“En ese instante gigantesco, he visto millones de actos deleitables o atroces¸ ninguno me asombró como el hecho de que todos ocuparan el mismo punto, sin superposición y sin transparencia. Lo que vieron mis ojos fue simultáneo: lo que transcribiré, sucesivo, porque el lenguaje lo es. Algo, sin embargo, recogeré.”
Jorge Luis Borges (Excerpt from the short story “The Aleph” (Borges, 2005, p. 285)).
Abstract
From the perspective of successive events, chemical reactions are expressed or thought about, in terms of the cause-effect category. In this work, I will firstly discuss some aspects of causation and interaction in chemistry, argue for the interaction, and propose an alternative or complementary representation scheme called “interaction diagram”, that allows representing chemical reactions through a geometric diagram. The understanding of this diagram facilitates the analysis of reactions in terms of the interaction, or reciprocal action, among the participating entities. Secondly, I will describe the model and provide examples and finally, I will discuss the scope and limitations of the current development status of the model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the short story “El Aleph”, Borges (2000) writes: “In that unbounded moment, I saw millions of delightful and horrible acts; none amazed me so much as the fact that all occupied the same point, without superposition and without transparency. What my eyes saw was simultaneous, what I shall write is successive, because language is successive. Something of it, though, I will capture.”Footnote 1 This phrase, which is included in Spanish as the epigraph, will serve as heuristic resource to express the main proposal of this work. Entities that participate in chemical reactions, as in the case of the mythical Aleph, could be considered as if “all occupied the same point, without superposition and without transparency” (Borges 2000). Although causation —a relationship between two events, cause and effect, related by a temporal succession— is the traditional and predominant way of approaching chemical reactions, it is insufficient to study every aspect of them. In fact, the causal perspective minimizes or completely neglects much of the conceptual richness of chemical phenomena, based on a complex network of simultaneous relationships. By contrast, the concept of reciprocal action or interaction makes it possible to account for the simultaneous interdependence between several entities. As a consequence, it provides a fertile framework to conceive chemical reactions. The main difficulty that the interactive approach faces is to describe simultaneity by means of language, since its very nature involves succession. As Borges clearly says: “What my eyes saw was simultaneous, what I shall write is successive, because language is successive. Something of it, though, I will capture” (Borges 2000).
The main aim of the present work is to propose a model of representation, that I will call “interaction diagram”, designed to overcome —at least partially— the inherent difficulty in representing the phenomenon of simultaneity in chemical reactions, which normally are presented in terms of cause and effect. In the second section, I will analyze general aspects of causation. In particular, the causal-interventionist model that has been proposed for the description of chemical reactivity will be recalled in the third section. In the fourth section, I will consider some aspects of the concept of reciprocal action or interaction, proposed as a category of relation by Kant. I will not focus here on the philosophical discussion of its foundations in Kantian terms, but I will take it as an “inspiring idea” to think about chemical reactivity. In the fifth section, the central one of the present work, I will introduce the “interaction diagrams”, describing its characteristics and discussing its foundations. I will provide several examples of reactions represented in the scheme. Lastly, in the sixth section, I will present the conclusions and final reflections of this work.
Causation and temporal succession
Causation describes a relationship between two events successive in time, according to which the first, “the cause”, produces the second, “the effect”. The purpose of this work is not to take a position regarding the many debates about this concept, but to explore its use in the context of the account of chemical reactivity.
