Abstract
Hypoxia affects fish’s survival, growth, and physiological metabolism processes. In this study, turbot plasma glucose and cortisol contents, hepatic glycolysis (hexokinase [HK], phosphofructokinase [PFK], pyruvate kinase [PK]) and lipolysis (fatty acid synthetase [FAS], lipoprotein lipase [LPL]) enzyme activities, anti-oxidant enzyme (superoxide dismutase [SOD], catalase [CAT], glutathione peroxidase [GSH-Px]) activities, malondialdehyde (MDA), lactate and glycogen contents, gill histological parameters (lamellar length [SLL], width [SLW], interlamellar distance [ID]), respiratory frequency (RF), the proportion of the secondary lamellae available for gas exchange (PAGE), and hifs (hif-1α, hif-2α, hif-3α) expression were determined during long-term hypoxia and reoxygenation. Results showed that long-term hypoxia (3.34 ± 0.17 mg L−1) significantly elevated plasma cortisol and glucose contents; increased hepatic HK, PK, PFK, FAS, and LPL activity; decreased hepatic glycogen, lactate contents, and lipid drop numbers; and caused changes of hepatocyte (vacuolation, pyknotic, and lytic nucleus) after treatment for 4 weeks. Hepatic SOD, CAT, GSH-Px activity, and MDA contents; lamellar perimeter, SLL, ID, RF, and PAGE; and hepatic hif-1α, hif-2α, and hif-3α manifested similar results. Meanwhile, hif-1α is significantly higher than hif-2α, and hif-3α. Interestingly, females and males demonstrated no sex dimorphism significantly different from the above parameters (except hepatic FAS, LPL activity, and lipid drop number) under hypoxia. The above parameters recovered to normal levels after reoxygenation treatment for 4 weeks. Thus, long-term hypoxia promotes turbot hepatic glycogenolysis and lipolysis, induces oxidative damage and stimulates hepatic antioxidant capacity, and alters gill morphology to satisfy insufficient energy demand and alleviate potential damage, while hif-1α plays critical roles in the above physiological process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxygen is essential for metabolism and physiological functions due to its use in cellular energy production and cofactor/substrate for many enzymes in metazoans including mammals and fishes (Semenza 2012; Choudhry and Harris 2018; Wang et al. 2023). Oxygen solubility in water is about 20 times lower than the oxygen capacity of air, and thus sufficient dissolved oxygen (DO) concentration is essential for the survival, growth, and development of aquatic organisms. However, DO levels in aquatic environments are affected and gradually decreased along with eutrophication (Levin and Breitburg 2015), global warming (Tomasetti and Gobler 2020), water pollution (Sarkar and Islam 2020), algal blooms (Bashir et al. 2020), extreme weather conditions (Stevens et al. 2006), and high-density aquaculture (Zhan et al. 2023). The level of global DO content exhibits a continuous and rapid declining trend, and the global hypoxic water area has increased > 15-fold since the 1960s (Laffoley and Baxter 2019). Numerous literatures reported that oxygen-minimum zones have expanded 4.5 million km2 and more than 700 coastal sites can have oxygen concentrations below 2 mg L−1 in the world (Breitburg et al. 2018; Limburg et al. 2020; Baltazar-Soares et al. 2023). Fish as water-living animals are prone to experience hypoxic conditions and severe hypoxia causes mass mortality and a decline in fishery production. Thus, hypoxia has increasingly received considerable attention during farming and fishing in the breeding companies and the fisheries science communities.
Hypoxia could affect behaviors, growth, development, physiological metabolism, and immune responses and even lead to the death in different fish species (Abdel-Tawwab et al. 2019; Saha et al. 2022; Jiang et al. 2023). Meanwhile, fish successfully evolved subtle regulator strategies to cope with situations of low oxygen through various physiological and biochemical changes (Zeng et al. 2020; Wang et al. 2023). It has been identified that glucose transporter contents and anaerobic glycolysis key enzyme activities significantly increased under hypoxia stress in large yellow croaker, golden pompano (Jiang et al. 2023), and other fish species (Abdel-Tawwab et al. 2019). Hypoxia was found to stimulate lipolysis and enhance fatty acid β-oxidation in fish to metabolize fat (Zhao et al. 2020). Fish could prevent and alleviate oxidative cellular damage by enhancing the antioxidant capacity to eliminate hypoxia-induced excessive accumulation of intracellular oxygen-free radicals which include superoxide anion (O2−) and H2O2. The increasing of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) activity was observed in large yellow croaker (Wang et al. 2017; Yang et al. 2017), scaleless carp (Chen et al. 2022), and black rockfish under hypoxia stress. Numerous genes are involved in the regulation of the hypoxia-induced physiological response and adaption in fish, while the expression of these genes was regulated by special transcription factors (Nikinmaa and Rees 2005). Hypoxia-inducible factors (HIFs), as a central regulator for detecting and adapting to cellular oxygen levels, transcriptionally activate genes modulating oxygen homeostasis and metabolic activation (Choudhry and Harris 2018). HIF-1α is a master transcriptional regulator for the expression of genes involved in the response to hypoxia in mammals and fish species (Kumar and Choi 2015; Xiao et al. 2015; Wang et al. 2023). Our previous study also found hif1α as the core gene involved in the regulation of anaerobic glucose metabolism by activating ldha under hypoxia stress in black rockfish. Thus, fish exhibit varied adaptations to variable oxygen concentration by a complex regulatory metabolism to maintain oxygen homeostasis.
Turbot is the most important cultured flatfish in Europe and East Asia for its high-quality flesh and rapid growth rate (Jia and Lei 2019). The annual production of turbot in China has been maintained at approximately 50,000 tons accounting for approximately 80% of the world’s total output of aqua-cultured turbot. As typical benthic flatfish, turbots spend most of their time on the bottom, and water DO fluctuation could affect their growth and metabolism. Long-term hypoxia exposure markedly depressed the growth and feeding conversion ratio of the turbot. Ma et al. (2023) found that acute hypoxia stress (2.0 ± 0.5 mg/L) for 24 h induces endoplasmic reticulum stress, causes lipid peroxidation and liver injury, and stimulates anaerobic glycolysis, whereas it suppresses lipid catabolism and protein synthesis in juvenile turbot. The differences in sex dimorphism for the hematological and biochemical parameters, hepatic antioxidant capacity, and gill histology under acute hypoxia stress were observed in our previous studies (Jia et al. 2021; Li et al. 2023). However, the detailed information about the physiological response of turbot under long-term hypoxia remained unknown. Do male and female turbots share the same or different regulating mechanisms to cope with long-term hypoxia-induced physiological damage? Thus, the changes of anaerobic glycolysis, hepatic antioxidant capacity, and histopathological alteration in gill, as well as HIF-α (hif-1α, hif-2α, hif-3α) mRNA levels of female and male turbot during long-term hypoxia and reoxygenation, were investigated to elucidate the possibly sex dimorphism differences of hypoxia tolerance mechanism in turbot. These findings will provide invaluable data to illustrate the underlying physiological response and potential adoption mechanism during long-term hypoxia stress, which will contribute to turbot effective management in captivity.
