Abstract
In the present work, we studied the effect of short-term acute hypoxia on the cellular composition of the blood and the head kidney of the black scorpionfish. Dissolved oxygen concentration was decreased from 8.5–8.7 mg O2 l−1 (normoxia) to 3–5 mg O2 l−1 (relative normoxia), 1–3 mg O2 l−1 (moderate hypoxia), and 0–1 mg O2 l−1 (acute hypoxia) within 1.5–2 h by bubbling of water with N2. Exposure period was 4 h, water temperature was adjusted to 14–16 °C, and photoperiod was 12 h (light). Short-time acute hypoxia induced a rapid release of blast and immature cells from the head kidney into the circulating blood of the black scorpionfish, which was associated with reduction in erythropoietic reserves in 2.5 times. The number of immature erythroid cells (pronormoblasts, basophilic and polychromatophilic normoblasts) significantly increased in blood, and the simultaneously relative decrease of the number of abnormal red blood cell (RBC) and the increase of the number of RBC ghosts (lysed RBCs) in circulating blood were observed. The significant correlation between methemoglobin concentration and the number of RBC ghosts was shown (R2 = 0.640 or r = 0.800). Hypoxia induced RBC swelling on 5–6% compared to control. The number of RBC ghosts in the blood is likely involved in the stimulation of erythropoietin production under hypoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blood oxygen–carrying capacity (BOC) is an essential parameter for the circulatory system which directly influences the level of the oxidative processes in tissues. The normal level of blood oxygen capacity ensures the balance between productive and destructive processes in the red blood cell (RBC) system (Houston 1990). Decreased BOC may be rapidly corrected by a release of RBCs from blood depot organs (Houston 1990). RBC formation in teleosts primarily occurs in the anterior part of the head kidney (pronephros) and is also partly observed in the spleen (Soldatov 2005a; Kondera 2011; Witeska 2013). Aged RBCs are utilized in the spleen (Soldatov 2005a; Witeska 2013), which also functions as red blood cell depot (Nilsson and Grove 1973; Muiswinkel et al. 1991; Houston et al. 1996). Aged and abnormal RBCs were shown to possess modified antigen complex, and therefore, these cells are effectively accumulated by the reticular tissue of the spleen (Drenckhahn 1988; Bartosz 1991).
Pronephros (head kidney) is considered the major hematopoietic organ in teleosts (Soldatov 2005a; Kondera 2011; Witeska 2013). Head kidney losses excretory function at early stages of ontogenesis, and its structure becomes close to mammalian bone marrow (Sordyl and Osterland 1991; Rodriguez 1995). Head kidney has a number of blood vessels, which are covered by a monolayer of epithelium with numerous sinuses (Liu et al. 2004; Abdel-Aziz et al. 2010; Santos et al. 2011). Connective tissue is well developed and is formed by reticular cells, collagen-rich reticular fibers, and melano-macrophage elements (Tatner and Manningm 1985; Meseguer et al. 1991, 1995). In fact, pronephros comprises all hemopoietic branches with erythroid line dominating above others (Botham and Manning 1981; Soldatov 2005a). Although there are some exceptions, for most of the teleostean species, erythropoiesis is active only during 3–4 months (usually following spawning) (Maslova et al. 1988) and is inhibited during the rest period of the annual cycle (Maslova et al. 1988).
Environmental hypoxia is frequently associated with the enhancement of erythroblast proliferation in pronephros of teleosts (Kulkeaw and Sugiyama 2012; Zhu et al. 2013). For aquatic organisms, dissolved oxygen concentration below 2.0 mg O2 l−1 is considered hypoxic (Rosenberg et al., 2001). This process is modulated by the production of erythropoietin in kidneys (Moritz et al. 1997; Lai et al. 2006), which was, for the first time, detected in fish using immunochemical analysis (Wickramasinghe 1993). The complete structure of erythropoietin gene is now annotated for Fugu rubripes (Chou et al. 2004). The production of erythropoietin and the enhancement of erythropoiesis in hemopoietic tissues are slow processes, which demand a certain period of time. The formation of new circulating RBCs usually occurs during 3–4 months (Soldatov 2005a), which is in line with a long lifespan period of teleostean RBCs determined by the inclusion of 3H-thymidine and fluorescent probes (270–310 days) (Zolotova 1987; Fischer et al. 1998). Despite the induction of erythropoietin production and proliferation of erythroblasts in hemopoietic sites is directly regulated by changes in circulating blood, the processes occurring there at the initial stages of hypoxia remain unclear.