Causation has been a central element for the conception of reality since Antiquity. Even those who challenged its objective existence, such as Hume, thought that it was an important subjective means to access the real domain. However, in the context of science, not all agree on the need to think in causal terms. For example, Bertrand Russell believed that causation had no role in scienceFootnote 2; according to him, this is very clear in Newtonian mechanics: “In the motions of mutually gravitating bodies, there is nothing that can be called a cause, and nothing that can be called an effect; there is merely a formula.” (Russell, 1992 p. 202). In this sense, it is worth emphasizing that Newton’s third law is usually called “Principle of Action and Reaction”, suggesting traditional causation: (i) first, a body exerts a force, the action, which acts as a cause that produces as its effect the modification of the behavior of another body, and (ii) then, the second body reacts with another force of equal magnitude and opposite direction, which acts as a cause that produces as its effect the modification of the behavior of the original body. As it is well known, this way of understanding the physical phenomenon suggested by the name “Principle of Action and Reaction” is wrong and wreaks havoc on the students’ understanding of classical mechanics. As Russell says, “it is erroneously supposed to do no harm”. In fact, Newton’s third law should be called “Principle of Interaction” in order to show that there are not two successive phenomena, action and reaction, but a single phenomenon, interaction, where two forces appear simultaneously, each acting on a different body. Physicists have already incorporated this terminology: for example, they no longer speak of fundamental forces, but of fundamental interactions.
Quantum mechanics itself has pretty much ridden itself from the cause-effect conundrum. We read in Tarasov’s 1980Basic Concepts of Quantum Mechanics (pp. 158–166):
In quantum mechanics the principle of causality refers to the possibilities of the realization of events (properties). In other words, in quantum mechanics it is not individually realized events that are causally related, but only the possibilities of the realization of these events. This is the essence of the quantum-mechanical meaning of causality. As Pauli stated in his Nobel lecture … the statements of quantum mechanics are dealing only with possibilities, not with actualities. They have the form, ‘This is not possible’, or ‘Either this or that is possible’, but they can never say, ‘that will actually happen then and there’.
Moreover, quantum mechanics abhors temporal succession during a quantum event. We read, again in Tarasov’s book (p. 50):
Let us consider the transition of an electron in an atom from level E1 to level E2 by absorbing a photon of energy ℏω = E2 - E1. We recall that the contradiction in transition was connected with the question whether the absorption of the photon precedes the transition of the electron or vice versa. It is easy to see that this question simply loses its meaning now. In fact, if we have a bound electron with energies E1 and E2 before and after interaction with radiation, respectively, then during the interaction we have one quantum-mechanical system including both the electron and the radiation. This system exists for a definite time (while the interaction with the radiation takes place) and, according to (3.2) [Heisenberg’s uncertainty relation], cannot have any definite energy. Hence it is meaningless to find out precisely what takes place in such a system. Strictly speaking, during the interaction of the electron with the photon there is no electron and no photon, but a single entity which must be treated as such, without going into details. This example shows that in quantum mechanics a physical process cannot be infinitely detailed in time. The question “what follows what”? cannot always be posed in the case of microphenomena. (The anomaly of quantum transition is completely removed by considering the principle of superposition of states).
Both modern science and contemporary philosophy have a completely different mind-set than the traditional causeeffect sequential thinking. Can this paradigm be also that of a chemical ontology? Can we get rid of an idea as rooted as causation and think in other terms? What differences and similarities does the reciprocal action in chemistry and physics have?
Causation in chemistry
Among the many concepts of causation discussed in the literature, in the present context it is interesting to consider James Woodward’s interventionist model, which was applied by Georgie Statham (2017) to study the manipulation of chemical reactions. According to Woodward (2003), the variable X is a cause of the variable Y if and only if an intervention that changes the value of X would result in a change in the value of Y. For example, smoking “is a cause” of cancer because a randomized experiment with proper statistical control clearly demonstrates that a group of subjects that smoked has a statistically-significantly higher incidence of cancer compared to the control group.
Interventionists represent causal systems by means of a set of exogenous and endogenous variables, structural equations, and background variables. The structural equation for each endogenous variable (i.e., each variable that is an effect of other variables in the graph) expresses the value of that variable as a function of its direct causes. Variables are connected by arrows representing the direct causal relationship between those variables. The background variables are kept fixed and can be considered to represent the context in which the system of structural equations (In Woodward's sense) is applied. Woodward’s explanation implies that it is possible to represent each causal relationship as part of a causal model with the above characteristics.
