Abstract
Organic amendments contribute significantly to the phosphorus (P) supply in agroecosystems. However, their long-term effects on specific P forms in soils are not completely understood. The objective of this study was to investigate the concentration of organic P forms and inorganic P pools in soil and the activity of enzymes involved in the P turnover in a long-term field experiment running since 1998 in Northern Germany as affected by P amendments. The following treatments with different P supplies were sampled in 2012, 14 years after the establishment of the experiment: control (no P), cattle manure (manure), biowaste compost (compost), and biowaste compost in combination with triple-superphosphate (compost + TSP). The classification of organic P forms by using enzyme additions to NaOH–EDTA soil extracts showed non-hydrolyzable organic P as the dominant form in soil followed by inositol hexakisphosphate (Ins6P)-like P. Non-hydrolyzable and total organic P concentrations in soil were highest in the combined compost + TSP treatment, which received the highest amount of inorganic P. The values of the bioavailable P pools (water-extractable P and double lactate-extractable P) were in accordance with the P balance (P addition with the amendments minus P removal with harvested crops) independently of the type of amendment. The results of this research suggest that the distribution of soil P forms is more reliant on the turnover processes in the soil than on the forms of P added.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Studies predict that resources of phosphate rock will be depleted within a short to medium duration of years (Rosmarin 2004; Cordell et al. 2009; Van Vuuren et al. 2010). Therefore, recycling organic residues as compost and manure in agriculture is an important practice to replenish soil phosphorus (P) pools, replacing inputs of mineral fertilizer and to improve soil properties (Eichler-Löbermann et al. 2008; Gopinath et al. 2008).
Organic amendments are composed of organic and inorganic P forms, but inorganic P is usually the principal P form. In a wide variety of organic wastes of different origins and treatments (including compost, pig slurries, sludge and digestate) organic P forms only account between 1 and 30 % of total P (García-Albacete et al. 2012; Sinaj et al. 2002). However, studies using manure from different animal species showed that organic P can amount to 80 % of total P (Pagliari and Laboski 2012; Pagliari and Laboski 2013). To improve the nutrient utilization in cropping systems and thus avoiding P losses a comprehensive understanding of the influences of organic amendments on soil fertility and soil P forms is necessary.
It is well documented that increase in organic matter enhances microbial biomass and microbial activity of soils (Ehlers et al. 2010; Krey et al. 2013). Thus, farming systems with higher inputs of organic amendments result in larger microbiological activity compared to soils receiving mainly mineral fertilizers. Higher microbial activities however, also represent higher P turnover in soil, by microbial immobilization of inorganic P, mineralization of organic P and microbial P synthesis (Richardson 2001; Richardson and Simpson 2011). The activity of microorganisms in soil can be estimated by the activity of intracellular enzymes, e.g. dehydrogenase (Nain et al. 2009). The hydrolysis of organic P like esters and anhydrides of phosphoric acid is catalysed by phosphatase in soil (Eivazi and Tabatabai 1977). Alkaline (AlP) and acid phosphatase (AcP) can be excreted by both, crops and microorganisms, whereas higher crops almost exclusively excrete AcP (Dick et al. 2000).
In the past, fertilizer recommendations were given with special attention on soil inorganic P pools (Steffens et al. 2010). However, organic P constitutes an important proportion of total P in soil (30–65 %; Harrison 1987) that can be available after being hydrolyzed to inorganic forms. The soil organic P fraction includes different chemical forms which differ in their susceptibility to degradation by soil enzymes (Bowman and Cole 1978), and thus in their potential availability for plants. Several investigations have studied the hydrolyzabiliy of organic P forms by adding combinations of phosphatase enzymes with different substrate specificities to soil extracts (He et al. 2004a; Turner et al. 2003a), soil water suspensions (Annaheim et al. 2013), and manures and composts (Annaheim 2013; He et al. 2004b; Pagliari and Laboski 2012). This technique requires relatively little laboratory equipment and low costs compared to other techniques like 31P-NMR spectroscopy. Comparison of P classes obtained by enzymatic hydrolysis and 31P nuclear magnetic resonance (NMR) spectroscopy in soil and manure extracts revealed similar results for both methods (He et al. 2007; Johnson and Hill 2010).
The effect of different long-term farming systems (conventional, biodynamic and bioorganic) on organic P forms was investigated by Keller et al. (2012) using incubations of alkaline soil extracts with acid phosphatase from potato (Solanum tuberosum L.), nuclease from Penicillium citrinum and phytase from Peniophora lycii. The authors concluded that the different farming systems did not affect the organic P fractions. In a field experiment in Switzerland Annaheim (2013) also found that forms of organic P remained largely unchanged in soils with contrasting fertilizer histories, including a mineral fertilizer (NPK) and different organic amendments (sludge, compost and manure). Our study contained a control without P supply, cattle manure, biowaste compost as well as a combined compost + TSP treatment. The objective of this study was to investigate the concentration of organic and inorganic P pools in soil, and to quantify the activity of enzymes involved in the P turnover after 14 years of treatment application under field conditions in Northern Germany. To estimate the enzymatic hydrolyzability of organic P, phosphatase enzymes with different substrate specificities were added to soil extracts.