The black scorpionfish (Scorpaena porcus L.) was chosen as the object of this study. It is highly tolerant to hypoxia benthic Black Sea species. Black scorpionfish represents the lowest critical and threshold oxygen concentrations among other regional teleosts (Parfenova 2004).
The aim of the present work was to determine the cellular composition as well as functional and morphological parameters of circulating red blood and the pronephros of the black scorpionfish, Scorpaena porcus L., upon exposure to acute short-time hypoxia.
Material and methods
Material
The object of the research was adult black scorpionfish, Scorpaena porcus L. (body lengths 14–19 cm, body weight 114–182 g) at resting physiological state (stages III–IV of gonad maturation). After capture with a seine, fish were immediately transferred (within 3 h) into the laboratory in 60-l plastic tanks equipped with aeration system. Black scorpionfish were acclimated to laboratory conditions in flowing-water system aquaria for 1 week to reduce stress. The water was delivered from a 10-mile sea zone. Animals were fed daily with a minced fish (6–7% of the body mass per specimen).
Experimental design
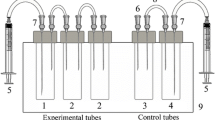
Hypoxia was established using the original experimental stand (total volume 13.5 l), which allowed maintaining a stable oxygen concentration and water temperature for one black scorpionfish. During the experimental period, water temperature was adjusted to 14–16 °C, and photoperiod was 12 h (light). After a 1-day adaptive period, dissolved oxygen concentration in the experimental tank was decreased by nitrogen gas (N2) bubbling from 8.5–8.7 mg O2 l−1 (normoxia) to 3–5 mg O2 l−1 (relative normoxia), 1–3 mg O2 l−1 (moderate hypoxia), and 0–1 mg O2 l−1 (acute hypoxia) within 1.5–2 h. The constant hypoxia level was maintained for 4 h using periodic aeration of water with O2. Dissolved oxygen concentration was monitored using DO Meter ST300D RU (“Ohaus” CШA).
Animals were treated with urethane (Soldatov 2005b) prior to tissue and blood sampling. Urethane was dissolved in the water of the experimental tank to reduce stress induced by capturing and surgery manipulations.
Laboratory measurements
Samples of the pronephros were mechanically ground up in the following solution: 128 mM NaCl, 3 mM KCl, 1.5 mM CaCl2, 1.5 mM MgCl2, 15 mM HEPES, and 2.2 mM d-glucose (pH 7.8) (Tiihonen and Nikinmaa 1991). Large fragments of tissue were removed. Cells were washed 3 times in 1.5 ml of the solution (5 min, 1500 rpm) on the centrifuge ELMI CM-50. Cell pellet was used for slide preparations. Three slides were made for each fish.
Approximately 2 ml of blood was obtained from the caudal artery using sterile syringe. Heparin (Richter, Hungary) was used to prevent blood coagulation. Morphometric analysis of the blood cells was conducted on slides. After the 24-h drying on air, slides with blood and pronephros samples were stained using combined Pappenheim method (May-Grünwald and Romanowsky-Giemsa) (Houston, 1990) and viewed on a light microscope (Biolar, Poland) equipped with a camera (Olympus C-7070, Japan). The percent ratio of immature and abnormal cells was calculated on 5000 cells per slide.
We measured the linear size of blood cells on the microphotographs using ImageJ 1.44p software (Girish and Vijayalakshmi 2004). The large and the small cellular diameter (C1 and C2) and similar nuclear dimensions (N1 and N2) were measured on 100 cells per slide (Fig. 1). Then, cellular volume (Vc) (Houchin et al. 1958), RBC thickness (h) (Chizhevsky 1959), nuclear volume (Vn) (Tasea 1976), and nucleo-cytoplasmic ratio (NCR) have been calculated using the following equations:
Methemoglobin concentration was measured on a spectrophotometer by the method of Evelyn and Malloy with modification of Kushakovsky (1970) (using acetone cyanohydrin).
A one-way analysis of variance (ANOVA) was used to compare the content of individual cell forms in the hematopoietic tissue of the head kidney and circulating blood, as well as the level of methemoglobin in the blood. We used PAST version 4.09 software (Hammer and Harper, 2006). The data were represented as mean ± standard error of the mean (SEM). The significant difference level was set at p < 0.05.