Statham (2017) applies the interventionist theory of causation to explain the manipulation strategies applied by organic chemists to regulate reaction conditions, both for reactions considered under kinetic and thermodynamic control, and also for those that can be considered under mixed control. He starts from considering that the main purpose of organic chemistry is synthesis and develops causal models to describe that phenomenon.Footnote 3 Statham’s proposal to follow Woodward’s interventionist theory of causation to account for chemical reactions is particularly adequate to chemistry, since it is a very different discipline from physics. For example, following Hacking´s terminology, the ultimate goal of chemistry is not to describe but to intervene in nature (Hacking 1983). Therefore, it seems reasonable to conceptualize the practice of chemistry by means of a theory that conceives causation in terms of intervention. But the perspective needs not be the same when the question is not about the practice of chemistry, but about the chemical world, independently of human intervention. In this case, is causation still the category that better explains how the chemical world works?
Reciprocal action or interaction
Different authors have tried to define the concept of interaction or reciprocal action in terms of causation, for example, as a bidirectional causal relation where the causes become effects and vice versa. This approach faces the problem that, since events are causes and effects at the same time, it should be admitted that a single event is previous and posterior in time to another one, which is a logical absurdity. Different attempts have been made to overcome this contradiction —for instance, by assuming that certain aspects of a substance act as “cause” whereas other play the role of “effect” (see Watkins 2005)—. However, these attempts hide the fundamental problem: Reciprocal action is not causation.
Within the framework of a transcendental philosophy such as that developed by Kant in the Critique of Pure Reason, the question is how to synthetize the multiplicity of perception, as described in transcendental aesthetics, so that the pure concepts of understanding acquire content and do not remain empty. In this process, reciprocal action is one of the ways of synthesizing that multiplicity (Salerno 2013): it is one of the elements of Kant’s table of categories, belonging to the class of relation besides substantiality and causality.
According to Torretti (2013), within the framework of Kantian philosophy, there are two temporal relationships that can be established between phenomena: succession and simultaneity. As time itself is not perceivable, these relationships cannot be characterized in temporal terms, but they must be defined by directly linking the phenomena to each other. The relation of succession is established according to the principle of causality, while the relation of simultaneity is established by the principle of interaction or reciprocal action.
Kant formulates the principle of causality as follows: “All alterations occur in accordance with the law of the connection of cause and effect.” (Kant B233; 1998. p. 304). In turn, he expresses the principle of interaction in the following terms: “All substances, insofar as they can be perceived in space as simultaneous, are in thoroughgoing interaction” (Kant B257; 1998. p. 316).
As Torretti observes, the category of causality is one of the basic concepts that, coming from common sense, enters physics to supply a scientific account of facts. By contrast, the category of interaction is a creation of Kant, inspired precisely by Newton’s third law of motion. Regarding the category of reciprocal action, Kant says: “Things are simultaneous if in empirical intuition the perception of one can follow the perception of the other reciprocally which in the temporal sequence of appearances, as has been shown in the case of the second principle happen” (Kant B257; 1998. p. 316).
Just as in Newton's third law there is no real difference between action and reaction since there are no not two processes involved, also in chemistry the difference between reactants and products is conventional. Although the difference seems natural, either out of habit or for practical reasons, there is no ontological distinction between reactants and products. Turning a pragmatic distinction into an ontological difference in chemical reactions is conceptually as damaging as conceiving Newton's third law in terms of two successive phenomena, action and reaction.
In this work we will not dwell on the ways of interpreting the Kantian category of reciprocal action, but instead, we will take it as an “inspiring notion” upon which we will build a conceptual framework useful for understanding chemistry. This framework aims to understand “the conditions of possibility of the objects of experience” in the study of chemical reactions and chemical reactivity. The category of interaction, taken in its most general aspects, will allow us to interpret chemical equilibrium and to clarify the limits and possibilities of chemists’ interventions onto chemical phenomena. The final goal is to emphasize the simultaneous interaction between all the species participating in a chemical reaction.