Materials and methods
Site characterization
Soil samples were taken in 2012 at the long-term field experiment established in autumn 1998 to study the effect of different organic amendments and inorganic fertilizers in single application and combination (see also Eichler-Löbermann et al. 2007). The site is located at the experimental station of Rostock University in Northern Germany in a maritime-influenced area about 15 km south of the Baltic Sea (54°3′41.47″N; 12°5′5.59″E). The mean annual precipitation is about 600 mm and the average annual temperature is 8.1 °C. The soil texture is loamy sand and according to the World Reference Base for Soil Resources, the soil is classified as Stagnic Cambisol. The bulk density (0–30 cm) was 1.38 kg L−1, and the soil organic matter (SOM) concentration was 2.50 %. The initial Pdl concentration of 42.2 mg P per kg soil indicated a suboptimal P supply according to the German soil-P classification (Kerschberger et al. 1997). Soil characteristics at the beginning of the experiment are given in Table 2.
Although the experiment originally had 9 treatments, this study focused on 4 treatments, which were: control (no P), cattle manure (manure), biowaste compost (compost), and biowaste compost in combination with mineral fertilizer (Triple-superphosphate TSP) (compost + TSP). Each treatment had 5 replications. The plots had a size of 30 m2 and were arranged in a randomized block design. Cattle manure and biowaste compost were applied every three years beginning in September 1998 at a rate of about 30 t ha−1. Consequently, the last application before sampling in 2012 was in 2010. The compost was produced in a compost facility near Rostock and provided as mature and sanitized compost on the basis of green garden and landscape residues. Mineral fertilizer was supplied annually as TSP at a rate of 21.8 kg P ha−1. The control did not receive any P since 1998. The plots of this study were cropped to spring oilseed rape (Brassica napus) 1999, spring wheat (Triticum aestivum) 2000, spring barley (Hordeum vulgare L.) 2001, spring oilseed rape 2002, winter wheat 2003, winter barley 2004, winter oilseed rape 2005, maize (Zea mais) 2006, maize 2007, maize 2008, green winter rye (Secale cereale) (as green manure) and sorghum (Sorghum bicolor) 2009, sorghum 2010, sunflower (Helianthus annuus) 2011, winter rye 2012. The P supply with the treatments and the P uptake of the crops resulted in different P balances (see Table 1). For oilseed rape and the cereals (1999–2005, 2012) only the grains of the plants were harvested and the straw remained on the field. For maize, sorghum and sunflower the whole crops were harvested and the plants were cut 10 cm above the soil. Plant residues were mulched into the soil after crop harvests. Afterwards the soil was ploughed at a depth of 25 cm either in autumn or in spring. The P removal was determined by multiplying the dry weight of harvested biomass with its P concentration. The P balances for the treatments were calculated by subtracting the amount P applied minus the P removed with the harvested crop parts (see Table 1).
Soil analysis
Six soil cores per plot were collected from the upper soil layer (0–30 cm) in September 2012. Samples were air-dried, sieved to 2 mm and stored at room temperature until analysis. Samples of the organic amendments of the last application (2010) before sampling were kept frozen at −18 °C until analysis. Various methods were used to characterize the different soil P pools. Water-extractable P (Pw) was measured according to Van der Paauw (1971) by extracting 12 g soil in 200 mL of ultrapure water. Phosphorus concentration in the water extracts was determined by the phosphomolybdate blue method via flow-injection analysis. Double lactate-extractable P (Pdl) was determined by extracting P from 12 g soil with 150 ml double lactate solution (C6H10CaO6·H2O + 10 N HCl) using the method of Hoffmann (1991). Phosphorus concentration in the dl extract was determined using the vanadate-molybdate method with a spectral photometer. Oxalate soluble P, Al and Fe (P OX , Al OX , Fe OX ) were extracted according to Schwertmann (1964) by shaking 2 g of soil with 100 mL of acid oxalate in the dark for 1 h and measured using inductively coupled plasma spectroscopy (ICP-OES, JY-238). The P sorption capacity (PSC (mmol kg−1) = (Al OX + Fe OX )/2) and the degree of P saturation (DPS (%) = P OX /PSC × 100) were calculated using the oxalate extractable P, Al and Fe according to Schoumans (2000). Total P(Pt) was extracted using the aqua regia method, where 6 ml HCl and 2 ml HNO3 are added to 0.5 g soil and digested in a microwave oven (Mars Xpress, CEM GmbH, Kamp-Lintfort, Germany) followed by ICP spectroscopy. SOM content was determined by dry ashing.