Results
Head kidney (pronephros)
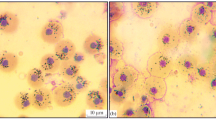
All hematopoietic lines have been identified in the black scorpionfish pronephros: myeloid, lymphoid, and erythroid branches. Erythroid line comprised mainly erythroblasts, which were round (diameter 9–10 µm) with large acidophilic soft-meshed euchromatin-rich nuclei and narrow basophilic cytoplasm (Fig. 2). Nuclei of erythroblasts contained 2–4 nucleoluses. Maturating stages of erythroid line were formed with pronormoblasts (Pro). These cells possessed morphological features close to those observed in erythroblasts. The specific feature of Pro was the presence of perinuclear zone. Hypoxia induced more than a 2.5-fold decrease in the number of Pro in pronephros (p < 0.01) (Table 1). In the black scorpionfish exposed to 5 mg O2 l−1, the number of Pro was more than 12.5% of total cell count, but in fish exposed to 0.52 mg O2 l−1, Pro comprised only 5%.
Myeloid hemopoietic line was formed by myeloblasts and maturating granular and agranular (monocytoblasts) cells (Fig. 2). Myeloblasts possessed a large euchromatin-rich nucleus with 1–3 nucleoluses and narrow-light basophilic cytoplasm and were morphologically close to erythroblasts (Fig. 2). Progenitors of granulocytes contained granules in cytoplasm (Fig. 2). A small number of promonocytes (less than 3%) and macrophages (up to 1%) were also observed in the pronephros (Fig. 2).
Hypoxia induced more than a 2.5-fold decrease in granulocyte number (p < 0.001) (Table 1). The number of myeloblasts also decreased from 9.5–11.5 to 6–8% of the total cell count. In fish exposed to severe hypoxia (dissolved oxygen concentration less than 1.2 mg O2 l−1), pronephros samples did not contain monocytes and macrophages.
Pronephros slides possessed a small number of megakaryocytes which did not exceed 0.5% of the total cell count. These cells have the largest diameter (20–22 µm) among other cells in pronephros, and their nuclei with coarse chromatin were situated in basophilic cytoplasm. These cells were not observed in the pronephros of the black scorpionfish exposed to dissolved oxygen concentration less than 1.5 mg O2 l−1.
We also observed the morphology of cells which was close to that of small lymphocytes (cellular diameter 4–5 µm, large nuclei, narrow basophilic cytoplasm) in the pronephros (Fig. 2). The number of these cells increased more than 2 times (p < 0.001) in contrast for other cell types. The number of plasma cells in the pronephros was initially low (up to 1%). At hypoxia (less than 1.2 mg O2 l−1), they disappeared from the pronephros completely.
Circulating blood
Opposite pronephros changes were observed in the blood of the black scorpionfish upon hypoxia. In the present study, we investigated erythroid cell line; therefore, the number of pronormoblasts (Pro) and basophilic and polychromatophilic normoblasts (BN and PN, respectively) was calculated (Fig. 3). BN are round-shaped cells with smaller nuclei and larger cytoplasm volume compared to Pro. Cytoplasm is also basophilic due to its high RNA content. PN are ellipsoid cells, which shape is determined by hemoglobin accumulation in cytoplasm and its binding to cellular membrane. Cytoplasm of PN becomes gray due to RNA and acidophilic hemoglobin. The level of heterochromatin in nuclei increases, indicating the decrease of functional activity of this organelle.
The number of immature erythroid cells in the blood of the black scorpionfish exposed to 1–3 mg O2 l−1 was more than 4 times higher (p < 0.01) compared to that in fish exposed to the dissolved oxygen concentration 3–5 mg O2 l−1 as well as in blood of animals following severe hypoxia treatment (0–1 mg O2 l−1) (Fig. 4). These changes were mainly caused by an increase of the number of erythroid progenitors (Pro and immature BN) and more differentiated cells (mature BN and PN).
Blood of the black scorpionfish also contained abnormal cells with nuclei invaginations, displacements, micronuclei, and dacryocyte (RBCs with cytoplasmic spiculae) (Fig. 3). As it is seen from the figure, the number of abnormal erythroid cells gradually decreased upon hypoxia. The decrease was about 30% (p < 0.05) compared to that observed at 3–5 mg O2 l−1 (Fig. 5). These changes were, for the most part, associated with the number of cells with invaginations of nuclei (R2 = 0.799). Other anomalies of the RBC structure did not significantly influence the parameter as their total number did not exceed 1% per slide.
The decrease in the number of cell abnormalities in circulating blood of the black scorpionfish was accompanied with more than an eightfold increase of methemoglobin content (p < 0.001) as well as the number of RBC ghosts (on about 70%) (p < 0.01) (Fig. 6). The correlative analysis between these parameters demonstrated a significant relation (R2 = 0.640 or r = 0.800). On the other hand, the number of RBC ghosts did not depend on changes in the number of any cellular abnormality.