Materials and methods
Fish and feeding conditions
Turbots were purchased from Tianyuan Aquatic Co., Ltd. (Yantai, Shandong, China). The healthy similar sizes (190.16 ± 7.3 g) of female and male fish were divided into two groups and allowed to acclimatize to the rearing conditions for 2 weeks. Water temperature was maintained at 18 ± 0.5 °C, salinity ranged from 29 to 30‰, and the pH varied from 7.5 to 8.0. Ammonia nitrogen was lower than 0.1 mg/L. The fish were fed commercial diets (Hai Do, Santong Bio-engineering Weifang Co., Ltd., China) by an automatic feeding machine (FishMom, South Korea) twice a day. The commercial pellets, containing 48.85% protein, 14.38% lipid, and 9.28% moisture, were fasted for 24 h prior to transfer to experimental tanks. A total of 300 experimental male and female fish were randomly distributed into 30 cylindrical fiberglass tanks (100 L, 10 fish per tank).
Experimental protocol and sampling
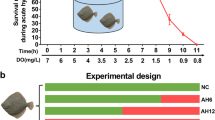
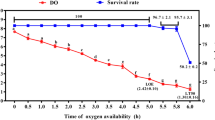
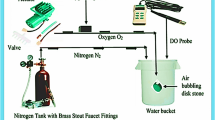
Critical oxygen tension (Pcrit) is defined as the point at which oxygen consumption rates are reduced below the resting oxygen requirement, and the fish has shifted to an oxy-conforming state (Timmerman and Chapman 2004). Our previous study has identified the DO of Pcrit in turbot was 3.34 ± 0.17 mg L−1 (Jia et al. 2021). Thus, we select 3.34 ± 0.17 mg L−1 DO used for long-term hypoxia treatment. Six tanks (three tanks female and three tanks male, 10 fish per tank) were provided with a continuous flow of water (6.5 L/min) and continuous aeration to maintain dissolved oxygen (DO) at/or near saturation used as the control group (7.21 ± 0.13 mg L−1). Our preliminary experiment found the physiological states and growth performance of female and female turbot demonstrated no significantly difference during 8 weeks culture which maintained normal DO (7.21 ± 0.13 mg L−1). Thus, we select the beginning group as the control group in the current study. Twenty-seven tanks were used to carry out the long-term chronic hypoxia and reoxygenation experiment. Our preliminary experiment has found water flow velocity could affect DO levels, the decrease of DO level together with a decrease of water flow velocity. Firstly, the aeration system of 27 tanks were closed and filled with N2 to reduce the DO concentration to the level required for the experiment, then maintain the DO level (3.34 ± 0.17 mg L−1) by regulating water flow velocity. Subsequently, the DO level in the tank water was monitored in real-time using a DO meter (YSI Pro-ODO, Germany). After hypoxia treatment for 2 and 4 weeks (T2W, T4W), the aeration system was restored and water flow velocity was upregulated to recover normal DO condition for 2 and 4 weeks (R2W, R4W). Respiratory frequency (the number of opercular movements per minute) of three fishes per tank was counted three times at 5 min (a total of nine times per group) throughout hypoxia and reoxygenation based on our previous study (Jia et al. 2021). The survival rate of experimental fish was calculated during hypoxia and reoxygenation. The scheme of hypoxia stress and reoxygenation process, the relationship between DO and water flow velocity, and the survival rate are shown in Fig. 1.
The scheme of hypoxia stress and reoxygenation in turbot. A Treated low DO at 3.34 ± 0.17 mg/L for 2 and 4 weeks and reoxygenation at 7.21 ± 0.13 mg/L for 2 and 4 weeks. B Relationship between DO and water flow velocity. C Survival rate of turbot during hypoxia and reoxygenation. Data are presented as means ± SEM. Bars with different superscripts are statistically different (P < 0.05, n = 9). DO, dissolved oxygen; T2W, treated for 2 weeks; T4W, treated for 4 weeks; R2W, reoxygenation 2 weeks; R4W, reoxygenation 4 weeks
The fish handling procedures were conducted according to the guidelines established by the Institutional Animal Care and Use Committee of the Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Science. The fish samples were anesthetized with 50 mg/L of tricaine methane sulfonate (MS-222, Sigma, St. Louis, MO) to avoid handling stress. Meanwhile, add 10 mg/L sodium bicarbonate (NaHCO3) to combat the MS-222-induced water acidity product. The fish were fully anesthetized within 5 min. Then, the blood samples of nine fish samples in each group (n = 9 from the control, T2W, T4W, R2W, and R4W group) were obtained from their caudal veins by using heparinized syringes. The blood was subsequently centrifuged at 1000 g for 10 min at 4 °C to separate the plasma, which was collected and stored at − 80 °C for the subsequent analysis of the cortisol and glucose.
After collecting blood, the fish were sacrificed. Tissues from the liver and gill were collected from nine fish in each group. For each fish, we collected three samples, and did triplicate technique repeats for each sample. The specimens of the liver were divided into three groups, one was snap-frozen in liquid nitrogen or preserved in RNAstore Reagent (Tiangen Biotech, Beijing, China) and then stored at − 80 °C until RNA extraction, and another was collected and stored at − 80 °C to analyze hepatic HK, PFK, PK, G-6-Pase, PEPCK, FAS, LPL, SOD, CAT, GSH-Px activity, and lactate, glycogen, and MDA content; the remaining liver was fixed in 4% paraformaldehyde overnight at 4 °C for histological observations, lipid droplet, and glycogen assay via hematoxylin and eosin (HE), oil red, and Periodic Acid-Schiff (PAS) staining method, respectively. The second right gill arch (total including three-gill arches in each gill) was fixed in 4% paraformaldehyde overnight at 4 °C for morphological observations.
Biochemical analysis and hepatic antioxidant status
Plasma cortisol levels were determined by radioimmunoassay (RIA) using cortisol radioimmunoassay (RIA) kits (Immunotech, Beckman Culture Company, France). Data were expressed as nanograms per milliliter. The plasma glucose levels were detected using commercially available kits (Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen City, China) in an Auto Chemistry Analyzer (BS-200, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen City, China). Glucose levels were expressed as millimoles per liter.
