Abstract
We examined neuronal responses of hypothalamic melanin-concentrating hormone (MCH) and corticotropin-releasing hormone (CRH) to background color in the self-fertilizing fish, Kryptolebias marmoratus. Fish were individually reared in lidless white or black cylindrical plastic containers for 15 days. The number of MCH-immunoreactive (ir) cell bodies in the nucleus lateralis tuberis (NLT) of the hypothalamus was significantly greater in the white-acclimated fish, while no significant differences were observed in the nucleus anterior tuberis (NAT) of the hypothalamus. Significant differences were not seen in the number of CRH-ir cell bodies in the NLT between the groups. The body of the white- and black-acclimated fish appeared lighter and darker, respectively, compared with the baseline color. In the black-acclimated fish, feeding activity was significantly greater with a tendency toward higher specific growth rate compared with the observations in white-acclimated fish. No significant inter-group cortisol level differences were observed. These results indicate that background color affects MCH neuronal activity in the NLT as well as body color adaptation but does not affect CRH neuronal activity in K. marmoratus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Background color has been suggested to influence body color in some teleost fish species (see Barton 2002). The body color of some teleost fish such as goldfish Carassius auratus (Cerdá-Reverter et al. 2006), tilapia Oreochromis mossambicus (Gröneveld et al. 1995), and barfin flounder Verasper moseri (Yamanome et al. 2007) becomes paler against a white background than against a black background. This phenomenon is induced by melanin-concentrating hormone (MCH). MCH was first found in the pituitary of the chum salmon Oncorhynchus keta, as a substance that made the skin color pale by concentrating melanin granules (Kawauchi et al. 1983). In general, MCH is produced in the hypothalamus, released from the pituitary, and reaches melanophores (Amano and Takahashi 2009). Indeed, MCH expression levels in the brain were higher in fish reared against white backgrounds than in those reared against black backgrounds (Gröneveld et al. 1995; Takahashi et al. 2004, 2007; Cerdá-Reverter et al. 2006). MCH projections inside the brain (i.e., those that do not project to the pituitary) are considered to be involved in the regulation of food intake in teleost fish (Amano and Takahashi 2009).
Background color has also been suggested to influence stress response in some teleost fish species (see Barton 2002). For example, plasma cortisol levels of the juvenile carnivorous freshwater catfish Lophiosilurus alexandri were higher when reared against a black background than against yellow, brown, and blue backgrounds (Costa et al. 2017). Plasma cortisol levels in goldfish were higher against a red background than against white and black backgrounds (Eslamloo et al. 2015). Since cortisol secretion is controlled by the hypothalamo-pituitary-interrenal (HPI) axis (Pankhurst 2011), it can be assumed that background color also affects corticotropin-releasing hormone (CRH) levels in the brain. We have recently found that, in the juvenile Japanese eel Anguilla japonica, hypothalamic CRH mRNA levels tended to be and plasma cortisol levels were significantly higher in the white-acclimated fish than in the black-acclimated fish (Amano et al. 2021).
Assuming that background color affects body color change and stress responses, MCH and CRH levels would be influenced by the background color. However, to date, neuronal responses of MCH and CRH to background color have not been reported in teleost fish.
The mangrove killifish Kryptolebias marmoratus (Cyprinodontiformes) is known to use self-fertilization for reproduction (Harrington 1961). Recently, K. ocellatus, the sister taxon of K. marmoratus, has also been reported as a self-fertilizer (Avise and Tatarenkov 2015). K. marmoratus thrives in wide ranges of water temperature, salinity, and dissolved oxygen and ammonia contents (see Taylor 2012), indicative of its high stress tolerance. We have recently found that the number of CRH-ir cell bodies in the hypothalamus and the levels of cortisol in the body were both increased following the induction of crowding stress in this fish (Amano et al. 2022).
The purpose of the present study was to elucidate the neuronal responses of MCH and CRH in the hypothalamus to background color in K. marmoratus. Since the distribution of CRH-immunoreactive (ir) cell bodies and fibers in the brain and pituitary has already been reported (Amano et al. 2022), in this study, we first examined the distribution of MCH-ir cell bodies and fibers by immunohistochemistry. Next, to clarify the neuronal responses of MCH and CRH in the hypothalamus to background color, dual-label immunohistochemistry was performed. Finally, fish were reared under white or black background conditions for 15 days, and the hypothalamic MCH-ir and CRH-ir cell body counts, body color, somatic growth, feeding activity, and cortisol levels were compared between white- and black-acclimated fish.