Representation of chemical reactions: “interaction diagrams”
When we represent —and think about— a chemical reaction, we have learned to consider that the substances that we call “reactants” first react with each other, and then give rise to the substances that we call “products.” That is, we describe reactions, both verbally, in writing or in symbolic language, in terms of cause and effect, in the temporal order that we perform certain actions in the laboratory. For example, we say: firstly, we add the substances A and B in the reaction vessel; then we add the solvent and the catalyst; afterwards, we apply temperature, pressure etc.; and finally, after a time t, we obtain the products C and D. This way of representing chemical reactions has strong limitations to understand reversibility, among other phenomena. Although we are aware of the non-sequential and complex interactions between molecules, in the traditional framework it is very difficult to express and transmit —and even fully understand— chemical reactions from the perspective of simultaneous interaction. In order to overcome the inherent limitation of causal scheme, sometimes the reverse narrative is used: besides the fact that A reacts with B to give C and D, C reacts with D to give A and B. But this strategy is analogous as that used in the attempts to conceptualize interaction in terms of causation instead of conceiving it as a new category.
The challenge is then, to present the network of interactions involved in the context of a reaction without appealing to the concept of causation and the temporal sequence that such a concept entails. For this purpose, I will use the strategy of representing the interacting substances arranged in a completely different way from that in which they appear in the traditional presentation. In particular, the substances will be ordered according an atemporal parameter, so that the idea of a temporal sequence is completely abandoned. Of course, since this timeless picture deeply diverges from the usual view, describing it is not easy. I find myself in a similar situation to the one narrated by Borges in the Aleph “Every language is an alphabet of symbols the employment of which assumes a past shared by interlocutors. How can one transmit to others the infinite Aleph, which my timorous memory can scarcely contain?” (Borges 2000, p. 129).
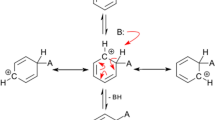
In the proposed model, which I call “interaction diagram”, the interacting substances are identified by numbers, which are represented by points placed in a system of concentric circles. Those circles correspond, from outside inwards, the reaction medium (blue),Footnote 4 the reactant entities (green), the intermediate entities (yellow), and the interaction nodes (red) (see Fig. 1). An interaction node, which has some analogy with the transition state,Footnote 5 is represented by a point characterized by the mass number that results from the sum of the mass numbers of the reactants that give rise to the node.Footnote 6 The node with the highest molar mass number will be located at the point corresponding to the angular value 2π, and the other nodes will be located at points defined by the proportional angle corresponding to their molar mass number. In this way, an angular scale is defined for molar mass numbers. Based on this scale, the interacting substances are located on the corresponding circle according to their mass number. Reactions are represented by lines, which connect the points representing the interacting substances with the corresponding interaction node.Footnote 7
In this diagram, the order of the interacting substances is decoupled from the order in which such substances appear in the classical expression of the reaction. This is because they are ordered by molar mass numbers, and not in terms of the traditional “cause-effect” relation (A reacts with B to give C and D, for example).
On the other hand, the reaction is presented in a global way, as “a whole”, encoded in a series of points that are joined by lines, forming a kind of “road network”. Each reaction is represented by a “geometric code”, which reflects the simultaneous interaction of the entities in the reaction. This code must be “read”, that is, it must be decoded in terms of everyday language, which is sequential. However, the possible “readings” are many,Footnote 8 and there is no privileged order for reading (such as left–right, top–bottom, or vice versa). Following the analogy of the road network, there are multiple logical paths to go through all the points. In some complex mechanisms, the number of possible reads can be very large, or even excessively large, but it can always be determined exactly. Any correct reading will start with the substances, in any order, corresponding to points joined by lines with the interaction node to which they “converge”, and then the value of the node is “read”.Footnote 9 Then, the substances corresponding to the points converging to the “second” node (one of them was already read in the first step) are read in a similar way, also without privileged order. It will continue in an analogous way, until finishing with the “last” node, which will close the reading. From this perspective, the usually called “intermediary elements” fulfill the function of connecting nodes, so that the term “intermediary” here has a different meaning from that of the classical representation (although it refers to the same kind of entity). In turn, the interaction nodes have a certain analogy with the symbol “ = ” (which in the past was used instead of arrows in chemical equations), in the sense that each node manifests the equality between the sum of the molar mass numbers of the substances corresponding to the “converging” lines and the sum of the molar mass numbers of the substances corresponding to the “diverging” lineFootnote 10 (the convergence-divergence distinction has no place in this system, but it can be helpful to express the idea). The molar mass number corresponding to the interaction node will, in general, be equal to that of a transition state, although conceptually the interaction node has a different meaning within this model.