Liver samples were homogenized in an ice bath by a liver and homogenate medium (pH 7.2, phosphate-buffered saline consists of 1.5 mmol/L monopotassium phosphate, 8 mmol/L sodium hydrogen phosphate, 135 mmol/L sodium chloride, 2.7 mmol/L potassium chloride) with a ratio of 1:5 (W:V) and then centrifuged at 4 °C for 10 min (4000 rpm). The supernatant was collected immediately for the following analysis. Hepatic HK, PFK, PK, G-6-Pase, PEPCK, FAS, LPL, SOD, CAT, GSH-Px activity, lactate, glycogen, and MDA content were measured using their corresponding commercial detection kits (A077-3–1, H244-1–2, A076-1–1, A027-1–1, A131-1–1, H231-1–1, A067-1–2, A001-3–2, A007-1–1, A005-1–2, A020-2–2, A043-1–1, A003-1–2) in accordance with the manufacturers’ instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The HK, PFK, PK, G-6-Pase, PEPCK, FAS, LPL, SOD, CAT, and GSH-Px activity were expressed as units per milligram of protein. MDA content was expressed as millimoles per milligram of protein. Glycogen and lactate were expressed as milligrams per gram of tissue.
Histological examination
The paraformaldehyde-fixed liver and gill tissues were washed three times with phosphate-buffered saline (PBS, pH 7.4), dehydrated through a graded ethanol series, cleared in xylene, and embedded in the paraffin, and then Sects. (5 μm) were cut using a microtome (RM2125, Leica). For HE staining, sections were deparaffinized in xylene (25 min) and rehydrated through successive 1-min washes in 100%, 95%, 80%, and 70% ethanol. The slices were then stained with hematoxylin (2 min); rinsed with distilled water, 50% ethanol, and distilled water for 15 min; stained with eosin for 1 min; and rinsed again with distilled water. The slides were then mounted with coverslips. After HE staining, the histological alterations were examined with a DP72 microscope (Olympus, Tokyo, Japan). Three electron micrographs from the liver per individual were randomly chosen and analyzed. The morphological alterations of hepatocytes were recorded as the percentage of affected nucleus and assigned to the categories: normal, vacuolation, pyknotic nucleus, double nucleus, lytic nucleus, and enlarged nucleus based on a previous study (Xie et al., 2023).
Five primary filaments from the second right gill arch per individual were randomly chosen, and 20 lamellae per filament were blindly selected and analyzed. The morphological alterations were recorded as the percentage of affected secondary lamellae and assigned to clubbing, hyperplasia, hypertrophy, and edema based on a previous study (Jia et al. 2021). Gill morphometric analysis was conducted using the protocol of Nero et al. (2006) and Li et al. (2023). Three gill filaments per fish were measured for perimeter SLL, SLW, ID, and ID using a free software program (Measure IT, Olympus).
The proportion of the secondary lamellae available for gas exchange (PAGE) was averaged for each filament of an individual and calculated as follows:
\(PAGE=\frac{(SLL)}{(SLL+BET)}{*}_{100}\)
Three electron micrographs from the liver per individual were randomly chosen and analyzed.
Oil red and PAS staining
Oil red and PAS staining were used to analyze the hepatic lipid droplet and glycogen content. Briefly, the paraformaldehyde-fixed liver tissue sections were washed three times with phosphate-buffered saline (PBS, pH 7.4), dehydrated in serial-graded ethanol, made transparent in xylene, and embedded in paraffin. The tissue was then sliced into 20-μm and 5 μm-thick sections by a microtome (Leica RM2125 RTS, German), respectively. The 20-μm and 5 μm-thick sections were stained following the protocol of the oil red and PAS staining kit (Nanjing Jiancheng Bioengineering Institute, China), respectively. The images of slides were examined with a DP72 microscope (Olympus, Tokyo, Japan). Image J software was used to identify and quantify lipid droplets and glycogen. Lipid droplets and glycogen were stained red and purple, respectively.
RNA extraction and real-time quantitative polymerase chain reaction
A two-step, real-time quantitative polymerase chain reaction (RT-PCR) was used to measure the hif-1α, hif-2α, and hif-3α. Total RNA was extracted from the liver of three turbots by Trizol reagent (GIBCOBRL, Carlsbad, CA, USA). Meanwhile, the total RNA was treated with DNase I (Qiagen) for 30 min at 37 ℃ to avoid contaminating the genomic DNA. Subsequently, total RNA (1 μg) was reverse transcribed by a Thermo One-step RT-PCR kit according to the manufacturer’s instructions. The mRNA expression levels of hif-1α, hif-2α, and hif-3α were assessed by real-time RT-PCR using TaKaRa RT-PCR Master Mix reagent and ABI7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The primer sequences and amplified length for above genes were designed and are listed in Table 1. A 20-μL reaction volume for amplification contained 10 μL of SYBR® Premix Ex Taq™ (Takara Bio., China), 0.8 μL of each primer (10 μM), 0.4 μL of ROX dye (50 ×), 2 μL of cDNA sample (25 ng/μL), and 6 μL of sterile distilled water. Initial denaturation was conducted at 95 °C for 10 s, followed by 40 cycles at 95 °C for 5 s and at 60 °C for 30 s. For normalization, four commonly used housekeeping genes (glyceraldehyde-3-phosphate dehydrogenase (gapdh), beta-actin (β-actin), elongation factor 1-alpha (ef1-α), and 18S ribosomal RNA (18 s)) were evaluated to compare the CT values of a subset of samples. The evaluation revealed that β-actin was the most constantly stable expressed housekeeping gene (Supplemental data, S1). Thus, the expression levels of hif-1α, hif-2α, and hif-3α gene were normalized to β-actin and expressed as a folded change relative to the expression level in the control according to the 2−ΔΔCT method. The primers used for qRT-PCR are listed in Table 1. All samples were amplified in triplicates.
Statistical analysis
Shapiro–Wilk normality test and Bartlett test were used to estimate whether the data complied or not with normal distribution and homogeneity of variance, respectively (Faraway 2005). The data were analyzed via two-way analysis of variance (ANOVA) and Tukey’s multiple range tests using SAS 8.0 software. All data are presented as the means ± standard error of the mean (mean ± SEM). In all statistical tests used, P < 0.05 was considered significantly different.