Materials and methods
Fish
Hermaphrodites from a single clonal lineage of K. marmoratus originated from a single fish sampled in Dangriga, Belize, and has been maintained for more than 10 generations at the Aquaculture Biology Laboratory at Nagasaki University (Kanamori et al. 2016) were used. All fish were held individually in translucent cylindrical plastic containers with 60 mL of brackish water (17 ppt) from birth and were reared under a controlled photoperiod (LD 14:10) and temperature (25 °C).
Immunohistochemistry for MCH
Underyearling and three-years old fish (total length (TL); 12.0–21.4 mm, standard length (SL); 10.5–18.0 mm, body weight (BW); 15–78 mg) were anesthetized by immersion in 0.05% 2-phenoxyethanol. Head regions without the lower jaw were separated and immediately fixed with Bouin’s fluid for 24 h at 4 °C. Subsequently, they were rinsed in cold 70% ethanol, dehydrated through a graded series of ethanol concentrations, cleared in benzene, and embedded in Surgipath Paraplast Plus (Leica Biosystems, Nussloch, Germany). Serial sagittal or frontal sections were cut at 8 or 10 μm, respectively, and mounted on adhesive glass slides (CRE-01, Matsunami, Osaka, Japan).
Immunohistochemistry was conducted according to Amano et al. (2016), using a rabbit polyclonal antibody against salmon MCH (Kawauchi et al. 1983). The mRNA sequences encoding MCH (MCH1 and MCH2) in the K. marmoratus have recently been updated in the NCBI database (NCBI Reference Sequence: XM_017438552.3, XM_017411545.3). The deduced amino acid sequence of K. marmoratus MCH1 is DTMRCMVGRVYRPCWEV (17 amino acid residues) and those of MCH2 is DKDLDMLRCMIGRVYRPCWEA (21 amino acid residues). K. marmoratus MCH1 is identical to salmon MCH (Kawauchi et al. 1983), and the sequence identity of K. marmoratus MCH2 with K. marmoratus MCH1 is 66.7%. Therefore, it is likely that the anti-MCH antibody recognizes K. marmoratus MCH1. The sections were incubated with the antibody diluted 4,000-fold with 0.1 M phosphate buffer (pH 7.4) containing 0.75% NaCl, 0.3% Triton X-100, and 0.02% bovine serum albumin (SP-5050; Vector Laboratories Inc., Burlingame, CA, USA) overnight at 4 °C. A Histofine immunostaining kit (SAB-PO (R), Nichirei Biosciences, Tokyo, Japan) was used for immunohistochemical reactions. MCH immunoreactivities were visualized by 3,3’-diaminobenzidine tetrahydrochloride (DAB, brown). Some frontal sections were counterstained with cresyl violet for the histological identification of nuclear boundaries. We referred the terminology for brain nuclei of Peter and Gill (1975) and Wullimann et al. (1996).
To confirm the specificity of the immunohistochemical reactions for MCH, control sections were incubated in antisera that had been pre-absorbed overnight at 4 °C with an excess amount of synthetic salmon MCH (25 μg MCH in 1 ml of diluted antiserum). The subsequent method was identical to that used for the experimental sections.
Dual-label immunohistochemistry for MCH and CRH
To clarify the neuronal responses of MCH and CRH and to count the number of cell bodies immunoreactive to MCH and CRH in the hypothalamus, dual-label immunohistochemistry was developed according to Amano et al. (2016, 2022), using the rabbit polyclonal antibody against salmon MCH (diluted 4,000-fold) (Kawauchi et al. 1983) and rabbit polyclonal antibody raised against human/mouse/rat CRH (diluted 1,000-fold) (Cat. # AB-02, Advanced Targeting Systems, San Diego, CA, USA). Immunoreactivities of MCH and CRH were visualized by DAB (brown) and nitro blue tetrazolium chloride, and 5-bromo-4-chloro-3-indolyl phosphate, toluidine salt (NBT/BCIP, blue), respectively.
Rearing experiment
Experimental fish and rearing conditions
Three-years old fish about 21 mm of TL, 17 mm of SL and 67 mg of BW were used. The rearing experiment was started on June 15 (day 0). First, to prevent the effects of anesthetization, representative fish were moved to a laboratory dish (5 × 5 × 2 cm) filled with about 10 mL of brackish water (approximately 5 mm height) and then photographed immediately from above. Next, 16 fish were anesthetized by immersion in 1.5% 2-phenoxyethanol. TL, SL and BW were measured, and the condition factor (CF) was calculated as BW (g) / SL (cm)3 × 1000. Then, fish were randomly moved to lidless white or black cylindrical plastic containers (one fish per container, eight containers each) which contained 60 mL of brackish water (17 ppt). Subsequently, fish were reared under the same conditions as described above until June 30 (day 15) by feeding to satiation with Artemia franciscana nauplii. One fish in the black-acclimated group died on day 3.