Morphometric parameters
Hypoxia induced an increase in RBC volume (Vc) and nuclear volume (Vn) on 5–6% and 10–11%, respectively (Table 2). However, the differences were not statistically significant (p > 0.05). NCR in all experimental series was 26–28.
Discussion
Short-term acute hypoxia reducing the number of blast and low-differentiated cells in hematopoietic tissue
We showed that the head kidney of the black scorpionfish contains blast cells of erythroid, myeloid, and megakaryocytic branches as well as maturing cells. Erythropoiesis significantly dominated among other hemopoietic lines accounting more than 12% of the total cell count at the dissolved oxygen concentration 3–5 mg O2 l−1. Short-term hypoxia induced substantial changes in the cellular composition of the pronephros by reducing the number of blast and low-differentiated cells of all hemopoietic branches. For the most part, these changes were associated with the erythroid line, as the number of erythroblasts and Pro decreased in more than 2.5 times. At the same time, the number of myeloblasts decreased only by 1.2–1.9-fold. Mature macrophages and plasmatic cells were not observed on slides during hypoxia, which is likely accompanied with their movement into the circulating blood.
On the other hand, the number of small lymphocyte-like cells increased, indicating that connective tissue selectively held this cell type in the pronephros. It is known that hematopoietic tissue may contain colony-forming cells, the morphology of which is close to that of small lymphocytes (Spangrude et al. 1988). Colony-forming cells usually possess low proliferative activity (Spangrude et al. 1988). Previously, we have shown that the number of cells undergoing division in this subpopulation of pronephros cells did not exceed 2% (Andreyeva et al. 2019). Therefore in the present work, we identified these cells as colony-forming elements.
The process of a rapid release of cells from pronephros to circulating blood is quite interesting. Acute hypoxia usually induces the adaptive responses which are aimed to increase blood oxygen–carrying capacity. In most species, hypoxic exposure is associated with a loss of spleen weight, which is also considered to perform hemopoietic activity (Soldatov 2005a; Witeska 2013). Similar to pronephros, the spleen also possesses developed reticular tissue which ensures its contraction (Muiswinkel et al. 1991). The spleen is innervated with cholinergic and adrenergic postganglionic fibers, which are parts of celiac nerve. In vitro adrenergic stimulation was shown to induce resistance to fluid flow in the perfused spleen (Nilsson and Grove 1973). Adrenalin injection causes similar response (Kita and Itazawa 1989). It is unknown whether similar mechanisms occur in the head kidney.
The involvement of the spleen in changing the cellular composition of the blood of the black scorpionfish should not be excluded from attention. The weight control of this organ was not carried out in these studies. However, the role of this process, apparently, is not so great. Most authors consider the spleen of teleostean fish as an organ of secondary (not primary) hematopoiesis (Soldatov, 2005a; Witeska, 2013).
Short-term acute hypoxia increases the number of immature red blood cells and lowers the number of abnormal erythroid cells in the blood
Changes in the blood cellular composition observed in the black scorpionfish exposed to hypoxia indicated the release of maturing blood cells from the hemopoietic tissue. This hypothesis is clearly confirmed by changes in the ratio of erythroid cells. We observed a significant proportional 4–fivefold increase in the number of immature RBCs at proliferating (Pro and immature BN) and differentiating (PN and mature BN) stages. Based on changes in the blood cellular composition, we may suppose the enhancement of proliferation of the erythroid line in the pronephros under acute hypoxia. However, this conclusion is premature when changes in cellular composition within the head kidney are taken into account.
Six percent of RBCs showed invaginations of nuclei. These anomalies are observed in the circulating blood of many teleostean species. Invaginations of nuclei are considered to appear due to a toxic effect of various chemical compounds (Ergene et al. 2007; Strunjak-Perovic et al. 2009b). Some authors intend that the number of nuclei invaginations and RBCs with micronuclei correlates (Strunjak-Perovic et al. 2009a; Anbumani and Mohankumar 2012). Invaginations of nuclei may precede micronuclei formation (Ayllon and Vazquez 2000; Kirschbaum et al. 2011). We did not observe such relations in the present work.
Hypoxic exposure was associated with a decrease in the number of abnormal RBCs in the black scorpionfish blood, which was also accompanied with an increase in the number of RBC ghosts indicating RBC lysis. The number of abnormal cells decreased on 55–56%, and the number of RBC ghosts declined similarly (59–60%). These results indicate that abnormal RBCs lysed under acute exposure to hypoxia. End products of the RBC lysis are considered to induce formation of erythropoietin in the kidney (Kulkeaw and Sugiyama 2012), which, in turn, enhances erythropoietic processes (Moritz et al. 1997). But, these responses require a longer period of time, which is confirmed by a restriction of erythropoiesis in pronephros observed in the present work.