Result
Survival rate and cortisol contents during hypoxia and reoxygenation
The survival rate of female and male varied slightly and remained high value during hypoxia and reoxygenation. The female and male turbot survival rate is more than 90% at T2W and T4W, and then decreases to 89% at R2W and R4W (Fig. 1C). However, no significant differences were found between the male and female turbot at control, T2W, T4W, R2W, and R4W (Fig. 1C, P > 0.05). The plasma cortisol levels varied significantly during hypoxia and reoxygenation (Fig. 2A). The cortisol levels significantly increased at T2W and T4W, with the highest values observed at T4W, and then drastically decreased after the reoxygenation treatment for 2 weeks (Fig. 2A, P < 0.05). No significant differences were found between the male and female turbot at control, T2W, T4W, R2W, and R4W (Fig. 2A, P > 0.05). Meanwhile, turbot plasma cortisol levels showed no significantly difference between R4W and the control group (Fig. 2A, P > 0.05). Plasma cortisol levels were significantly affected by treatment time but not by gender (Fig. 2A, P < 0.05). No significant interaction effect of treatment time and gender on plasma cortisol was observed (Fig. 2, P > 0.05). Meanwhile, the water amonia, nitrites, and nitrate content were lower than 0.1 mg/L in hypoxia tanks (Supplemental data, S2). The growth performance manifested no significant difference between control and treatment groups during hypoxia and reoxygenation (Supplemental data, S3).
Carbohydrate metabolism of turbot during hypoxia stress and reoxygenation. A–B Changes in plasma cortisol glucose. C–F Hepatic HK, PFK, and PK activity and lactate contents. G Hepatic glycogen. H–I PAS staining and mean density of glycogen in liver. Data are presented as means ± SEM. Bars with different superscripts are statistically different (P < 0.05, n = 9). HK, hexokinase; PFK, phosphorfrutokinase; PK, pyruvate kinase; PAS, Periodic Acid Schiff. T2W: treated for 2 weeks; T4W: treated for 4 weeks; R2W: reoxygenation 2 weeks; R4W: reoxygenation 4 weeks. NS, non-significant at P > 0.05; **P < 0.01
The alteration of glycolysis and gluconeogenesis during hypoxia and reoxygenation
The plasma glucose contents and hepatic HK, PFK, and PK activity manifested similar patterns to plasma cortisol, with the highest values at T4W during hypoxia and reoxygenation (Fig. 2B–E). Meanwhile, plasma glucose contents and hepatic HK, PFK, and PK activity showed no significant difference between R4W and the control group (Fig. 2B–E P > 0.05). Hepatic lactate content significantly decreased at T2W and T4W; the lowest values were observed at T4W, and then drastically increased after the reoxygenation treatment for 4 weeks (Fig. 2F, P < 0.05). Hepatic glycogen contents showed similar patterns to hepatic lactate, significantly decreased at T2W, T4W, and R2W (Fig. 2G, P < 0.05). PAS staining results showed a decrease in glycogen content for female fish at T2W, T4W, and R2W, and male fish remained unchanged at T2W and decreased at T4W and R2W (Fig. 2H, I, P < 0.05). No significant differences in hepatic HK, PK, and PFK activity, hepatic lactate, glycogen, and plasma glucose contents were found between the male and female turbot at control, T2W, T4W, R2W, and R4W (Fig. 2B–H, P > 0.05). The aforementioned glycolysis and gluconeogenesis-related parameters demonstrated no significant difference at R4W compared to the control group (Fig. 2, P > 0.05). There is no significant interaction effect of treatment time and gender on these parameters (Fig. 2, P > 0.05); only treatment time significantly affects these parameters during hypoxia and reoxygenation (Fig. 2, P < 0.05).
The alternation of lipid metabolism during hypoxia and reoxygenation
Hepatic FAS and LPL activity significantly increased at T2W and T4W, with the highest values observed at T4W (Fig. 3A, B, P < 0.05), and then gradually recover to normal levels after the reoxygenation treatment for 12 h and 24 h (Fig. 3A, B, P > 0.05). Female hepatic FAS activity was significantly higher than male hepatic FAS activity at T2W and T4W, whereas female hepatic LPL activity was significantly lower than male hepatic FAS activity at T2W (Fig. 3A, B, P < 0.05). No significant differences were found between the male and female turbot at control, R2W, and R4W. Oil red staining results showed significantly fewer lipid droplets in the liver at T2W, T4W, and R2W compared with the control group (Fig. 3C, D, P < 0.05). No significant difference was observed between the female and male fish at control, T2W, R2W, and R4W (Fig. 3A, B, C, P > 0.05). Treatment time and not gender significantly affected the number of hepatic lipid droplets, FAS, and LPL activity (Fig. 3C, P < 0.05). In addition, we observed no significant interaction effect of treatment time and gender on the hepatic lipid droplets, FAS, and LPL activity (Fig. 3, P > 0.05).
Lipid catabolism of turbot during hypoxia and reoxygenation. A–B Hepatic FAS and LPL activity. C–D The number of lipid droplet and Oil red O staining of hepatocyte. Data are presented as means ± SEM. Bars with different superscripts are statistically different (P < 0.05, n = 9). FAS, fatty acid synthetase; LPL, lipoprotein lipase. T2W: treated for 2 weeks; T4W: treated for 4 weeks; R2W: reoxygenation 2 weeks; R4W: reoxygenation 4 weeks. NS, non-significant at P > 0.05; **P < 0.01
Hepatic antioxidant capabilities and histological analysis
In comparison with the control group, hypoxia stress significantly increased the hepatic SOD, GSH-Px, and CAT activity at T2W and T4W (Fig. 4A–C, P < 0.05). Meanwhile, hepatic MDA contents significantly increased at T4W (Fig. 4D, P < 0.05). Histological observation manifested that hypoxia stress caused significantly higher areas of vacuolation and numbers of pyknotic, double, lytic, and enlarged nucleus in the female and male liver compared to the control group, with the highest values observed at T4W (Fig. 5A–E, P < 0.05). Meanwhile, a significantly high percentage of vacuolation, pyknotic nucleus, and lytic nucleus in the male and female liver were observed at T4W (Fig. 5F, G, P < 0.05). The above parameters returned to normal levels after reoxygenation treatment for 24 h in female and male turbots, while male and female turbots demonstrated no significant difference in these parameters throughout hypoxia stress and reoxygenation (Fig. 5, P > 0.05). Treatment time and not gender significantly affected hepatic SOD, GSH-Px, CAT activity, MDA contents, and histological alternation (Fig. 4, Fig. 5A–E, P < 0.05).