Measurement of food intake
Measurement of food intake was conducted on June 24 (day 9). The fish were moved to concolorous cylindrical plastic containers filled with 60 mL of fresh brackish water (17 ppt) containing a certain number (200 individuals) of A. franciscana nauplii. Ten minutes later, fish were again moved to another concolorous cylindrical plastic containers filled with 60 mL of fresh brackish water (17 ppt). The number of A. franciscana nauplii eaten in 10 min was calculated by subtracting the number of uneaten nauplii from that of initial number (200) of nauplii. Then, the fish were again fed A. franciscana nauplii until satiation.
Fish sampling
Fish were sampled on June 30 (day 15). Photographs of representative fish were taken as described above. Then, fish were anesthetized by immersion in 1.5% 2-phenoxyethanol. TL, SL and BW were measured, and the CF was calculated. Head regions without the lower jaw were separated and immediately fixed with Bouin’s fluid for 24 h at 4 °C for dual-label immunohistochemistry for MCH and CRH. The remaining body (without head region) was weighed and put into a 1.5 mL plastic tube and stored at -80 °C until analysis for cortisol. Specific growth rate (SGR) was calculated as follows: SGR (%) = {ln (final BW)–ln (initial BW)} × 100/day.
Counting of MCH-ir and CRH-ir cell bodies in the hypothalamus
Dual-label immunohistochemistry for MCH and CRH was conducted as described above. The number of MCH-ir cell bodies in the nucleus lateralis tuberis (NLT) and the nucleus anterior tuberis (NAT) of the hypothalamus, and CRH-ir cell bodies in the NLT of the hypothalamus was separately counted according to Abercrombie (1946) and Amano et al. (2022). Since MCH-ir cell bodies in the NLT tend to form tight clusters, counting the number of MCH-ir cell bodies is difficult as Cánepa et al. (2006) reported. Thus, in some sections, we measured the nuclear area of labeled MCH-ir cell bodies, and the number of MCH-ir cell bodies was calculated by dividing the nuclear area of labeled MCH-ir cell bodies in average area of single MCH-ir cell body (77.5 μm2) using the Image J software provided free of charge by National Institute of Health, USA, via the Internet. We eliminated two fish (one fish per each group), because the sections were in poor condition.
Cortisol measurement
Cortisol levels in the body without head region were measured by a time-resolved fluoroimmunoassay (TR-FIA) for cortisol (Yamada et al. 2002), as described in detail in Amano et al. (2022). Briefly, the samples were sonicated in an assay buffer, and the homogenate was centrifuged at 10,000 g for 30 min at 4 °C. Cortisol was extracted from the resulting supernatant using diethyl ether. For TR-FIA, cortisol-BSA conjugate was immobilized in the wells of a 96-well microtiter plate at 4 °C overnight. After three washes with 0.9% saline, the wells were blocked with 0.1% BSA at room temperature for 1 h. After three washes, the standard or extracted samples and anti-cortisol serum were dispensed into the wells. After the immunoreaction at 4 °C overnight and three washes, europium (Eu)-labeled anti-rabbit IgG goat IgG (Eu-IgG), was added to the wells, and the plate was shaken at room temperature for 1 h. Eu was dissociated from the steroid complex, primary antibody, and Eu-IgG by adding an enhancement solution, and the intensity of Eu was measured using a time-resolved fluorometer (Infinite F500, Tecan Austria GmbH, Austria).
Statistical analysis
Data were tested for normality by Kolmogorov–Smirnov test and for equal variance by Bartlett test. Then, data between two groups were compared: Student’ t-test for the parametric data with equal variance, Welch’s t-test for the parametric data with unequal variance, Mann–Whitney U-test for the nonparametric data, respectively. The paired t-test was used to compare initial and final levels in TL, BW, and CF. In all analyses, p-values < 0.05 were considered significant.
Results
Immunohistochemistry for MCH
The distribution of MCH-ir cell bodies and fibers is summarized in Figs. 1 (sagittal section) and 2 (frontal section). MCH-ir cell bodies were detected in the NLT (Figs. 2a, b, 3a–h, 4a–d, 5a, b) and in the NAT of the hypothalamus (Figs. 2c, d, 3g, h, 4e–h). MCH-ir cell bodies in the NLT formed tight clusters (Figs. 3a, b, 5a, b). MCH-ir cell bodies in the NLT (8.6 ± 0.4 μm (mean ± SEM, n = 17) in diameter) seemed larger than those in the NAT (6.3 ± 0.4 μm (n = 8) in diameter). MCH-ir fibers were observed mainly in the hypothalamus and a small number of MCH-ir fibers were detected in the telencephalon (Fig. 1a). MCH-ir cell bodies in the NLT were observed to send fibers to the pituitary (Figs. 3i, j, 5d, e); MCH-ir fibers were mainly distributed in the neurohypophysial tissues within the pars intermedia (PI) (Figs. 3i, j, 5d, e).