Several processes may underlie the lysis of abnormal RBCs in response to acute hypoxia. Our results allow supposing two main reasons. We observed the substantial correlation between the number of RBC ghosts and methemoglobin concentration (R2 = 0.640 or r = 0.800). Oxidation of hemoglobin to its ferryl form is usually associated with the release of superoxide anion radical (∙O2−) which enhances the oxidizing processes in the cell. This process might primarily occur in abnormal cells with non-effective antioxidant enzyme complex. Methemoglobin formation has been widely observed under hypoxic conditions in higher and lower vertebrates (Olander and Parr 1978; Arnaud et al. 1979; Affonso et al. 2002; Chen et al. 2017) during in vivo and in vitro experiments (Soldatov et al., 2020). Hypoxia induces hemoglobin deoxygenation and then formation of ferryl form (Mansouri 1981; Jensen et al. 1998). Ferro ion of deoxygenated hemoglobin possesses high-spin state (4 unpaired electrons). Binding with oxygen induces a shift of ferrum state to the low-spin state with all electrons paired. Therefore, changes in hemoglobin form followed by a gradual-spin crossover to low-spin state of Fe2+ may be associated with the release of electron and oxidation of ferrum. In this case, oxygen becomes an electron acceptor and transits to ∙O2−.
Among other reasons involved in the lysis of abnormal RBCs in the blood of the black scorpionfish under hypoxia, RBC swelling should also be taken into account. The increase of RBC volume upon hypoxic exposure has been postulated in many works (Soivio et al. 1974; Nikinmaa et al. 1987; Holk 1996; Jensen et al. 1998). Here, we observed an increase of RBC volume on 5–6%, which agrees with previously published data (Valenzuela et al. 2005). Swelling is involved in the regulation of intracellular pH level and is achieved through the activation of Na+/H+ antiporter (Tufts 1992). RBC swelling is a cAMP-dependent process, which is mediated by blood catecholamines binding to cellular β-adrenoreceptors (Ferguson and Boutilier 1988; Salama and Nikinmaa 1990; Val et al. 1997). RBC swelling was also observed in vitro (Andreyeva et al. 2017) and, in this case, was likely modulated by a slight decrease of the intracellular pH, which, in turn, activated the Na+/H+ antiporter via increased affinity of inner cellular membrane to H+. In the present work, we may expect catecholamine release as exposure to hypoxia was relatively abrupt. Obviously, abnormal RBCs might lyse first.
Thus, short-time acute hypoxia induced a rapid release of blast and immature cells from the head kidney into the circulating blood of the black scorpionfish. This response restricted erythropoietic reserves in 2.5 times. The number of immature erythroid cells (pronormoblasts, basophilic and polychromatophilic normoblasts) significantly increased in blood, which was accompanied by a relative decrease of the number of abnormal RBC and the increase of the number of RBC ghosts in the circulating blood. The significant correlation between methemoglobin concentration and the number of RBC ghosts was observed (R2 = 0.640 or r = 0.800). Hypoxia induced RBC swelling on 5–6% compared to control. The number of RBC ghosts in the blood is suggested to modulate the production of erythropoietin in conditions of hypoxia.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abdel-Aziz E-SH, Abdu SBS, Ali TE-S, Fouad HF (2010) Haemopoiesis in the head kidney of tilapia, Oreochromis niloticus (Teleostei: Cichlidae): a morphological (optical and ultrastructural) study. Fish Physiol Biochem 36:323–336. https://doi.org/10.1007/s10695-008-9297-z
Affonso EG, Polez VL, Corrêa CF, Mazon AF, Araújo MR, Moraes G, Rantin FT (2002) Blood parameters and metabolites in the teleost fish Colossoma macropomum exposed to sulfide or hypoxia. Comp Biochem Physiol Part c Toxicol Pharmacol 133:375–382. https://doi.org/10.1016/S1532-0456(02)00127-8