Hepatic antioxidant parameters of turbot during hypoxia and reoxygenation. A SOD activity. B GSH-Px activity. C CAT activity. D MDA levels. Data are presented as means ± SEM. Bars with different superscripts are statistically different (P < 0.05, n = 9). SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; CAT, catalase; MDA, malondialdehyde; T2W: treated for 2 weeks; T4W: treated for 4 weeks; R2W: reoxygenation 2 weeks; R4W: reoxygenation 4 weeks. NS, non-significant at P > 0.05; **P < 0.01
Change in hepatic histopathology during hypoxia and reoxygenation. Morphological change includes A vacuolation, B pyknotic nucleus, C double nucleus, D lytic nucleus, E enlarged nucleus and the percentage of each structure in F male and G female. Data are presented as means ± SEM. Bars with different superscripts are statistically different (P < 0.05, n = 9)
Gill histomorphology, respiratory frequency, and PAGE
The changes of turbot gill histology were observed during hypoxia and reoxygenation. The lamellar perimeter, SLW, SLL, ID, and BET measurements are shown in Fig. 6A. Lamellar perimeter, SLL, and ID significantly increased at T2W and T4W (Fig. 6B, D, and E, P < 0.05) with the highest levels observed at T4W. Then, the levels decreased at R2W and returned to normal at R4W during hypoxia and reoxygenation. By contrast, SLW significantly decreased at T2W and T4W, and the lowest value was obtained at T4W (Fig. 6C, P < 0.05). Subsequently, it drastically increased at R2W and recovered to nearly normal levels at R4W (Fig. 6C, P > 0.05). Interestingly, the value of male SLL was significantly higher than that of female SLL at T2W, T4W, and R2W, whereas there was no significant difference between the control and R4W. Respiratory frequency and PAGE manifested similar results to ID during hypoxia and reoxygenation (Fig. 6F, G). Hypoxia stress significantly increased the respiratory frequency and PAGE at T2W, T4W, and R2W, the highest value observed at T4W (Fig. 6F, G, P < 0.05), and then recovered to normal at R4W (Fig. 6F, G, P > 0.05). The male and female fish showed no difference in perimeter, SLL, ID, respiratory frequency, and PAGE during hypoxia and reoxygenation (Fig. 6A, D, E, F, G, P > 0.05). Treatment time significantly affected these parameters (Fig. 6B–G, P < 0.05), and the significant interaction effect of treatment time and gender on SLW and ID was observed during hypoxia and reoxygenation (Fig. 6C, E, P < 0.05). However, perimeter, SLL, respiratory frequency, and PAGE were not affected by gender (Fig. 6B, D, F, G, P > 0.05). Lamellar clubbing, hyperplasia, and hypertrophy were the main histological alterations of gill filaments during hypoxia and reoxygenation (Fig. 6H). The number of female and male turbot gill filaments significantly increased in terms of the percentage of lamellar clubbing hyperplasia hypertrophy at T2W and T4W, then gradually decreased at R2W, and recovered to nearly normal at R4W (Fig. 6I, J).
Gill histological alternation of turbot during hypoxia and reoxygenation. A Normal gill histology. B–G Perimeter, SLW, SLL, ID, respiratory frequency, and PAGE. H–J Histological alteration and proration of secondary gill filaments of and female and male turbot. Data are presented as means ± SEM. Bars with different superscripts are statistically different (P < 0.05, n = 9). SLL, secondary lamellar length; ID, interlamellar distance; SLW, secondary lamellar width; BET, basal epithelial thickness; PAGE: The proportion of the secondary lamellae available for gas exchange; T2W: treated for 2 weeks; T4W: treated for 4 weeks; R2W: reoxygenation 2 weeks; R4W: reoxygenation 4 weeks. NS, non-significant at P > 0.05; **P < 0.01
Gene expression
Compared with the control group, hypoxia stress significantly increased the mRNA levels of hepatic hif1-α, hif2-α, and hif3-α at T2W, T4W, and R2W, the highest value obtained at T4W (Fig. 7A–C, P < 0.05), which returned to nearly normal levels at R4W (Fig. 7A–C, P > 0.05). In addition, hif1-α was significantly higher than hif2-α and hif3-α at T4W (Fig. 7D, P < 0.05). No significant differences of hepatic hif1-α, hif2-α, and hif3-α were found between the male and female turbot at control, T2W, T4W, R2W, and R4W throughout hypoxia and reoxygenation (Fig. 7A–C, P > 0.05).
Relative hif-1α, hif-2α, and hif-3α mRNA expression levels in liver of turbot during hypoxia and reoxygenation. A–C Hepatic hif-1α, hif-2α, and hif-3α mRNA in male and female turbot under hypoxia and reoxygenation. C Heatmap of hif-1α, hif-2α, and hif-3α mRNA expression. Bars with different superscript are statistically different (P < 0.05, n = 9)
Discussion
This study first represents the physiological response of female and male turbot subject to long-term chronic hypoxia stress and reoxygenation. Long-term chronic hypoxia significantly increases the plasma cortisol and glucose levels in the current study. Similar results were reported in turbot (Pichavant et al. 2002), crucian carp (Sula and Aliko 2017), Nile tilapia (Li et al. 2018), rainbow trout (Léger et al. 2021), and golden pompano (Jiang et al. 2023) in response to hypoxia stress. Hypoxia could activate anaerobic glycolysis and break down glucose into pyruvate by glycolytic key rate-limiting enzymes including HK, PFK, and PK, which transported to the mitochondria oxidized to acetyl CoA and entered the TCA cycle and produced ATP (Lyssiotis and Kimmelman 2017; Farhat et al. 2021). Meanwhile, glycogen as an essential energy source was consumed to provide sufficient glucose used for hypoxia-induced anaerobic metabolism in numerous fish species (Jiang et al. 2023; Oliveira et al. 2004; Bacca et al. 2005). Long-term chronic hypoxia stress significantly stimulated turbot hepatic HK, PFK, and PK activity and decreased the hepatic glycogen contents in the current study. These results suggest hypoxia stress activate turbot anaerobic glycolysis and stimulate glycogen degradation to maintain energy supply. Lactate levels are usually upregulated in a time-dose manners subject to hypoxia stress in fish (Pichavant et al 2002; Weber et al. 2016; Yang et al. 2019). Interestingly, turbot hepatic lactate content significantly decreased under long-term chronic hypoxia stress treatment for 2 and 4 weeks in this study. Fagernes et al. (2017) reported pyruvate dehydrogenase enzyme catalyzes the conversion of pyruvate to acetaldehyde under hypoxia conditions, and then alcohol dehydrogenase undertakes the conversion of acetaldehyde to ethanol, which is swiftly eliminated through the gills. Therefore, we speculate turbot hepatic lactate could be converted into ethanol by uncertain pathways and avoid lactate acidosis under long-term hypoxia stress. Meanwhile, no significant differences in sex dimorphism of these parameters were found throughout long-term hypoxia stress in the current study. These findings suggest female and male turbot take similar anaerobic glycolysis mechanisms and provide sufficient energy to cope with long-term hypoxia stress.