Schematic illustration of the distribution of MCH-ir cell bodies (closed circles) and fibers from a parasagittal section (a) to a midsagittal section (d) of the K. marmoratus brain. Large and small closed circles indicate MCH-ir cell bodies in the NLT and NAT, respectively. For the abbreviations of brain regions, see the text. Left indicates the rostral. Bar indicates 500 μm
Schematic illustration of the localization of MCH-ir cell bodies (closed circles) and fibers in a frontal section of the NLT and NAT of the hypothalamus from anterior (a) to posterior (d). Large and small closed circles indicate MCH-ir cell bodies in the NLT and NAT, respectively. For the abbreviations of brain nuclei and regions, see the text. Top indicates the dorsal. Bar indicates 500 μm
Immunohistochemistry images for MCH-ir cell bodies and fibers in a parasagittal section (a, b) to a midsagittal section (i, j) of the same individual. Arrowheads and arrows indicate MCH-ir cell bodies in the NLT and the NAT, respectively. (b), (d), (f), (h), and (j) are higher magnification of boxed areas in (a), (c), (e), (g), and (i), respectively. For the abbreviations of brain nuclei and regions, see the text. Left indicates the rostral. Bars indicate 50 μm
Immunohistochemistry images for MCH-ir cell bodies and fibers in a frontal section of the NLT and NAT of the hypothalamus from anterior (a, b) to posterior (g, h) of the same individual. Arrowheads and arrows indicate MCH-ir cell bodies in the NLT and NAT, respectively. (b), (d), (f), and (h) are higher magnification of boxed areas in (a), (c), (e), and (g), respectively. For the abbreviations of brain nuclei and regions, see the text. Top indicates the dorsal. Bar indicates 50 μm
(a) Sagittal section through the NLT of the hypothalamus. (b) Higher magnification of boxed areas in (a). MCH-ir cell bodies (arrowheads) and fibers are observed. (c) Adjacent section of (b). No MCH-ir cell bodies and fibers are observed when the anti-MCH antibody was pre-absorbed overnight at 4 °C with an excess amount of synthetic MCH. (d) Sagittal section through the pituitary. (e) Higher magnification of boxed areas in (d). MCH-ir cell fibers originated from the NLT are observed (double arrowhead). MCH-ir fibers (arrowheads) are mainly observed in the neurohypophysial tissues within the pars intermedia. (f) Adjacent section of (e). No MCH-ir fibers are observed when the anti-MCH antibody was pre-absorbed overnight at 4 °C with an excess amount of synthetic MCH. Left indicates the rostral. Bars indicate 50 μm
No MCH-ir cell bodies or fibers were detected when the anti-MCH antibody was pre-absorbed overnight at 4 °C with an excess amount of synthetic MCH (Figs. 5c, f), demonstrating the specificity of the immunoreaction.
Dual-label immunohistochemistry for MCH and CRH
Using dual-label immunohistochemistry for MCH and CRH, MCH-ir and CRH-ir cell bodies were found to be distinguishable from each other in the hypothalamus (Figs. 6a, b). Reciprocal connections between the CRH neurons and MCH neurons in the hypothalamus were not observed.
Rearing experiment
Body color
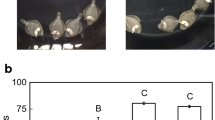
Compared to initial (Fig. 7a), the body color of the white-acclimated fish appeared lighter (Fig. 7b) and that of black-acclimated fish darker (Fig. 7c).
Somatic growth
At the start of the experiment (initial), no significant differences were observed between groups in TL (Student’s t-test, t = 0.226, df = 13, p = 0.8249), BW (Student’s t-test, t = 0.237, df = 13, p = 0.8162), and CF (Student’s t-test, t = 0.748, df = 13, p = 0.4680) (Fig. 8).
(a) Total length (mm), (b) body weight (mg), and (c) condition factor (CF) of the two groups. Each value is expressed as the mean and standard error (bar). Number indicates the fish employed. ††(p < 0.01) and †††(p < 0.001) indicate the level of statistical difference between the initial and final sampling
At the sampling time (final), no significant differences were observed between groups in TL (Student’s t-test, t = 1.025, df = 13, p = 0.3242) and BW (Student’s t-test, t = 724, df = 13, p = 0.4869) (Figs. 8a, b). CF tended to be higher in the black-acclimated fish than that in the white-acclimated fish (Student’s t-test, t = 1.925, df = 13, p = 0.0764) (Fig. 8c).