Anbumani S, Mohankumar MN (2012) Gamma radiation induced micronuclei and erythrocyte cellular abnormalities in the fish Catla catla. Aquat Toxicol 122–123:125–132. https://doi.org/10.1016/j.aquatox.2012.06.001
Andreyeva AY, Kukhareva TA, Soldatov AA (2019) Cellular composition and proliferation levels in the hematopoietic tissue of black scorpionfish (Scorpaena porcus L.) head kidney and spleen during the spawning and wintering periods. Anat Rec 302:1136–1142. https://doi.org/10.1002/ar.24031
Andreyeva AY, Soldatov AA, Mukhanov VS (2017) The influence of acute hypoxia on the functional and morphological state of the black scorpionfish red blood cell. In Vitro Cel Develop Biol Anim 53:312–319. https://doi.org/10.1007/s11626-016-0111-4
Arnaud J, Quilici JC, Gutierrez N, Beard J, Vergnesa H (1979) Methaemoglobin erythrocyte reducing systems in high-altitude natives. Ann Hum Biol 6:585–592. https://doi.org/10.1080/03014467900003951
Ayllon F, Vazquez G-E (2000) Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna: an assessment of the fish micronucleus test. Mutation Res 467:177–186. https://doi.org/10.1016/S1383-5718(00)00033-4
Bartosz G (1991) Erythrocyte aging: physical and chemical membrane changes. Gerontology 37:33–67. https://doi.org/10.1159/000213251
Botham JW, Manning MJ (1981) The histogenesis of the lymphoid organs of the carp Cyprinus carpio L. and the ontogenetic development of allograft reactivity. J Fish Biol 19:403–414. https://doi.org/10.1111/j.1095-8649.1981.tb05844.x
Chen N, Wu M, Tang G-P, Wang H-J, Huang C-X, Wu X-J (2017) Effects of acute hypoxia and reoxygenation on physiological and immune responses and redox balance of Wuchang bream (Megalobrama amblycephala Yih, 1955). Front Physiol 8:1–9. https://doi.org/10.3389/fphys.2017.00375
Chizhevsky AL (1959) Structural analysis of moving blood. USSR Academy of Science, Moscow (in Russian)
Chou C-F, Tohari S, Brenner S, Venkatesh B (2004) Erythropoietin gene from a teleost fish, Fugu rubripes. Blood 104:1498–1503. https://doi.org/10.1182/blood-2003-10-3404
Drenckhahn D (1988) Removal of old and abnormal red blood cells from circulation: mechanical and immunologic mechanisms. In: Blood cells, rheology, and aging. Springer, Berlin, Heidelberg, pp. 62–72. https://doi.org/10.1007/978-3-642-71790-1_7
Ergene S, Cavas T, Celik A, Koleli N, Kaya F, Karahan A (2007) Monitoring of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): genotoxic damage in relation to water pollution. Ecotoxicol 16:385–394. https://doi.org/10.1007/s10646-007-0142-4
Ferguson RA, Boutilier RG (1988) Metabolic energy production during adrenergic pH regulation in red cells of the Atlantic salmon, Salmo salar. Respir Physiol J 74:65–76. https://doi.org/10.1016/0034-5687(88)90141-7
Fischer U, Ototake M, Nakanishi T (1998) Life span of circulating blood cells in ginbuna crucian carp (Carassius auratus langsdorfii). Fish Shellfish Immunol 8:339–349. https://doi.org/10.1006/fsim.1998.0144
Girish V, Vijayalakshmi A (2004) Affordable image analysis using NIH Image/ImageJ. Indian J Cancer 41:41–47
Hammer Ø, Harper DAT (2006) Paleontological data analysis. Blackwell. https://doi.org/10.1002/jqs.1107
Holk K (1996) Effects of isotonic swelling on the intracellular Bohr factor and the oxygen affinity of trout and carp blood. Fish Physiol Biochem 15:371–375. https://doi.org/10.1007/BF01875579
Houchin DN, Munn JI, Parnell BL (1958) A method for the measurement of red cell dimensions and calculation of mean corpuscular volume and surface area. Blood 13:1185–1191. https://doi.org/10.1182/blood.V13.12.1185.1185
Houston AH (1990) Blood and circulation. In Methods for fish biology. N-Y.: Am Fish Soc pp. 273–334.