Lipids are also energy storage materials that supply energy to the body for the growth and development of vertebrates (Jean-Michel 2011). FAS is the main lipogenic enzyme that produces fatty acids, and LPL hydrolyzes triglycerides in plasma lipoproteins and supplies free fatty acids for storage or oxidation (Salmerón, 2018). Lipid droplets (LDs) are intracellular organelles specialized for the storage of energy in the form of neutral lipids and are involved in lipid metabolism (Welte and Gould 2017). In general, the fatty acid synthesis pathway is inhibited, whereas fatty acid β-oxidation is increased to release ATP to meet energy demands under long-term hypoxia status (Wang et al. 2012; Zhao et al. 2020). In this study, hypoxia stress significantly stimulates hepatic LPL activity. The elevated LPL content indicated that the oxidative energy supply of triglycerides was activated during long-term hypoxia stress in turbot. In addition, fewer lipid droplets were observed after turbot treatment for 2 and 4 weeks. Female hepatic LPL activity and lipid droplets are lower than those of males, but female hepatic FAS activity is higher than that of males. These results suggest lipids were involved in the long-term hypoxia-induced energy metabolism process in turbot, males activate more lipolysis than females. Li et al (2018) reported acute hypoxia stress mainly stimulates glycolysis, and long-term hypoxia stress increases lipolysis in Nile tilapia. However, there is a decrease in lipolytic activity of common carp. Thus, these results and glycolysis data suggested both lipids and glucose metabolism are synchronously involved in the regulation of the energy supply process and guarantee efficiency enough to produce energy during turbot long-term hypoxia stress.
Hypoxia could induce and produce excessive ROS and oxidative stress, impair the antioxidant oxidative system, cause DNA damage, lipid peroxidation, and severe damage to cellular structure (Wang et al. 2023). Fish’s defensive antioxidant system maintains the dynamic balance of the production and elimination of intracellular free radicals, and protects cells from oxidant stress by enhancing the antioxidant capacity to actively respond to hypoxia stress (Lushchak et al. 2011). MDA is a strong biotoxin and damages the cell structure and functions as the main component of lipid peroxides. We found the percentage of hepatic MDA levels, vacuolation, pyknotic, and lytic nucleus significantly increased during long-term hypoxia stress in the current study. The appearance of abnormal hepatocyte and MDA levels suggests long-term hypoxia stress that resulted in the hepatic oxidative damage of turbot. However, fish have evolved “the preparation for oxidative stress” response strategy via activation of the antioxidant system and attenuates the hypoxia-induced negative effects (Welker et al. 2013). The activity of hepatic SOD, CAT, and GSH-Px significantly increased after hypoxia stress, indicating that the turbot-activated antioxidant abilities cope with oxidative stress in the current study. Similar results were reported in black rockfish, large yellow croaker (wang et al. 2017), and other fish species (Wang et al. 2023). Female and male fish manifested similar results; all parameters returned to normal condition after reoxygenation treatment for 4 weeks. Thus, long-term hypoxia induces hepatic oxidative stress, alters antioxidant capabilities, impairs hepatocyte structure, and affects its physiological function in turbot, with no significant differences in sex dimorphism.
Hypoxia stress significantly caused the gill morphological alternation in numerous farming fish species including black rockfish (Jia et al. 2021), scaleless carp (Chen et al. 2022), and rohu Labeo rohita. In the present study, lamellar clubbing, hypertrophy, hyperplasia, and edema occurred after hypoxia treatment for 2 and 4 weeks, and their proportions gradually decreased and nearly recovered to the normal state after reoxygenation for 4 weeks. Mallatt (1985) reported lamellar hyperplasia could increase the epithelial area for oxygen diffusion between the blood and external medium as a defense mechanism. Fishes could regulate their functional respiratory surface area by changing a fraction of the total lamellar area perfused with blood. Hypoxia stress can greatly increase the respiratory surface area and stimulate the capacity for oxygen uptake in crucian carp and goldfish. Similar results have been found in hypoxia-sensitive blunt snout bream and black rockfish (Jia et al. 2021). Fish stimulate an immediate increase in ventilation volume via an increase in ventilation rate and/or amplitude in response to hypoxia stress (Perry et al. 2009). Meanwhile, the fish will continue branchial respiration by positioning their mouths to skim the air/water interface, which is richer in oxygen. In the current study, long-term hypoxia stress significantly increased lamellar perimeters, SLL, ID, respiratory frequency, and PAGE. These results indicated that hypoxia stress induced lamellar morphological alternation, stimulated respiratory frequency and PAGE, helped stimulate oxygen uptake capacity, and alleviated hypoxia stress in turbot.
The primary transcriptional regulator of oxygen homeostasis in response to hypoxic stress is mediated by the hypoxia-inducible factors (HIFs) in mammals and teleost (Majmundar et al. 2010; Pelster and egg 2018; Luo et al. 2023). HIF-1α has been best characterized and recognized as a master regulator of hypoxic signaling in numerous fish species (Xiao 2015). Terova et al. (2008) found the hepatic hif-1α mRNAs were significantly upregulated under both acute (1.9 mg/L DO for 4 h) and chronic (4.3 mg/L DO for 15 days) hypoxia situations in sea bass. Similar results were reported in Eurasian perch (Rimoldi et al. 2012), largemouth bass (Sun et al. 2020), and golden pompano (Jiang et al. 2023). In the present study, hepatic hif-1α, hif-2α, and hif-3α mRNAs were significantly upregulated under long-term hypoxia exposure; hif-1α obtained the highest values, which returned to basal values after reoxygenation 4 weeks. Meanwhile, the mRNA levels of female hif-1α, hif-2α, and hif-3α are significantly higher than those of males under long-term hypoxia stress. These results suggested that hif-1α is the main isoform involved in the regulation of hypoxia-induced metabolic adjustments and maintaining homeostasis. Combining the above hepatic FAS, LPL activity, and Oil red data, we speculate the different roles of HIF-1α for male and female fish exist in the regulation of genes involved in lipid metabolism. However, the detailed mechanism needs to be further studied.
In summary, long-term hypoxia stress elevated plasma cortisol and glucose contents; increased hepatic HK, PK, PFK, FAS, and LPL activity; decreased hepatic glycogen contents and lipid drop numbers; caused pathological changes of hepatocyte and oxidative stress; induced alterations in gill morphology; stimulated respiratory frequency and PAGE; and upregulated the transcription of hepatic hif-1α, hif-2α, and hif-3α in turbot. The above parameters recovered to normal levels after reoxygenation treatment for 4 weeks. Interestingly, females and males demonstrated no significantly difference of these parameters (except hepatic FAS, LPL activity, and lipid drop number) during hypoxia. These results suggested turbot promotes hepatic glycogenolysis and lipolysis, stimulates hepatic antioxidant capacity, reversibly remodels the gill morphology, and initiates a positive physiological response to satisfy long-term hypoxia stress-induced energy demand and alleviate potential damage. Furthermore, hif-1α plays key roles throughout the above physiological process under hypoxia and reoxygenation in turbot. These findings and our previous studies (Jia et al. 2021; Li et al. 2023) provide more data for fully understanding the mechanism of turbot hypoxia tolerance and helping for guide us in the breeding of hypoxia-tolerant species/strains.