During the experiment, BW significantly increased in both the white- (paired t-test, t = 8.653, df = 7, p < 0.001) and the black-acclimated fish (paired t-test, t = 5.513, df = 6, p = 0.0015) (Fig. 8b). TL and CF did not significantly change in either group (Figs. 8a, c).
Feeding activity
The number of A. franciscana nauplii eaten in 10 min was significantly greater in the black-acclimated fish than that in the white-acclimated fish (Student’s t-test, t = 2.316, df = 13, p = 0.0375) (Fig. 9a).
(a) Amount of feed (number of nauplii eaten in 10 min), (b) SGR (%), and (c) cortisol levels in the body without head region (pg/g tissue) of the two groups. Each value is expressed as the mean and standard error (bar). Number indicates the fish employed. * (p < 0.05) indicates the level of statistical difference between the two groups
SGR
SGR tended to be higher in the black-acclimated fish group than that in the white-acclimated fish group (Student’s t-test, t = 1.736, df = 13, p = 0.1061) (Fig. 9b).
Levels of cortisol in the body
No significant differences were observed in cortisol levels in the body without head region (pg/g tissue) between the white-acclimated fish group than those in the black-acclimated fish group (Welch’s t-test, t = 0.907, df = 8.295, p = 0.3902) (Fig. 9c).
Number of MCH-ir cell bodies and CRH-ir cell bodies in the hypothalamus
The number of MCH-ir cell bodies in the NLT of the hypothalamus was significantly greater in the white-acclimated fish group than that in the black-acclimated fish (Student’s t-test, t = 2.432, df = 11, p = 0.0333) (Fig. 10a). No significant differences in the number of MCH-ir cell bodies in the NAT of the hypothalamus were observed between the groups (Student’s t-test, t = 0.599, df = 11, p = 0.5614) (Fig. 10b).
(a) The number of MCH-ir cell bodies in the NLT, and (b) the number of MCH-ir cell bodies in the NAT of the hypothalamus of the two groups. Each value is expressed as the mean and standard error (bar). Number indicates the fish employed. * (p < 0.05) indicates the level of statistical difference between the two groups
Moreover, no significant inter-group variation was seen in the number of CRH-ir cell bodies in the NLT of the hypothalamus (Mann–Whitney U test, U = 16, p = 0.4751) (Fig. 11).
Box plots of the number of CRH-ir cell bodies in the NLT of the hypothalamus of the two groups. Line inside the box indicates median. Lower and upper box boundaries indicate 25th and 75th percentiles, respectively. Lower and upper error lines indicate the minimum value and the maximum value, respectively. Numbers indicate the number of fish employed
Discussion
In this study, we primarily clarified the distribution of MCH-ir cell bodies and fibers in the brain and pituitary gland of K. marmoratus. MCH-ir cell bodies are located in the NLT and the NAT of the hypothalamus, and the projections of MCH-ir fibers from these loci are different. The distribution of MCH-ir cell bodies and fibers has been examined in many teleost fish; the overall distribution is almost the same and the main group of MCH-ir cell bodies is detected in the NLT and a smaller group is observed close to the lateral ventricular recess (LVR) (see Diniz and Bittencourt 2019). It is considered that MCH neurons in the NLT project to the pituitary, release MCH in the pituitary, and make the skin paler by concentrating melanin granules (Bird and Baker 1989; Gröneveld et al. 1995; Suzuki et al. 1995). MCH-ir cell bodies above the LVR are suggested to project mainly to non-hypophyseal targets (see Diniz and Bittencourt 2019). In barfin flounder, MCH neurons located above the LVR may be associated with food intake or stress response rather than adaptation to background color (Takahashi et al. 2004). In goldfish, MCH neurons located in the dorsal part of the nucleus recessus lateralis (NRLd) are suggested to be involved in inhibition of appetite (Matsuda et al. 2006, 2007). In K. marmoratus, MCH-ir cell bodies in the NLT project to the pituitary and those in the NAT of the hypothalamus seem to project to non-hypophyseal areas. Although MCH-ir cell bodies have not been observed in the area above the LVR in K. marmoratus, it is suggested that MCH in the NAT functions in the brain possibly regulating food intake.