Dehydrogenase (DHA) activity was determined according to Thalmann (1968) by suspending 1 g soil in 0.8 % triphenyltetrazoliumchloride solution followed by incubation at 37 °C for 24 h. Most microorganisms are able to reduce triphenyltetrazoliumchloride to triphenylformazan (TPF). The released TPF was extracted with acetone and measured photometrically at 546 nm. Results were expressed as µg TPF per g soil released within 1 h (µg TPF g−1 h−1). The activities of acid and alkaline phosphatases (phosphoric monoester hydrolases, AcP and AlP) were measured according to Tabatabai and Bremner (1969). The activities were expressed as µg p-nitrophenol released from a pre-given p-nitrophenyl phosphate solution in 1 g soil after incubation at 37 °C for 1 h (µg p-nitrophenol g−1 h−1).
NaOH–EDTA extraction and enzymatic incubation assay
Ten grams of fresh compost and manure were added to 100 mL of 0.25 M NaOH–0.05 M Na2EDTA for 16 h (Cade-Menun and Preston 1996) centrifuged and filtered (Whatman 4). The extracts were diluted 200 times before colorimetric measurement of inorganic P with malachite green at 623 nm (Ohno and Zibilske 1991). For total P, a persulfate digestion (Tiessen et al. 1993) was performed with 1 mL of NaOH–EDTA extracts and 10 mL of digestion mix (6 g NH4-persulfate in 100 mL 0.9 M H2SO4). In the neutralized digests total P was determined by colorimetric measurement. Organic P was calculated as the difference between total P and inorganic P. Extractions were repeated 3 times.
The extraction and analysis of soil samples followed the same procedure. Ten grams of dry soil were treated for 16 h with 0.25 M NaOH–0.05 M Na2EDTA in a soil-to-solution ratio of 1:10 (w:v). For inorganic P, the extracts were diluted 10 times for colorimetric measurement. Total extractable P was determined as described for the organic amendments above and organic P was calculated by the difference between total P and inorganic P.
The enzymatic hydrolyzability of organic P in the NaOH–EDTA extracts from soil, compost and manure was determined following the protocol of Keller et al. (2012) with minor modifications. Three enzyme solutions were prepared: acid phosphatase from potato (Sigma P1146; 50 UN diluted in 15 mL H2O); nuclease from Penicillium citrinum (Sigma N8630; 0.167 mg dissolved in 1 mL H2O); and a commercial granulated phytase prepared from Peniophora lycii (RONOZYME; 0.25 g dissolved in 50 mL H2O, centrifuged, and the supernatant filtered at 0.2 µm). Aliquots of the enzyme solutions (0.5 mL) were dispensed and stored at −18 °C in a freezer until use. The incubations were conducted in micro-centrifuge vials achieving a final volume of 320 µL. 40 µL of NaOH–EDTA extract were added to 60 µL of 1 M MES buffer (2-(N-morpholino) ethanesulfonic acid; at pH 5.2) and incubated with 20 µL of acid phosphatase alone or in combination with 20 µL of nuclease solution, or with 40 µL of phytase alone. The final volume was reached by adding H2O. The incubations were performed at 30 °C for 24 h (Annaheim et al. 2013). After incubation, inorganic P in the incubation mix was determined using the malachite green method with a spectral photometer.
Acid phosphatase hydrolyzes simple phosphomonoesters and phosphoanhydrides (He et al. 2004a), while phytase additionally hydrolyzes inositol hexakiphosphate (Ins6P) and RNA (Annaheim et al. 2013). Nuclease is used to achieve the hydrolysis of diester bonds in nucleic acids, whose organic P is then hydrolyzed by acid phosphatase (He et al. 2004a). Three model substrates were used to verify the effectiveness of the three enzyme preparations in the buffered solutions. Acid phosphatase was shown to be active against glycerolphosphate, with about 90 % of hydrolized P. The combination of acid phosphatase and nuclease hydrolized 74 % of P from DNA and the phytase preparation hydrolyzed 83 % of Ins6P. Vials containing only soil extract or only enzyme in the buffered solution were run simultaneously with the samples along with an internal calibration curve (0–1.25 µg orthophosphate). No inorganic P release was detected in the vials incubated with the enzyme preparations alone. Enzyme labile P was calculated as the difference of inorganic P measured in the soil extracts incubated with the enzymes and the P measured in the vials without enzyme (4 analytical replications each). An orthophosphate spike (0.4 µg P) was added to the soil extracts (4 analytical replicates) in parallel with the samples to check the recovery of hydrolyzed P.
Enzyme labile-P was classified into simple monoester-like P (P released by acid phosphatase); DNA-like P (difference of P released by acid phosphatase in combination with nuclease and acid phosphatase alone); and Ins6P-like P (difference of P released by phytase and by acid phosphatase). Non-hydrolyzed P was calculated as the difference between organic P and the sum of the three fractions of enzyme-labile P.