Data availability
Not applicable.
No datasets were generated or analysed during the current study.
Code availability
Not applicable.
References
Abdel-Tawwab M, Monier MN, Hoseinifar SH, Faggio C (2019) Fish response to hypoxia stress: growth, physiological, and immunological biomarkers. Fish Physiol Biochem 45(3):997–1013
Bacca H, Huvet A, Fabioux C, Daniel JY, Delaporte M, Pouvreau S, Wormhoud AV, Moal J (2005) Molecular cloning and seasonal expression of oyster glycogen phosphorylase and glycogen synthase genes. Comp Biochem. Physiol Part B Biochem Mol. Biol 140(4):635–646
Baltazar-Soares M, Lima ARA, Silva G, Gaget E (2023) Towards a unified ecoevolutionary framework for fisheries management: coupling advances in next generation sequencing with species distribution modelling. Front Mar Sci 9:1–9
Bashir I, Lone F, Bhat RA, Mir SA, Dar ZA, Dar SA (2020) Concerns and threats of contamination on aquatic ecosystems. In: Bioremediation and Biotechnology, Springer, pp 1–26
Breitburg D, Levin LA, Oschlies A, Gr´egoire M, Chavez FP, Conley DJ et al (2018) Declining oxygen in the global ocean and coastal waters. Science 359:6371
Chen FJ, Ling X, Zhao YT, Fu SY (2022) Hypoxia-induced oxidative stress and apoptosis in gills of scaleless carp (Gymnocypris przewalskii). Fish Physiol Biochem 48:911–924
Choudhry H, Harris AL (2018) Advances in hypoxia-inducible factor biology. Cell Metab 27(2):281–298
Fagernes CE, Stensløkken KO, Røhr ÅK, Berenbrink M, Ellefsen S, Nilsson GE (2017) Extreme anoxia tolerance in crucian carp and goldfish through neofunctionalization of duplicated genes creating a new ethanol-producing pyruvate decarboxylase pathway. Sci Rep 7(1):7884
Faraway JJ (2005) Linear models with R. Chapman & Hall/CRC, Boca Raton
Farhat E, Cheng H, Romestaing C, Pamenter M, Weber JM (2021) Goldfish response to chronic hypoxia: mitochondrial respiration, fuel preference and energy metabolism. Metabolites 11(3):187
Heinrichs-Caldas W, Campos DF, Paula-Silva MN, Almeida-Val VMF (2019) Oxygen-dependent distinct expression of hif-1α gene in aerobic and anaerobic tissues of the Amazon Oscar. Astronotus Crassipinnis Comp Biochem Physiol B 227:31–38
Jean-Michel W (2011) Metabolic fuels: regulating fluxes to select mix. J Exp Biol 214:286–294
Jia YD, Lei JL (2019) Molecular function of gonadotrophins and their receptors in the ovarian development of turbot (Scophthalmus maximus). Gen Comp Endocrinol 277(17):19
Jia YD, Wang JW, Gao YT, Huang B (2021) Hypoxia tolerance, hematological, and biochemical response in juvenile turbot (Scophthalmus maximus L). Aquaculture 535:736380
Jiang T, Sun JL, Gu Y, Yao FC, Liang YS, Liu YF, Zhang KX, Song FB, Zhou L, Wang ZW, Gui JF, Luo J (2023) Hypoxia alters glucose and lipid metabolisms in golden pompano (Trachinotus blochii). Aquaculture 562:738747
Kumar H, Choi DK (2015) Hypoxia inducible factor pathway and physiological adaptation: a cell survival pathway? Mediators Inflamm 2015:584758
Laffoley D, Baxter JM (2019) Ocean deoxygenation: everyone’s problem: causes, impacts, consequences and solutions, Gland, Switzerland, IUCN
Léger JAD, Athanasio CG, Zhera A, Chauhan MF, Simmons DBD (2021) Hypoxic responses in Oncorhynchus mykiss involve angiogenesis, lipid, and lactate metabolism, which may be triggered by the cortisol stress response and epigenetic methylation. Comp Biochem Physiol Part D Genom Proteom 39:100860
Levin LA, Breitburg DL (2015) Linking coasts and seas to address ocean deoxygenation. Nat Clim Chang 5(5):401–403
Li MX, Wang XD, Qi CL, Li E, Du ZY, Qin JG, Chen LQ (2018) Metabolic response of Nile tilapia (Oreochromis niloticus) to acute and chronic hypoxia stress. Aquaculture 495:187–195
Li FX, Gao YT, Wang JW, Xie T, Zhang JR, Jia YD (2023) Gender differences in the hematology, hepatic antioxidant capacity, and gill histology of turbot (Scophthalmus maximus) under hypoxic stress. J Fish Sci China 30(7):878–890
Limburg KE, Breitburg D, Swaney DP, Jacinto G (2020) Ocean deoxygenation: a primer. One. Earth 2(1):24–29
LuoLiSang MTH (2023) The role of hypoxia-inducible factor 1α in hepatic lipid metabolism. J Mol Med 101:487–500
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Lyssiotis CA, Kimmelman AC (2017) Metabolic interactions in the tumor microenvironment. Trends Cell Biol 27:863–875
Majmundar AJ, Wong WJ, Simon MC (2010) Hypoxia inducible factors and the response to hypoxic stress. Mol Cell 22(40):294–309
Nikinmaa M, Rees BB (2005) Oxygen-dependent gene expression in fishes. Am J Physiol Regul Integr Comp Physiol 288:R1079–R1090
Oliveira GT, Rossi IC, Kucharski LC et al (2004) Hepatopancreas gluconeogenesis and glycogen content during fasting in crabs previously maintained on a high-protein or carbohydrate-rich diet. Compar Biochem Physiol Part A 137(2):383–390
Pelster B, Egg M (2018) Hypoxia-inducible transcription factors in fish: expression, function and interconnection with the circadian clock. J Exp Biol 221(13):jeb163709
Perry SF, Jonz MG, Gilmour KM (2009) Oxygen sensing and the hypoxic ventilatory response. In: Richards JG, Farrell AP, Brauner CJ (eds) Hypoxia. Academic Press, London, pp 193–253
Pichavant, K., Maxime, V., Th´ ebault, M.T., Ollivier, H., Garnier, J.P., Bousquet, B., Diouris, M., Boeuf, G., Nonnotte, G. 2002. Effects of hypoxia and subsequent recovery on turbot Scophthalmus maximus: hormonal changes and anaerobic metabolism. Mar. Ecol. Prog. Ser. 225, 275-285.