Houston AH, Roberts WC, Kennington JA (1996) Hematological response in fish: pronephric and splenic involvements in the goldfish, Carassius auratus L. Fish Physiol Biochem 15:481–489. https://doi.org/10.1007/BF01874922
Jensen FB, Fago A, Weber RE (1998) Hemoglobin structure and function. Fish Physiol Biochem 17:1–40
Kirschbaum AA, Seriani R, Pereira CDS, Assunção A, Abessa DM, Rotundo S, Seriani MM (2011) Hematology, micronuclei and nuclear abnormalities in fishes from São Francisco River, Minas Gerais State, Brazil. Acta Scientiarum Biol Sci Maringa 33:107–112. https://doi.org/10.4025/actascibiolsci.v33i1.7117
Kita J, ItazawaY, (1989) Release of erythrocytes from the spleen during exercise and splenic constriction by adrenaline infusion in the rainbow trout. Jap J Ichthyol. 36:48–52. https://doi.org/10.11369/jji1950.36.48
Kondera E (2011) Haematopoiesis in the head kidney of common carp (Cyprinus carpio L.): a morphological study. Fish Physiol Biochem 37:355–362. https://doi.org/10.1007/s10695-010-9432-5
Kulkeaw K, Sugiyama D (2012) Zebrafish erythropoiesis and the utility of fish as models of anemia. Stem Cell Res Therapy 3:55. https://doi.org/10.1186/scrt146
Kushakovsky MS (1970) Methemoglobinemia. In: Handbook of functional diagnostics. Moscow: Medicine, pp. 423–427. (in Russian)
Lai JCC, Kakuta I, Mok HOL, Rummer JL, Randall D (2006) Effects of moderate and substantial hypoxia on erythropoietin levels in rainbow trout kidney and spleen. J Exp Biol 209:2734–2738. https://doi.org/10.1242/jeb.02279
Liu Y, Jiang G, Zhang S (2004) Ontogeny of the lymphoid organs of Japanese flounder, Paralichthys olivaceus. J Ocean Univ 3:161–165. https://doi.org/10.1007/s11802-004-0028-5
Mansouri A (1981) Methemoglobin formation and reduction in relation to hemoglobin oxygen affinity. Experientia 37:95–96. https://doi.org/10.1007/BF01965591
Maslova MN, Soldatov AA, Tavrovskaya TV (1988) Seasonal dynamics in the state of the red blood system of several Black Sea fish. J Evolut Biochem Physiol 24:398–402
Meseguer J, Esteban MA, Agulleiro B (1991) Stromal cells, macrophages and lymphoid cells in the head-kidney of sea bass (Dicentrarchus labrax L.). An Ultrastructural Study Arc Histol Cytol 54:299–309. https://doi.org/10.1679/aohc.54.299
Meseguer J, Lopez-Ruiz A, Garcia-Ayala A (1995) Reticulo-endothelial stroma of the head-kidney form the seawater teleost gilthead seabream (Sparus aurata L.): an ultrastructural and cytochemical study. Anat Rec 241:303–309. https://doi.org/10.1002/ar.1092410303
Moritz KM, Lim GB, Wintour EM (1997) Developmental regulation of erythropoietin and erythropoiesis. Am J Physiol 273:R1829–R1844. https://doi.org/10.1152/ajpregu.1997.273.6.R1829
Muiswinkel WB, Lamers CHJ, Rombait JHWM (1991) Structural and functional aspects of the spleen in bony fish. Res Immunol 142:962–966. https://doi.org/10.1016/0923-2494(91)90093-X
Nikinmaa M, Cech JJ, Ryhaenen L, Salama A (1987) Red cell function of carp (Cyprinus carpio) in acute hypoxia. J Exp Biol 47:53–58
Nilsson S, Grove D (1973) Autonomic nerve control of the spleen in a fish, Gadus morhua. Acta Physiol Scandinavica 89:98–104
Olander CP, Parr CE (1978) Methemoglobin in hypoxic rats. Experientia 33:1656–1657. https://doi.org/10.1007/BF01934055
Parfenova IA (2004) Critical and threshold oxygen tensions in marine fish of different tolerance to external hypoxia. In VII International Scientific and Practical Conference of Students, Postgraduates and Young Scientists «Ecology. A man. Society». Kiev, May 13–15. 2004, Kiev, pp. 53. (in Russian)
Rosenberg R, Nilsson HC, Diaz RJ (2001) Response of benthic fauna and changing sediment redox profiles over a hypoxic gradient. Estuar Coast Shelf Sci 53:343–350. https://doi.org/10.1006/ecss.2001.0810
Rodriguez FA (1995) Basic hematology of Oncorhynchus mykiss (Salmonidae), in Cundinamarca, Colombia. Rev Biol Trop 4:283–288
Salama A, Nikinmaa M (1990) Effect of oxygen tension on catecholamine-induced formation of cAMP and on swelling of carp red blood cells. Am J Physiol 259:C723–C726. https://doi.org/10.1152/ajpcell.1990.259.5.C723
Santos AA, Gutierre RC, Antoniazzi MM, Ranzani MJTP, Silva MRR, Oshima CTF, Egami MI (2011) Morphocytochemical, immunohistochemical and ultrastructural characterization of the head kidney of fat snook Centropomus parallelus. J Fish Biol 79:1685–1707. https://doi.org/10.1111/j.1095-8649.2011.02718.x
