nitrogen from manures permitted in organic farming. Eur J Agron 25:328–335
Rodrigues MA, Santos H, Ruivo S, Arrobas M (2010a) Slow-release N fertilisers are not an alternative to urea for fertilisation of autumn-grown tall cabbage. Eur J Agron 32(2):137–143
Rodrigues MA, Gomes V, Dias LG, Pires J, Aguiar CF, Arrobas M (2010b) Evaluation of soil nitrogen availability by growing tufts of nitrophilic species in an intensively grazed biodiverse legume-rich pasture. Span J Agric Res 8(4):1058–1067
Rodrigues MA, Lopes JI, Pavão F, Cabanas JE, Arrobas M (2011) Effect of soil management on olive yield and nutritional status of trees in rainfed orchards. Commun Soil Sci Plant Anal 42:993–2011
Rodrigues MA, Correia CM, Claro AM, Ferreira IQ, Barbosa JC, Moutinho-Pereira JM, Bacelar EA, Fernandes-Silva AA, Arrobas M (2013) Soil nitrogen availability in olive orchards after mulching legume cover crop residues. Sci Hort 156:45–51
Sackett TE, Classen AT, Sanders NJ (2010) Linking soil food web structure to above- and belowground ecosystem processes: a meta-analysis. Oikos 119:1984–1992
Schnier HF (1994) Nitrogen-15 recovery fraction in flooded tropical rice as affected by added nitrogen interaction. Eur J Agron 3(2):161–167
Snoeck D, Zapata F, Domenach A-M (2000) Isotopic evidence of the transfer of nitrogen fixed by legumes to coffee trees. Biotechnol Agron Soc Environ 4(2):95–100
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1(4):301–307
Tomasi N, Weisskopf L, Renella G, Landi L, Pinton R, Varanini Z, Nannipieri P, Torrent J, Martinoia E, Cesco S (2008) Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol Biochem 40:1971–1974
Uhde-Stone C, Gilbert G, Johnson JM-F, Litjens R, Zinn KE, Temple SJ, Vance CP, Allan DL (2003) Acclimation of white lupin to phosphorus deficiency involves enhanced expression of genes related to organic acid metabolism. Plant Soil 248:99–116
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Varennes A, Torres MO, Cunha-Queda C, Goss MJ, Carranca C (2007) Nitrogen conservation in soil and crop residues as affected by crop rotation and soil disturbance under Mediterranean conditions. Biol Fertil Soils 44:49–58
Walinga I, van Vark W, Houba V, van der Lee J (1989) Soil and plant analysis: Part 7—plant analysis procedures. Wageningen Agricultural University, Wageningen
Wang X, Tang C, Guppy CN, Sale PWG (2010) Cotton, wheat and white lupin differ in phosphorus acquisition from sparingly soluble sources. Environ Exp Bot 69:267–272
Zhu Y, Yan F, Zörb C, Schubert S (2005) A link between citrate and proton release by proteoid roots of white lupin (Lupinus albus L.) grown under phosphorus-deficient conditions? Plant Cell Physiol 46(6):892–901
Zotarelli L, Zatorre P, Boddey M, Urquiaga S, Jantalia P, Franchin C, Alves R (2012) Influence of no-tillage and frequency of a green manure legume in crop rotations for balancing N outputs and preserving soil organic C stocks. Field Crop Res 132:185–195
Acknowledgments
Supported by PRODER under the project OlivaTMAD – Rede Temática de Informação e Divulgação da Fileira Olivícola em Trás-os-Montes e Alto Douro - Medida 4.2.2.2. The authors also thank Rita Diz, Ana Veiga Pinto, David Cabral and José Rocha for laboratorial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, M.Â., Dimande, P., Pereira, E.L. et al. Early-maturing annual legumes: an option for cover cropping in rainfed olive orchards. Nutr Cycl Agroecosyst 103, 153–166 (2015). https://doi.org/10.1007/s10705-015-9730-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-015-9730-5