The concept of interaction node is the central element in the articulation of the reading “grammar” of the reaction. From the traditional cause-effect perspective it is said: “A reacts with B, to give C and D”. If the reaction is reversed, as in the traditional way of understanding reversibility, the cause-effect relation is conceived in the opposite direction. So, there are two cause-effect relations, one in each direction. By contrast, from the perspective proposed in this work, the reaction is described by saying: “A, B, C and D (in any order) react in X”, where X is the interaction node. In this way, the reversibility of reactions is not conceived as a superposition of “reactions in both directions”, but as an implicit property of reactions understood as interactions.
On this basis, we can define the concept of interactionFootnote 11 in chemical reactions as follows: “interaction in chemical reactions is the simultaneous relationship between substances around an interaction node, independently of the order of perception.” This definition has a close analogy with how Kant describes interaction in the third analogy of experience:
“Thus I can direct my perception first to the moon and subsequently to the earth, or, conversely, first to the earth and them subsequently to the moon, and on this account, since the perceptions of these objects can follow each other reciprocally, I say that they exist simultaneously. Now simultaneity is the existence of the manifold at the same time.” Kant B257 (Kant 1998. p. 316).
This abstract presentation of the proposal will become clearer in the following examples of reactions, represented both as a succession of equations (as usual), and through the interaction diagrams proposed in this work.Footnote 12
Figure 2 shows the representations of the dissociation of sulfuric acid, which is generally represented in two steps, with two so-called “successive” dissociations, the first is strong and the second weak. As it is well known, the dissociation process must be understood as a whole, regardless of the fact that in some situations the quantities of some of the entities may be so low that they can be neglected in the calculations. In the interaction diagram, the five participating entities interact through the two nodes (identified by the molar mass numbers 115.1 and 116.1).Footnote 13 As any diagram, this one can be read sequentially in multiple ways. Two of them are (nodes in red):
-
a)
98.1–18.0–97.1–19–116.1–97.1–18.0–96.1–19.0–115.1
which can also be expressed in formulas as.
H2SO4-H2O-HSO4‒-H3O+-Node (116.1)-HSO4‒-H2O-SO4−2-H3O+-Node (115.1).
-
b)
18.0–96.1–19.0–97.1–115.1–18.0–97.1–19.0–98.1–116.1,
which can also be expressed in formulas as.
H2O–SO4−2–H3O+–HSO4‒–Node (115.1)–H2O–HSO4‒–H3O+–H2SO4–Node (116.1).Footnote 14
According to the mechanism exposed in Pimentel et al. (1966) p.135, the mechanism of the hydrogen bromide oxidation reaction in a gaseous medium is usually described by three successive equations, and can be represented by an interaction diagram with four reactant entities, two intermediate entities, and three interaction nodes (see Fig. 3).