In K. marmoratus, CRH-ir cell bodies have been detected not only in the NLT but also in several brain regions, and CRH-ir fibers originating from the NLT project to adrenocorticotropic hormone cells in the pituitary, showing that CRH in the NLT is involved in the stress response through the HPI axis (Amano et al. 2022). In addition, it is indicated that the physiological function of MCH in the NLT and the NAT differs, as noted above. Thus, to evaluate responses of each neuronal population to background color, it is not appropriate to measure the expression levels of MCH and CRH in the whole brain using a quantitative reverse-transcription PCR. Accordingly, in this study, we conducted dual-label immunohistochemistry for MCH and CRH and estimated the MCH-ir and CRH-ir cell body counts in the hypothalamus separately.
The body color of the white-acclimated fish appeared lighter and that of black-acclimated fish darker, compared with the baseline color. The number of MCH-ir cell bodies in the NLT of the hypothalamus was significantly greater in the white-acclimated fish group. Thus, it is hypothesized that under white background conditions, the synthesis and secretion of MCH from the pituitary increased, although it is difficult to measure plasma MCH levels in K. marmoratus because of its small body size.
In contrast to MCH, there was no significant inter-group variation in the number of CRH-ir cell bodies in the NLT of the hypothalamus. Furthermore, no significant differences were observed in the cortisol levels of the two groups. Incidentally, the number of CRH-ir cell bodies in the NLT of the hypothalamus and cortisol levels in the body were significantly increased following the induction of crowding stress in K. marmoratus (Amano et al. 2022). Thus, it is suggested that background color causes less stress to the HPI axis than crowding stress in K. marmoratus. The reason why the number of CRH-ir cell bodies and whole-body cortisol levels did not differ between white- and black-acclimated groups might be due to the possibility that the fish adapted to the new environment and that their stress response subsequently returned to normal levels. For example, in the three-spined stickleback Gasterosteus aculeatus, during 10-days of confinement stress, although whole-body cortisol levels remained significantly higher in the stressed fish than in the control fish at the end of the experiment (day 10), the cortisol levels of the stressed fish reached a maximum level within 4 days and declined afterward (Pottinger et al. 2002). However, in olive flounder Paralichthys olivaceus juveniles reared under a white or dark-green tank for 120 days, plasma cortisol levels increased throughout the experimental period in both groups, and plasma cortisol levels were significantly higher in the white-acclimated fish than in the dark-green-acclimated fish on both days 60 and 120 (Kang and Kim 2013). More precise studies would be necessary to further clarify this crucial observation.
The relationship between cortisol and MCH has been reported in rainbow trout Oncorhynchus mykiss (Baker 1994; Green et al. 1991); increased MCH levels in the pituitary of white-acclimated fish indirectly decreased cortisol levels by depressing CRH release and thereby, its downstream processes (Baker 1994). In the present study, the body color of white-acclimated fish appeared lighter than that of black-acclimated fish. Therefore, it is possible that pituitary MCH levels were higher in white-acclimated fish, as previously reported in the rainbow trout (Green et al. 1991). However, no significant differences were observed in the levels of cortisol in the body between the groups. This difference may be explained by the results of dual-label immunohistochemistry, which revealed no reciprocal connections between the CRH neurons and MCH neurons in the hypothalamus of K. marmoratus, while such connections may be present in the rainbow trout.
In conclusion, the present study indicates that the background color affects MCH neuronal activity in the NLT and body color change in K. marmoratus. Future studies should be conducted to clarify the effects of background color on CRH neuronal activity and stress-related hormones.