Statistical analyses
Analyses of variance were performed using the General Linear Models (GLM) of PASW Statistics 18 software (SPSS Statistics). For all statistical analyses, a p value of 0.05 was used to determine significance. In case of significant effects, Duncan multiple range test was performed to compare means of soil parameters. Descriptive statistics of soil and organic amendments parameters were calculated using the same software.
Results and discussion
Effect of long-term P amendment practice on inorganic and total soil P pools
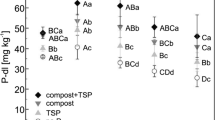
The treatments applied in combination with crop P uptake affected soil bio-available P contents (Pw and Pdl, see Table 2). In relation to the initial Pdl concentration of about 42 mg per kg soil in 1998 the values dropped significantly in the treatment without any P supply and the manure treatment, remained almost stable in the compost treatment and increased after the combined compost + TSP application. Organic residues usually contain considerable amounts of soluble inorganic P which contribute to the fast release of P after incorporation into soil. The control treatment without any P supply during the last 14 years showed very low P concentration; Pdl concentration of only 29.7 mg per kg soil correspond to strong suboptimal P status according to the German soil P classification (Kerschberger et al. 1997). The clear responses of Pdl values to the P application (correlation coefficient r = 0.97, p < 0.01) make these parameters a suitable tool for fertilizer recommendations. High correlations between P applied and Pdl and Pw concentrations in the soil were also found previously in 2004 (correlation coefficient r = 0.73 and 0.77, p < 0.01), where more than 50 % of the variation of Pw and Pdl values could be explained by the P supply (Eichler-Löbermann et al. 2007). No differences were found between treatments regarding the P-sorption capacity (PSC) in 2012 (Table 2). However, the compost + TSP treatment resulted in the highest degree of P saturation (DPS) with values of more than 50 %. DPS values higher than 40 % are critical with respect to P losses from sandy soils (Schoumans 2000).
The differences of the Pt contents in soil from the beginning of the experiment and after 14 years (Δ Pt, kg ha−1) were positively correlated with the P balances given in Table 1 (r = 0.75**, n = 20). However, Δ Pt was not identical with the P balances. In the treatments which have received P the Pt contents in 0 to 30 cm were lower than expected. The comparison of Δ Pt and the P balances (kg ha−1) showed for the manure treatment: −112 versus −62, for compost treatment: −66 versus −24, and for the compost + TSP treatment: +50 vs. +258. This could be explained by a downward movement of P into deeper soil layers, which was also described by Oehl et al. (2002). Whereas for the manure and compost treatment the differences between Δ Pt and the P balances were moderate, much higher Pt values were expected in the compost + TSP treatment and about 200 kg ha−1 is mathematically missing in the topsoil. This result however, confirms other studies which showed that P movement with percolating water or in preferential flow paths increased with higher fertilizer inputs and following higher bioavailable P contents in the topsoil (Godlinski et al. 2004). On the other hand, in the control the Pt contents in 0–30 cm were higher than expected (Δ Pt vs. P balances (kg ha−1): −232 versus −345). This can be explained by a upwards movement of P from the subsoil to the topsoil under P deficient conditions which was also shown by previous long-term studies by Oehl et al. (2002) and Gransee and Merbach (2000).
Effect of long-term P amendment practice on activities of enzymes
The long-term P amendment practice also affected the activity of enzymes involved in the soil nutrient turnover. In the amended plots (which also showed higher SOM contents) we found higher microbial activities (DHA) in comparison to the control. The availability of organic substrates usually promotes the growth of microbial populations and microbial P (Bünemann et al. 2006; Fliessbach et al. 2007). Furthermore, the application of compost alone or in combination with TSP enhanced the activity of AlP (about 70 µg g−1 h−1, p-np) in comparison to the control (about 40 µg g−1 h−1, p-np). This may be explained with higher microbial activity (since the synthesis and excretion of AlP is coupled to microbial activity or population size) and the slight increase of soil pH (from 5.30 in the control to 5.44 in the manure plots and 5.53 in the compost plots). However, the application of compost or manure had no significant effect on the activity of AcP. This was also observed after 10 years experimental time in this field experiment (Krey et al. 2013) and confirms findings from another field experiment where the long-term application of manure also enhanced the activity of AlP, but not of AcP in the soil (Parham et al. 2002). Phosphatases contribute to mineralization of hydrolyzable organic P in soil, whereas considerably parts of total organic P may be non-hydrolyzable, as shown in this study (see chapter "Effect of long-term P amendment practice on inorganic and total soil P pools") (Table 3).