Note that the HBr is in the vertex of 3 lines, since it participates in the 3 “steps” of the mechanism.Footnote 15 In this interaction
-
a)
32.8–80.9–112.9–112.9–80.9–112.9–96.9–193.8–96.9–18.0–80.9–79.9–177.8
which can also be expressed in formulas as
O2–HBr–HOOBr–Node (112.9)–HBr–HOOBr–HOBr–Node (193.8)–HOOBr–H2O–HBr–Br2–Node177.8
-
b)
18.0–79.9–80.0–96.9–177.8–32.0–80.9–112.9–112.9–96.9–80.9–112.9–193.8
which can also be expressed in formulas as
H2O–Br2–HBr–HOBr–Node (177.8)–O2–HBr–HOOBr–Node (112.9)–HOBr–HBr–HOOBr–Node (193.8).
diagram, the reaction can be “decoded” by starting the reading by any of the lines that join any of the 4 reactants that converge to an interaction node, and can conclude when all the nodes were read. For example, two possible readings are:
It is interesting to highlight that, among the many possible orders of reading, none of them is preferred or privileged. The network of interactions integrates all the possibilities of simultaneous interactions.
Figure 4 shows the usual representation and the interaction diagram of an esterification in an acid medium.Footnote 16 In this case, formic acid reacts with methanol, which —using the usual language— “produces” the corresponding ester (methyl methanoate), and water. In general, esterification reactions (one of the most important in organic chemistry) produce similar patterns in the interaction diagrams, although the molar mass numbers and therefore the relative positions will change. Note that, since there are four reactants, four intermediates, and four interaction nodes (three of them coincident), the number of possible “reads” significantly increases.
For illustrative purposes, I will present a possible reframing that includes stoichiometry. In Fig. 5, the HBr oxidation reaction previously shown in Fig. 3 (4HBr + O2 2H2O + 2Br2) is represented. The in-plane interaction diagram is spatially projected onto a cylinder. The green lines are proportional to the stoichiometric coefficients of the reaction. In this work I do not advance in the particularities of the spatial representations of the model.
Finally, interaction diagrams find a particularly useful application in the context of teaching, when explaining chemical equilibrium, one of the most difficult topics in introductory chemistry courses. As already noted, chemical reactions are expressed and thought of as sequences of cause-effect relationships. This representative framework, when applied to chemical equilibrium, introduces serious difficulties.
In the case of chemical equilibrium, a generic reaction is presented as:
and it is defined as the equality between the forward and the reverse reaction rates. Here the products are, at the same time, reactants, and vice versa. In a cause-effect framework, all species are simultaneously “before” and “after”, since all of them are considered both causes and effects of each other. The classical way of understanding and representing reactions is particularly perplexing in this case. In addition, this form of representation leads to an interpretation of the reaction as an isolated phenomenon, which leads to ignoring the complex context in which the chemical phenomenon occurs. This situation results in the consolidation of erroneous interpretations and “alternative conceptions” of chemical equilibrium in students. Raviolo and Martínez Aznar (2003) carry out an exhaustive study of the problem, and present the many difficulties encountered in the traditional explanation of chemical equilibrium such as non-differentiation or non-acceptance of reversible chemical reactions, non-admission of the coexistence of all species, compartmentalization of equilibrium, among others (Raviolo and Martinez Aznar 2003). The perspective underlying the interaction diagrams can be an effective strategy in the context of teaching, since it avoids the logical inconsistencies associated with bidirectional causality, clearly reveals reversibility, and helps to obtain a more complete picture of the conceptual complexity of reactions in chemical equilibrium.
Conclusions and final thoughts
In chemistry, phenomena are usually interpreted from the cause-effect perspective: reactions are conceived as successive in time, each one linked to the following one by a causal relation. Although this image, which embodies the idea of “causal chain”, has relevant practical applications, it can also become a significant obstacle to devising new knowledge or to properly integrating established knowledge. As the instrument by which we transmit and give meaning to all knowledge is language, that has the structure of a successive order, there is a natural tendency to consolidate the cause-effect view without further questioning.
In this work, a model for representing chemical reactions is presented, and is called “interaction diagram”. This model tries to block —or, at least, to attenuate— the tendency to understand chemical reactions from the “cause-effect” perspective by representing them in a simultaneous framework. This representation is intended to be an effective complement to the traditional representation, and thus to broaden the global understanding of the chemical phenomenon.