Data availability
The datasets and materials used during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Abbreviations
- C:
-
Cerebellum
- CP:
-
Central posterior thalamic nucleus
- GA:
-
Corpus glomerulosus pars anterior
- GR:
-
Corpus glomerulosus pars rotunda
- HB:
-
Habenula
- Hyp:
-
Hypothalamus
- LI:
-
Inferior lobe
- M:
-
Medulla oblongata
- NAT:
-
Nucleus anterior tuberis
- NC:
-
Nucleus corticalis
- NLT:
-
Nucleus lateralis tuberis
- NRL:
-
Nucleus recessi lateralis
- OB:
-
Olfactory bulb
- OT:
-
Optic tectum
- PG:
-
Preglomerular complex
- PGZ:
-
Periventricular gray zone of optic tectum
- Pit:
-
Pituitary
- T:
-
Telencephalon
- TE:
-
Tectum
- TL:
-
Torus longitudinalis
- TLa:
-
Torus lateralis
- TS:
-
Torus semicircularis
- VM:
-
Ventromedial thalamic nucleus
References
Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94:239–247. https://doi.org/10.1002/ar.1090940210
Amano M, Takahashi A (2009) Melanin-concentrating hormone: A neuropeptide hormone affecting the relationship between photic environment and fish with special reference to background color and food intake regulation. Peptides 30:1979–1984. https://doi.org/10.1016/j.peptides.2009.05.022
Amano M, Amiya N, Yokoyama T, Onikubo K, Yamamoto N, Takahashi A (2016) Immunohistochemical detection of corticotropin-releasing hormone (CRH) in the brain and pituitary of the hagfish, Eptatretus burgeri. Gen Comp Endocrinol 236:174–180. https://doi.org/10.1016/j.ygcen.2016.07.018
Amano M, Amiya N, Mizusawa K, Chiba H (2021) Effects of background color and rearing density on stress-related hormones in the juvenile Japanese eel Anguilla japonica. Fish Sci 87:521–528. https://doi.org/10.1007/s12562-021-01527-4
Amano M, Amiya N, Fukushima K, Hagio H, Yamamoto N, Sakakura Y (2022) Effects of crowding stress on the hypothalamo-pituitary-interrenal axis of the self-fertilizing fish Kryptolebias marmoratus. Comp Biochem Physiol A 264:111110. https://doi.org/10.1016/j.cbpa.2021.111110
Avise JC, Tatarenkov A (2015) Population genetics and evolution of the mangrove rivulus Kryptolebias marmoratus, the world’s only self-fertilizing hermaphroditic vertebrate. J Fish Biol 87:519–538. https://doi.org/10.1111/jfb.12741
Baker BI (1994) Melanin-concentrating hormone updated functional considerations. Trends Endocrinol Metab 5:120–126. https://doi.org/10.1016/1043-2760(94)90093-0
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integ Comp Biol 42:517–525. https://doi.org/10.1093/icb/42.3.517
Bird DJ, Baker BI (1989) An immunological study of the secretory activity of neurons producing melanin-concentrating hormone in a teleost. Neuroscience 28:245–251. https://doi.org/10.1016/0306-4522(89)90248-0
Cánepa MM, Pandolfi M, Maggese MC, Vissio PG (2006) Involvement of somatolactin in background adaptation of the cichlid fish Cichlasoma dimerus. J Exp Zool 305A:410–419. https://doi.org/10.1002/jez.a.273
Cerdá-Reverter JM, Canosa LF, Peter RE (2006) Regulation of the hypothalamic melanin-concentrating hormone neurons by sex steroids in the goldfish: possible role in the modulation of luteinizing hormone secretion. Neuroendocrinol 84:364–377. https://doi.org/10.1159/000098334
Costa DC, Mattioli CC, Silva WS, Takata R, Leme FOP, Oliveira AL, Luz RK (2017) The effect of environmental colour on the growth, metabolism, physiology and skin pigmentation of the carnivorous freshwater catfish Lophiosilurus alexandri. J Fish Biol 90:922–935. https://doi.org/10.1111/jfb.13208
Diniz GB, Bittencourt JC (2019) The melanin-concentrating hormone (MCH) system: a tale of two peptides. Front Neurosci 13:1280. https://doi.org/10.3389/fnins.2019.01280
Eslamloo K, Akhavan SR, Eslamifar A, Henry MA (2015) Effects of background colour on growth performance, skin pigmentation, physiological condition and innate immune responses of goldfish, Carassius auratus. Aqua Res 46:202–215. https://doi.org/10.1111/are.12177
Green JA, Baker BI, Kawauchi H (1991) The effect of rearing rainbow trout on black or white backgrounds on their secretion of melanin-concentrating hormones and their sensitivity to stress. J Endocrinol 128:267–274. https://doi.org/10.1677/joe.0.1280267
Gröneveld D, Balm PHM, Martens GJM, Bonga SEW (1995) Differential melanin-concentrating hormone gene expression in two hypothalamic nuclei of the teleost tilapia in response to environmental changes. J Neuroendocrinol 7:527–533. https://doi.org/10.1111/j.1365-2826.1995.tb00789.x
Harrington RW Jr (1961) Oviparous hermaphroditic fish with internal self-fertilization. Science 134:1749–1750. https://doi.org/10.1126/science.134.3492.1749