NaOH–EDTA extractable P forms in manure and compost
Inorganic P was the major fraction of P present in the NaOH–EDTA extracts of both amendments (Table 4). Organic P accounted only for 12 and 18 % of total P extracted with NaOH–EDTA for compost and manure respectively. These proportions of organic P are within the range proposed by Sinaj et al. (2002) for compost (12–33 %) and by Pagliari and Laboski (2012) for manure (12–24 %). The concentration of both inorganic and organic P in NaOH–EDTA extracts was higher in manure than in compost. All organic P in the NaOH–EDTA extracts compost was enzymatically hydrolyzable, with DNA-like P being the main form detected (9 %). The concentration of Ins6P-like P was lower (7 %) and monoester-like P was not detected. Annaheim (2013) also reported a complete hydrolysis of all organic P in compost obtained from green waste, whereas the concentration of Ins6P-like P was 10 %, of DNA-like P 6 % and of monoester-like P 3 %. In the manure extract, 60 % of organic P was hydrolyzed by enzyme addition. Monoester-like P was the major form of the total NaOH–EDTA extracted P (10.7 %), the DNA-like P fraction was only 0.2 % and Ins6P-like P was not detected. Annaheim (2013) also obtained a dominance of the monoester-like P fraction when analysing manure (13.3 % monoester-like P). The high proportion of monoester-like P was also shown for dairy manure with no bedding in water (He et al. 2006) and NaOH–EDTA (He et al. 2007) extracts. However, He et al. (2007) reported higher proportions of hydrolyzable organic P and found higher proportions of DNA-like P and Ins6P-like P (6.4 and 10.1 % of total NaOH–EDTA extracted P) as compared to our results. These differences could be due to the presence of straw in the manure used in our study. Furthermore, it is important to point out that the composition of manures and composts may vary from one sampling date to another even if the material originates from the same facility (Schoumans et al. 2010). For this study the characterization of the organic P forms in compost and manure was performed using the amendments applied in 2010. Although, the main nutrient concentrations of manure and compost were similar in all application years (both materials were obtained from the same facilities since the start of the experiment in 1998—see also Krey et al. 2013), the concentration of organic P forms can be different and these results should be considered as approximations when interpreting the results.
Effect of long-term P amendment practice on organic and inorganic P forms in the NaOH–EDTA soil extracts
The extraction of soil with NaOH–EDTA showed a complete recovery of the total P in the topsoil. The concentration of the different P forms in the NaOH–EDTA soil extracts differed with respect to the amendment used (Table 5). The compost + TSP treatment was found to have the highest inorganic and total organic P contents. Generally, in this study the ratio of inorganic P:organic P was higher in the organic amendments than in the soil (Table 4). Organic P applied to fields can be mineralized, but inorganic P can also be immobilized into microbial and plant biomass and thus converted into organic P (Bünemann et al. 2008; Richardson and Simpson 2011). Soil analyses of the combined compost + TSP treatment, which showed the highest total organic P contents and the lowest inorganic P:organic P ratio (1.14) in soil underline this process. A conversion of inorganic into organic soil P forms in this experiment is also supported by the fact, that during the experimental time only about 50 kg ha−1 organic P were applied with the organic amendments (calculation on the basis of the characteristic of the organic amendments in 2010). The rest of total P supply (see Table 1) was in inorganic form. The 50 kg ha−1 corresponds to a soil P concentration of about 12 mg kg−1 (soil bulk density 1.35 g cm−3, 0–30 cm), however, the difference between the concentration of organic P in soil in the compost + TSP treatment and the control treatment in 2012 was more than 60 mg kg−1. Nutrients are also provided with crop residues (Eichler et al. 2004; Noack et al. 2012). However, in our experiment the amount of organic P applied can be expected to be low, since straw remained at the field only from 1999 until 2005 and in 2012 when cereals were cultivated, whereas in the other years the whole plants were removed from the field (see chapter "Site characterization". Thus, organic P applied with residues can explain differences in organic soil P contents between the treatments only partly. In addition, Randhawa et al. (2005) showed that plant residues can increase the P mineralization in soil, and plant residues must not result in higher amount of organic P in soil.
The amendments did not increase the enzyme hydrolyzable organic P pools compared with the control (Table 5). The proportion of hydrolyzed (the sum of simple monoester, DNA and Ins6P like P) to total organic P extracted by NaOH–EDTA ranged from 26 % (compost + TSP) to 50 % (compost). The maximum release achieved was similar to that observed by Annaheim (2013) (37–50 %) applying an inorganic fertilizer and different organic amendments, and higher than that reported by Johnson and Hill (2010) (25–36 %) for soils amended with poultry manure. He et al. (2004a) found that as little as 29 % and as much as 49 % of organic P was enzymatically hydrolyzable in soils with and without long-term swine manure application extracted with NaOH (previously extracted with water and NaHCO3).