From this alternative perspective, each reaction has a “geometric code”, which reflects the simultaneous interactions of the entities in the reaction. Decoding that code will produce a very large (but precisely definable) set of possible equivalent readings from a small set of interacting entities. This strategy shows the simultaneity of possible and correct decoding, and allows us to visualize the reaction from the perspective of simultaneous interaction, instead of the classical cause-effect perspective. Moreover, the fact that different reactions have topologically similar geometric codes supplies valuable information for organizing reactions in kinds of similar features. For these reasons, this representation is intended to be an effective complement to the traditional representation, and thus to broaden the global understanding of chemical phenomena.
This proposal is a first step towards a more developed model, that might include other quantitative aspects of the phenomenon of chemical reaction: for instance, it is possible to add energetic and stoichiometric parameters. Moreover, since the reactivity patterns obtained in the interaction diagrams focus on a formal perspective, could be related to Paneth's notion of basic substance (see Paneth 1931; Scerri 2005). Thus, they could contribute to the foundation of the concept of periodicity tree, a symmetric closed structure presented as a possible substitute for the traditional idea of group in the periodic system based on triads of atomic numbers (Zambon 2018, 2019).
Of course, the proposal of the present paper is an audacious conjecture. Nevertheless, this should not be considered as a shortcoming if we follow Popper's methodological advice “Prefer an attempt to solve an interesting problem by a bold conjecture, even (and especially) if it soon turns out to be false, to any recital of a sequence of irrelevant truisms. […] this is the way in which we can learn from our mistakes and that in finding that our conjecture was false, we shall have learnt much about the truth and shall have got nearer to the truth.” (Popper 1963, p.231).
Notes
According to Andrew Hurley’s translation.
A well-known quote from Russell expresses: “The law of causation, […] is a relic of a bygone age, surviving, like the monarchy, only because it is erroneously supposed to do no harm.” (Russell, 1992, p. 193).
This is the commonly accepted manner of considering the ultimate objective of organic chemistry.
In this work I will not focus on that circle, but it may be important to highlight some aspects of the reactions. I will only express that, if any substance of the reaction medium (e.g., water in an aqueous solution) participates in the reaction mechanism, it will be in the circle that corresponds to its function in the reaction (reactant or intermediate).
Although they are analogous concepts, they are not equivalent. On the one hand, the transition state is thought of in the framework of a succession between products and reactants while the interaction node is supported by the framework of simultaneity. Furthermore, the interaction node, unlike the transition state, is a concept of a strictly formal nature. In some reactions where it is not possible to establish a safe transition state, such as concerted addition reactions between a diene and a dienophile, the position of the interaction node will correspond to the sum of the molecular masses of the reacting entities.
One could also use the molecular number or another significant parameter for the reaction one wants to represent, but here I will use the mass number because it is —I think— the most intuitive one.
Here all the elements are represented on a surface. However, the representation could be extended in space so that lines, perpendicular to the plane, are drawn from each point. These lines can represent, for example, the specific energy level or the stoichiometric coefficient. In this way, the model would become a cylinder (or a concentric series of cylinders). Although I will not advance in the development of cylindrical coordinates in this work further than what is proposed in Fig. 5. I mention them to point out the projection of this proposal, since they open the way to address aspects of greater complexity.
E.g. for a reaction between 4 entities with a single interaction node, there are 24 possible readings or permutations (4!).
The node could also be read at the beginning, the important aspect is to cover all the nodes and therefore the species that interact in each one of them.
The principle of conservation of mass is implicit in the interaction node, so that it is not necessary to make it explicit as in the traditional representation of reactions.
Usually, interaction in chemistry is defined in an unclear way, as an imprecise kind of relationship or agreement between entities.
In the following diagrams the position of the points is approximate, but the relative order is correct.
Note that HSO4− "joins" the 2 nodes, it would fulfill the function of an intermediary, but it would also be a reactant in the dissociation. For those cases, in this system, the location is given primacy as the reactant.