Rimoldi S, Terova G, Ceccuzzi P, Marelli S, Antonini M, Saroglia M (2012) HIF-1a mRNA levels in Eurasian perch (Perca fluviatilis) exposed to acute and chronic hypoxia. Mol Biol Rep 39:4009–4015
Saha N, Koner D, Sharma R (2022) Environmental hypoxia: a threat to the gonadal development and reproduction in bony fishes. Aqua Fish 7:572–582
Salmerón C (2018) Adipogenesis in fish. J Exp Biol 221:1–18
Sarkar B, Islam A (2020) Drivers of water pollution and evaluating its ecological stress with special reference to macrovertebrates (fish community structure): a case of Churni River. India Environ Monit Assess 192:1–31
Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148:399–408
Stevens PW, Blewett DA, Casey JP (2006) Short-term effects of a low dissolved oxygen event on estuarine fish assemblages following the passage of hurricane Charley. Estuaries Coasts 29(06):997–1003
Sula E, Aliko V (2017) Effects of stressors on hematological and immunological response in the fresh water crucian carp fish (Carassius carassius). Albanian J Agric Sci S1:583–590
Sun JL, Zhao LL, Wu H, Liu Q, Liao L, Luo J, Lian WQ, Cui C, Jin L, Ma JD, Li MZ, Yang S (2020) Acute hypoxia changes the mode of glucose and lipid utilization in the liver of the largemouth bass (Micropterus salmoides). Sci Total Env 713:135157
Terova G, Rimoldi S, Corà S, Bernardini G, Gornati R, Saroglia M (2008) Acute and chronic hypoxia affects HIF-1α mRNA levels in sea bass (Dicentrarchus labrax). Aquaculture 279:150–159
Timmerman CM, Chapman LJ (2004) Behavioral and physiological compensation for chronic hypoxia in the sailfin molly (Poecilia latipinna). Physiol Biochem Zool 77:601–610
Tomasetti SJ, Gobler CJ (2020) Dissolved oxygen and pH criteria leave fisheries at risk. Science 368:372–373
Wang X, Wang L, Yao C, Qiu L, Zhang H, Zhi Z, Song L (2012) Alternation of immune parameters and cellular energy allocation of Chlamys farreri under ammonia-N exposure and vibrio anguillarum challenge. Fish Shellfsh Immunol 32(5):741–749
Wang QF, Shen WL, Hou CC, Liu C, Wu XF, Zhu JQ (2017) Physiological responses and changes in gene expression in the large yellow croaker Larimichthys crocea following exposure to hypoxia. Chemosphere 169:418–427
Wang C, Wu X, Hu X et al (2020) Hypoxia-inducible factor 1α from a high-altitude fish enhances cytoprotection and elevates nitric oxide production in hypoxic environment. Fish Physiol Biochem 46(01):39–49
Wang ZX, Pu D, Zheng J, Li P, Lü H, Wei X, Li M, Li D, Gao L (2023) Hypoxia-induced physiological responses in fish: from organism to tissue to molecular levels. Ecotoxicol Environ Safe 267:115609
Weber JM, Choi K, Gonzalez A, Omlin T (2016) Metabolic fuel kinetics in fish: swimming, hypoxia and muscle membranes. J Exp Biol 219:250–258
Welker AF, Moreira DC, Campos EG, Hermes-Lima M (2013) Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp Biochem Physiol A Mol Integr Physiol 165:384–404
Welte MA, Gould AP (2017) Lipid droplet functions beyond energy storage. BBA- Mol Cell Biol Lip 1862:1260–1272
Xiao WH (2015) The hypoxia signaling pathway and hypoxic adaptation in fishes. Sci China Life Sci 58(2):148–155
Xie T, Gao Y, Qin H, Zhang J, Li M, Gao Y, Guan C, Jia Y (2023) Physiological response of spotted knifejaw (Oplegnathus punctatus) during transportation in offshore aquaculture net pen. Aquaculture 563:739029
Yang S, Yan T, Wu H, Xiao Q, Fu HM, Luo J, Zhou J, Zhao LL, Wang Y, Yang SY, Sun JL, Ye X, Li SJ (2017) Acute hypoxic stress: effect on blood parameters, antioxidant enzymes, and expression of HIF-1 alpha and GLUT-1 genes in largemouth bass. (Micropterus salmoides). Fish Shell Immunol 67:449–45
Yang S, Wu H, He K, Yan T, Zhou J, Zhao LL et al (2019) Response of AMP activated protein kinase and lactate metabolism of largemouth bass (Micropterus salmoides) under acute hypoxic stress. Sci Total Environ 666:1071–1079
Zeng L, Ai CX, Zhang JS et al (2020) Pre-hypoxia exposure inhibited copper toxicity by improving energy metabolism, antioxidant defence and mitophagy in the liver of the large yellow croaker Larimichthys crocea. Sci Total Environ 708:134961
Zhan Y, Ning B, Sun J, Chang Y (2023) Living in a hypoxic world: a review of the impacts of hypoxia on aquaculture. Mar Pollut Bull 194:115207
Zhao LL, Sun JL, Liang J, Liu Q, Luo J, Li ZQ, Yang S (2020) Enhancing lipid metabolism and inducing antioxidant and immune responses to adapt to acute hypoxic stress in Schizothorax prenanti. Aquaculture 519:734933
Acknowledgements
We sincerely thank Wenlei Gao (Tianyuan Aquatic Co., Ltd.) for providing technical help during the experiments.
Funding
This study was supported by the Central Public-interest Scientific Institution Basal Research Fund, YSFRI, CAFS (2023TD81, 20603022020008, 20603022023018).
Author information
Authors and Affiliations
Contributions
Jia Yudong: conceptualization; supervision; writing—original draft; reviewing and editing; project administration; validation; funding acquisition. Feng Wang: methodology, investigation, software, data curation, formal analysis, visualization. Shuaiyu Chen: investigation, formal analysis; Jiawei Wang: investigation, software, formal analysis. Yuntao Gao: investigation, methodology. All authors approved the final version of the revised manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
All procedures were conducted according to the guidelines established by the Institutional Animal Care and Use Committee of the Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Science.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, Y., Wang, F., Chen, S. et al. Long-term hypoxia-induced physiological response in turbot Scophthalmus maximus L. Fish Physiol Biochem (2024). https://doi.org/10.1007/s10695-024-01398-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10695-024-01398-3