Ins6P-like P was the main form of enzymatically hydrolyzable P in all treatments ranging from 19 % (compost + TSP) to 38 % (manure) of organic P extracted with NaOH–EDTA. Several studies reported that Ins6P-like P is the major hydrolyzable form in most soils using different extractants like water (Turner et al. 2002), NaOH (He et al. 2004a, 2008) or NaOH–EDTA (Keller et al. 2012). This abundance can be explained by a strong affinity of Ins6P to soil surfaces which protect it from degradation, causing its accumulation in soil (Celi et al. 1999, Steffens et al. 2010). Based on the characterization of the organic amendments applied in 2010, plots amended with compost have received the highest amounts of Ins6P-like P, while plots amended with manure have received the lowest, as Ins6P-like P was not detected in the manure NaOH–EDTA extract (Table 4). However, the concentration of Ins6P-like P in soil in the plots with compost addition was not higher than in the plots with manure addition. This suggests that inputs of Ins6P-like P via organic amendment may have no or only little influence on the amount of this form in the topsoil. Crop residues and harvest losses can be also a source of Ins6P in soil (Noack et al. 2012). However, as stated before, the amount of organic P provided with plant residues must have been low, and Ins6P-like inputs by plant residues should be proportional to crop yield and P uptake, so this form of organic P also would be expected to be higher in the compost + TSP plots than in other treatments. Ins6P-like P accumulation in soil could be also from microbial origin (Makarov et al. 2002). In our study we found a positive correlation between the activity of DHA and the Ins6P-like P concentration in soil (r = 0.588*, n = 19) which can support this fact. Further analyses (e.g. 31P NMR) of the NaOH–EDTA extract could help to interpret the high amount of not hydrolyzable P in the compost + TSP treatment, together with the decrease in Ins6P.
Monoester-like P and DNA-like P forms were also detected in NaOH–EDTA soil extracts but in a lower concentration and no differences between treatments were found. The proportion of simple monoester-like P to organic P ranged from about 2 % (control) to 8 % (compost) while the proportion of DNA-like P to organic P ranged from 4 % (compost + TSP) to 11 % (manure). Compost addition represents an important input of DNA-like P to soil, whereas cattle manure contains important amounts of simple monoester-like P. However, the low percentage of these forms of P in the topsoil may reflect the previous degradation (Dick and Tabatabai 1978) or the leaching to deeper soil layers (Anderson and Magdoff 2005). In addition, some orthophosphate diesters could have been degraded rapidly in NaOH–EDTA extracts, which may underestimate the concentration of DNA-like P and limit the characterization of soil organic P with this method applied (Makarov et al. 2002; Turner et al. 2003b).
A large fraction of soil organic P remained non-hydrolyzed in NaOH–EDTA extracts (47–73 %). This fraction includes more complex forms associated with humic substances (Brannon and Sommers 1985) not accessible for hydrolytic enzymes. These proportions are in accordance with other similar studies (Keller et al. 2012, He et al. 2004a).
Conclusions
The long-term amendment practice affected the organic and inorganic P forms in soil. However, the amount of organic P applied with the amendments cannot explain the concentration of organic P in soil. Likely, the organic P forms in soil are a result of the multifaceted P turnover processes in soil which are affected by plants, microorganisms and abiotic factors. Higher ratio of P organic:P inorganic in soil than in the organic amendments supported this. To include further treatments and field experiments can help to gain a better understanding of factors affecting organic P classes in soil. The results showed that the application of P with organic amendments can be considered to be an adequate P source since the high-soluble contents of inorganic P in soil (Pw and Pdl) could be warranted for a period of 14 years, when the P supplied is balanced with the P removed by harvested crop products.