If molecular numbers are to be used instead of molar mass numbers, only integers would appear. The molecular number is an extension of the atomic number to the molecule, proposed by Allen in 1918 to address the study of esotericism (Galbis Pérez, 2004).
Note that the HBr has 3 lines, since in the mechanism, it participates in the 3 “steps”. It could be considered both an intermediate or a reactant, in this approach it is located in the circle of reactants.
Representations of other organic reactions are not presented in this introductory work. This point may be the subject of a future paper.
References
Borges, J.L.: Obras Completas. Emecé, Buenos Aires (2005)
Borges, J. L. (2005). Obras Completas. Emecé, Buenos Aires
Galbis Pérez, J.: Panorama Actual de la Química Farmacéutica. Publicaciones de la Universidad de Sevilla, Madrid (2004)
Hacking, I.: Representing and intervening: introductory topics in the philosophy of natural science. Cambridge University Press, Cambridge (1983)
Jauregui, C.: “Reflexiones acerca de la acción recíproca y la unidad necesaria. Sobre la interpretación de E. Watkins del modelo causal Kantiano”, Tópicos, 52: ene-jun (2017).
Kant, I.: Critique of pure reason. In: Guyer, P., Wood, A.W. (eds.) Cambridge University Press, New York (1998)
Paneth, F.A.: The epistemological status of the concept of element. Found. Chem. 5, 113–145 (1931)
Pimentel, G., et al.: Química. Una ciencia experimental. Editorial Reverte, México (1966)
Popper, K.: Conjectures and refutations, The growth of scientific knowledge. Harper and Row, New York (1963)
Raviolo, A., Martínez Aznar, M.: Una revisión sobre las concepciones alternativas de los estudiantes en relación con el equilibrio químico. Clasificación y síntesis de sugerencias didácticas. Educación Química 14(3), 159–165 (2003)
Russell, B.: “On the Notion of Cause, orig.1912. In: Slater, J. (ed.) The collected papers of bertrand Russell v6 logical and philosophical papers 1909–1913, pp. 193–210. Routledge Press, London (1992)
Salerno, G.: “Comunidad: acción recíproca”, Universidade Estadual de Campinas (Sociedade Kant Brasileira); Kant E-Prints; 8; 1; 6–2013; 153–167 (2013)
Scerri, E.: “Some aspects of the metaphysics of chemistry and the nature of the elements.” HYLE – Int J. Phil. Chem. 11, 127–145 (2005)
Statham, G.: The manipulation of chemical reactions: probing the limits of interventionism. Synthese 194, 4815–4838 (2017)
Tarasov, L.V.: Basic concepts of quantum mechanics (English Translation). Mir Publishers, Moscow (1980)
Torretti, R.: Manuel Kant. Ediciones Universidad Diego Portales, Santiago de Chile (2013)
Watkins, E.: Kant and the Metaphysics of Causality. Cambridge University Press, Cambridge (2005)
Woodward, J.: Making things happen: a theory of causal explanation. Oxford University Press, Oxford (2003)
Zambon, A.: A representation of the periodic system based on atomic-number triads. Found. Chem. 20(1), 51–74 (2018)
Zambon, A.: Periodicity trees in a secondary criterion of periodic classification: Its implications for science teaching and communication. Substantia 3(2), 101–114 (2019)
Acknowledgements
I want to acknowledge especially Prof. Olimpia Lombardi for her persistent support, Prof. Cherif Matta for his valuable contributions. Also to Lic. Andrea Blengini for her valuable help in the translation and presentation, Alejandro Aguado at the Universidad Nacional de la Patagonia Print for his special assistance with the spatial graphic, and the anonymous reviewers for their valuable comments and suggestions. This paper was possible thanks to the Grant No. PICT-2018-04519 by the Agencia Nacional de Promoción Científica y Tecnológica and Universidad Nacional de la Patagonia San Juan Bosco, Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zambon, A. Chemical reactivity: cause-effect or interaction?. Found Chem 24, 375–387 (2022). https://doi.org/10.1007/s10698-022-09430-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10698-022-09430-1