Kanamori A, Sugita Y, Yuasa Y, Suzuki T, Kawamura K, Uno Y, Kamimura K, Matsuda Y, Wilson CA, Amores A, Postlethwait JH, Suga K, Sakakura Y (2016) A genetic map for the only self-fertilizing vertebrate. G3 Genes Genomes Genetics 6:1095–1106. https://doi.org/10.1534/g3.115.022699
Kang D-Y, Kim H-C (2013) Influence of density and background color to stress response, appetite, growth, and blind-side hypermelanosis of flounder, Paralichthys olivaceus. Fish Physiol Biochem 39:221–232. https://doi.org/10.1007/s10696-012-9693-2
Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI (1983) Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature 305:321–323. https://doi.org/10.1038/305321a0
Matsuda K, Shimakura SI, Maruyama K, Miura T, Uchiyama M, Kawauchi H, Shioda S, Takahashi A (2006) Central administration of melanin-concentrating hormone (MCH) suppresses food intake, but not locomotor activity, in the goldfish, Carassius auratus. Neurosci Lett 399:259–263. https://doi.org/10.1016/j.neulet.2006.02.005
Matsuda K, Shimakura SI, Miura T, Maruyama K, Uchiyama M, Kawauchi H, Shioda S, Takahashi A (2007) Feeding-induced changes of melanin-concentrating hormone (MCH)-like immunoreactivity in goldfish brain. Cell Tissue Res 328:375–382. https://doi.org/10.1007/s00441-006-0347-5
Pankhurst NW (2011) The endocrinology of stress in fish: an environmental perspective. Gen Comp Endocrinol 170:265–275. https://doi.org/10.1016/j.ygcen.2010.07.017
Peter RE, Gill VE (1975) A stereotaxic atlas and technique for forebrain nuclei in the goldfish, Carassius auratus. J Comp Neurol 159:69–102. https://doi.org/10.1002/cne.901590106
Pottinger TG, Carrick TR, Yeomans WE (2002) The three-spined stickleback as an environmental sentinel: effects of stressors on whole-body physiological indices. J Fish Biol 61:207–229. https://doi.org/10.1006/jfbi.2002.2034
Suzuki M, Narnaware YK, Baker BI, Levy A (1995) Influence of environmental colour and diurnal phase on MCH gene expression in the trout. J Neuroendocrinol 7:319–328. https://doi.org/10.1111/j.1365-2826.1995.tb00764.x
Takahashi A, Tsuchiya K, Yamanome T, Amano M, Yasuda A, Yamamori K, Kawauchi H (2004) Possible involvement of melanin-concentrating hormone in food intake in a teleost, barfin flounder. Peptides 25:1613–1622. https://doi.org/10.1016/j.peptides.2004.02.022
Takahashi A, Kosugi T, Kobayashi Y, Yamanome T, Schiöth BH, Kawauchi H (2007) The melanin-concentrating hormone receptor 2 (MCH-R2) mediates the effect of MCH to control body color for background adaptation in the barfin flounder. Gen Comp Endocrinol 151:210–219. https://doi.org/10.1016/j.ygcen.2007.01.011
Taylor DS (2012) Twenty-four years in the mud: what have we learned about the natural history and ecology of the mangrove rivulus, Kryptolebias marmoratus? Integr Comp Biol 52:724–736. https://doi.org/10.1093/icb/ics062
Wullimann MF, Rupp B, Reichert H (1996) Neuroanatomy of the zebrafish brain: a topological atlas. Birkhӓuser Verlag, Basel, Switzerland
Yamada H, Satoh R, Fujimoto Y, Takaji K, Hakuba T, Chiba H, Iwata M (2002) Circadian changes in serum concentrations of steroids in Japanese char Salvelinus leucomaenis at the stage of final maturation. Zool Sci 19:891–898. https://doi.org/10.2108/zsj.19.891
Yamanome T, Chiba H, Takahashi A (2007) Melanocyte-stimulating hormone facilitates hypermelanosis on blind side of barfin flounder, a pleuronectiform fish. Aquaculture 270:505–511. https://doi.org/10.1016/j.aquaculture.2007.05.037
Acknowledgements
We thank Ms. Ayae Kuriu, Mr. Aoi Kato, and Mr. Takashi Sato of the School of Marine Biosciences, Kitasato University, for their help in this study.
Funding
This study was supported in part by JSPS KAKENHI Grant Number JP23380115.
Author information
Authors and Affiliations
Contributions
Masafumi Amano and Yoshitaka Sakakura designed the study; Masafumi Amano, Noriko Amiya, and Naoyuki Yamamoto conducted immunohistochemistry; Naoyuki Yamamoto drew the brain atlas; Masafumi Amano and Yoshitaka Sakakura conducted the rearing experiment; Masafumi Amano drafted the manuscript and all authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Fish maintenance and sacrifice were performed following the guidelines of the animal care committee of Nagasaki University and Kitasato University.
Conflict of interest
The authors declare no conflict of interest.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amano, M., Amiya, N., Yamamoto, N. et al. Neuronal responses of melanin-concentrating hormone and corticotropin-releasing hormone to background color in the self-fertilizing fish, Kryptolebias marmoratus. Fish Physiol Biochem 49, 385–398 (2023). https://doi.org/10.1007/s10695-023-01178-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-023-01178-5