References
Anderson BH, Magdoff FR (2005) Relative movement and soil fixation of soluble organic and inorganic phosphorus. J Environ Qual 34:2228–2233
Annaheim KE (2013) Use of enzyme additions to characterize the nature and hydrolysability of soil organic phosphorus. Ph.D. thesis, ETH Zurich, Switzerland
Annaheim KE, Rufener C, Frossard E, Bünemann E (2013) Hydrolysis of organic phosphorus in soil water suspensions after addition of phosphatase enzymes. Biol Fertil Soils 49:1203–1213
Bowman RA, Cole C (1978) Transformations of organic phosphorus substrates in soils as evaluated by NaHCO3 extraction. Soil Sci 125:49–54
Brannon CA, Sommers LE (1985) Stability and mineralization of organic phosphorus incorporated into model humic polymers. Soil Biol Biochem 17:221–227
Bünemann E, Schwenke G, Van Zwieten L (2006) Impact of agricultural inputs on soil organisms—a review. Soil Res 44:379–406
Bünemann E, Smernik RJ, Marschner P, McNeill AM (2008) Microbial synthesis of organic and condensed forms of phosphorus in acid and calcareous soils. Soil Biol Biochem 40:932–946
Cade-Menun B, Preston CM (1996) A comparison of soil extraction procedures for 31P NMR spectroscopy. Soil Sci 161:770–785
Celi L, Lamacchia S, Marsan FA, Barberis E (1999) Interaction of inositol hexaphosphate on clays: adsorption and charging phenomena. Soil Sci 164:574–585
Cordell D, Drangert J, White S (2009) The story of phosphorus: Global food security and food for thought. Global Environ Change 19:292–305
Dick W, Tabatabai M (1978) Hydrolysis of organic and inorganic phosphorus compounds added to soils. Geoderma 21:175–182
Dick W, Cheng L, Wang P (2000) Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol Biochem 32:1915–1919
Ehlers K, Bakken LR, Frostegård Å, Frossard E, Bünemann EK (2010) Phosphorus limitation in a Ferralsol: impact on microbial activity and cell internal P pools. Soil Biol Biochem 42:558–566
Eichler B, Zachow B, Bartsch S, Köppen D, Schnug E (2004) Influence of catch cropping on nitrate contents in soil and soil solution. Landbauforsch Volkenrode 54:7–12
Eichler-Löbermann B, Köhne S, Köppen D (2007) Effect of organic, inorganic, and combined organic and inorganic P fertilization on plant P uptake and soil P pools. J Plant Nutr Soil Sci 170:623–628
Eichler-Löbermann B, Köhne S, Kowalski B, Schnug E (2008) Effect of catch cropping on phosphorus bioavailability in comparison to organic and inorganic fertilization. J Plant Nutr 31:659–676
Eivazi F, Tabatabai M (1977) Phosphatases in soils. Soil Biol Biochem 9:167–172
Fliessbach A, Oberholzer H, Gunst L, Mäder P (2007) Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric Ecosyst Environ 118:273–284
García-Albacete M, Martín A, Cartagena MC (2012) Fractionation of phosphorus biowastes: characterisation and environmental risk. Waste Manag 32:1061–1068
Godlinski F, Leinweber P, Meissner R, Seeger J (2004) Phosphorus status of soil and leaching losses: results from operating and dismantled lysimeters after 15 experimental years. Nutr Cycl Agroecosyst 68:47–57
Gopinath KA, Saha S, Mina BL, Pande H, Kundu S, Gupta HS (2008) Influence of organic amendments on growth, yield and quality of wheat and on soil properties during transition to organic production. Nutr Cycl Agroecosyst 82:51–60
Gransee A, Merbach W (2000) Phosphorus dynamics in a long-term P fertilization trial on Luvic Phaeozem at Halle. J Plant Nutr Soil Sci 163:353–357
Harrison AF (1987) Soil organic phosphorus: a review of world literature. CAB International Wallingford, UK
He Z, Griffin TS, Honeycutt CW (2004a) Enzymatic hydrolysis of organic phosphorus in swine manure and soil. J Environ Qual 33:367–372
He Z, Griffin TS, Honeycutt CW (2004b) Phosphorus distribution in dairy manures. J Environ Qual 33:1528–1534
He Z, Toor GS, Honeycutt CW, Sims JT (2006) An enzymatic hydrolysis approach for characterizing labile phosphorus forms in dairy manure under mild assay conditions. Bioresour Technol 97(14):1660–1668
He Z, Cade-Menun BJ, Toor GS, Fortuna A, Honeycutt CW, Sims JT (2007) Comparison of phosphorus forms in wet and dried animal manures by solution phosphorus-31 nuclear magnetic resonance spectroscopy and enzymatic hydrolysis. J Environ Qual 36:1086–1095
He Z, Honeycutt CW, Cade-Menun BJ, Senwo ZN, Tazisong IA (2008) Phosphorus in poultry litter and soil: enzymatic and nuclear magnetic resonance characterization. Soil Sci Soc Am J 72:1425–1433
Hoffmann G (1991) Die Untersuchung von Böden, in: VDLUFA-Methodenbuch, 4 Aufl, Bd 1, VDLUFA-Verlag, Darmstadt
Johnson NR, Hill JE (2010) Phosphorus species composition of poultry manure-amended soil using high-throughput enzymatic hydrolysis. Soil Sci Soc Am J 74:1786–1791
Keller M, Oberson A, Annaheim KE, Tamburini F, Mäder P, Mayer J, Frossard E, Bünemann EK (2012) Phosphorus forms and enzymatic hydrolyzability of organic phosphorus in soils after 30 years of organic and conventional farming. J Plant Nutr Soil Sci 175:385–393
Kerschberger M, Hege L, Jungk A (1997) Phosphordüngung nach Bodenuntersuchung und Pflanzenbedarf. VDLUFA Standpunkt, VDLUFA Verlag
Krey T, Vassilev N, Baum C, Eichler-Löbermann B (2013) Effects of long-term phosphorus application and plant-growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. Eur J Soil Biol 55:124–130
Makarov M, Haumaier L, Zech W (2002) Nature of soil organic phosphorus: an assessment of peak assignments in the diester region of 31P NMR spectra. Soil Biol Biochem 34:1467–1477
Nain L, Rana A, Joshi M, Jadhav SD, Kumar D, Shivay SY, Paul S, Prasanna R (2009) Evaluation of synergistic effects of bacterial and cyanobacterial strains as biofertilizers for wheat. Plant Soil 331:217–230
Noack SR, McLaughlin MJ, Smermik RJ, McBeath TM, Armstrong RD (2012) Crop residue phosphorus: speciation and potential bio-availability. Plant Soil 359:375–385
Oehl F, Oberson A, Tagmann H, Besson J, Dubois D, Mäder P, Roth H, Frossard E (2002) Phosphorus budget and phosphorus availability in soils under organic and conventional farming. Nutr Cycl Agroecosyst 62:25–35
Ohno T, Zibilske LM (1991) Determination of low concentrations of phosphorus in soil extracts using malachite green. Soil Sci Soc Am J 55:892–895
Pagliari PH, Laboski CA (2012) Investigation of the inorganic and organic phosphorus forms in animal manure. J Environ Qual 41:901–910
Pagliari PH, Laboski CA (2013) Dairy manure treatment effects on manure phosphorus fractionation and changes in soil test phosphorus. Biol Fertil Soils 1–13
Parham JA, Deng SP, Raun WR, Johnson GV (2002) Long-term cattle manure application in soil I Effect on soil phosphorus levels, microbial biomass C, and dehydrogenase and phosphatase activities. Biol Fertil Soils 35:328–337
Randhawa P, Condron L, Di HJ, Sinaj S, McLenaghen RD (2005) Effect of green manure addition on soil organic phosphorus mineralisation. Nutr Cycl Agroecosyst 73:181–189
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct Plant Biol 28:897–906
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability. Update on microbial phosphorus. Plant Physiol 156:989–996
Rosmarin A (2004) The precarious geopolitics of phosphorus. Down to Earth: Science and Environment Fortnightly (30 June), pp 27–31
Schoumans OF (2000) Determination of the degree of phosphate saturation in noncalcareous soils. In: Pierzynski GM (ed) Methods for phosphorus analysis for soils, sediments, residuals, and water. Southern Cooperative Series Bulletin 396, North Carolina, pp 31–34
Schoumans OF, Rulkens WH, Oenema O, Ehlert PAI (2010) Phosphorus recovery from animal manure. Alterra report 2158. ISSN:1566-7197
Schwertmann U (1964) Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Z Pflanzenern Bodenk 105:194–202
Sinaj S, Traore O, Frossard E (2002) Effect of compost and soil properties on the availability of compost phosphate for white clover (Trifolium repens L.). Nutr Cycl Agroecosyst 62:89–102
Steffens D, Leppin T, Luschin-Ebengreuth N, Min Yang Z, Schubert S (2010) Organic soil phosphorus considerably contributes to plant nutrition but is neglected by routine soil-testing methods. J Plant Nutr Soil Sci 173:765–771
Tabatabai M, Bremner J (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Thalmann A (1968) Zur Methodik der bestimmung der dehydrogenaseaktivität im boden mittels triphenyltetrazoliumchlorid (TTC). Landwirtsch Forsch 21:249–258
Tiessen H, Moir J, Carter M (1993) Characterization of available P by sequential extraction. In: Carter MR (ed) Soil sampling and methods of analysis. CRC, Boca Raton, pp 75–86
Turner BL, McKelvie ID, Haygarth PM (2002) Characterisation of water-extractable soil organic phosphorus by phosphatase hydrolysis. Soil Biol Biochem 34:27–35
Turner BL, Cade-Menun BJ, Westermann DT (2003a) Organic phosphorus composition and potential bioavailability in semi-arid arable soils of the western United States. Soil Sci Soc Am J 67:1168–1179
Turner BL, Mahieu N, Condron LM (2003b) Phosphorus-31 nuclear magnetic resonance spectral assignments of phosphorus compounds in soil NaOH–EDTA extracts. Soil Sci Soc Am J 67:497–510
Van der Paauw F (1971) An effective water extraction method for the determination of plant-available soil phosphorus. Plant Soil 34:467–481
Van Vuuren DP, Bouwman A, Beusen A (2010) Phosphorus demand for the 1970–2100 period: a scenario analysis of resource depletion. Global Environ Change 20:428–439
Acknowledgments
M.I. Requejo is thankful for the grant conceded by the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, Spanish Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Requejo, M.I., Eichler-Löbermann, B. Organic and inorganic phosphorus forms in soil as affected by long-term application of organic amendments. Nutr Cycl Agroecosyst 100, 245–255 (2014). https://doi.org/10.1007/s10705-014-9642-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-014-9642-9