Soivio A, Nyholm K, Westman K (1974) Changes in haematocrit values in blood samples treated with and without oxygen: a comparative study with four salmonid species. J Fish Biol 6:763–769. https://doi.org/10.1111/j.1095-8649.1974.tb05118.x
Soldatov AA (2005a) Peculiarities of organization and functioning of the fish red blood system. J Evolut Biochem Physiol 41:272–281. https://doi.org/10.1007/s10893-005-0060-0
Soldatov AA (2005b) Physiological aspects of effects of urethane anesthesia on the organism of marine fishes. Hydrobiol J 41:113–126. https://doi.org/10.1615/HydrobJ.v41.i1.130
Soldatov AA, Andreeva AY, Kukhareva TA, Andreyenko TI (2020) Methemoglobin and the activities of catalase and superoxide dismutase in nucleated erythrocytes of Scorpaena porcus (Linnaeus, 1758) under experimental hypoxia (in vitro). Biophysics (russian Federation) 65:452–459. https://doi.org/10.1134/S0006350920030197
Sordyl H, Osterland A (1991) Erythropoiesis in the head kidney of rainbow trout. Fischerei-Forschung 29:61–63
Spangrude GJ, Heimfeld S, Weissman IL (1988) Purification and characterization of mouse hematopoietic stem cells. Sci 241:58–62. https://doi.org/10.1126/science.2898810
Strunjak-Perovic I, Coz-Rakovak R, Popovik TN, Jadan M (2009a) Seasonality of nuclear abnormalities in gilthead sea bream Sparus aurata (L.) erythrocytes. Fish Physiol Biochem 35:287–296. https://doi.org/10.1007/s10695-008-9208-3
Strunjak-Perovic I, Popovik TN, Coz-Rakovak R, Jadan M (2009b) Nuclear abnormalities of marine fish erythrocytes. J Fish Biol 74:2239–2249. https://doi.org/10.1111/j.1095-8649.2009.02232.x
Tasea C (1976) Introducere in morfologia cantitativa cito-histologica. Bucuresti. Editura Academiei R.S.R.
Tatner MF, Manningm MJ (1985) The ontogenetic development of the reticuloendothelial system in the rainbow trout, Salmo gairdneri Richardson. J Fish Diseases 8:35–41. https://doi.org/10.1111/j.1365-2761.1985.tb01214.x
Tiihonen K, Nikinmaa M (1991) Short communication: Substrate utilization by carp (Cyprinus carpio) erythrocytes. J Exp Biol 161:509–514
Tufts B (1992) In vitro evidence for sodium-dependent pH regulation in sea lamprey (Petromyzon marinus) red blood cells. Can J Zool 70:411–416. https://doi.org/10.1139/z92-062
Val AL, De Menezes GC, Wood CM (1997) Red blood cell adrenergic responses in Amazonian teleosts. J Fish Biol 52:83–93. https://doi.org/10.1111/j.1095-8649.1998.tb01554.x
Valenzuela A, Silva V, Tarifeño E, Klempau A (2005) Effect of acute hypoxia in trout (Oncorhynchus mykiss) on immature erythrocyte release and production of oxidative radicals. Fish Physiol Biochem 31:65–72. https://doi.org/10.1007/s10695-005-5288-5
Wickramasinghe SN (1993) Erythropoietin and the human kidney: evidence for an evolutionary link from studies of Salmo gairdneri. Comp Biochem Physiol A 104A:63–65. https://doi.org/10.1016/0300-9629(93)90009-s
Witeska M (2013) Erythrocytes in teleost fishes: a review. Zool Ecol 23:275–281. https://doi.org/10.1080/21658005.2013.846963
Zhu CD, Wang ZH, Yan B (2013) Strategies for hypoxia adaptation in fish species: a review. J Comp Physiol B 183:1005–1013. https://doi.org/10.1007/s00360-013-0762-3
Zolotova TE (1987) Experimental study of hematopoiesis in fish: abstract of PhD dissertation. Moscow State University, Moscow (in Russian)
Funding
The study received the State assignment 121041400077–1 and a grant from the Russian Foundation for Basic Research (grant 20–04-00037A).
Author information
Authors and Affiliations
Contributions
A.A.S.: pivotal idea, task setting, experimental design, writing and editing the manuscript, and determination of methemoglobin concentration; T.A.K.: setting up the experiments, analysis of the head kidney preparations, statistical data processing, and preparing the illustrations; V.N.R.: setting up the experiments, analysis of the blood preparations, statistical data processing, and preparing the illustrations; E.S.K.: setting up the experiments, statistical data processing, and preparing illustrations; A.Y.A.: setting up the experiments and English translation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures using fish were accomplished in accordance with the European Communities Council Directive (2010/63/EU) and approved by the local Institutional Animal Care and Use Committee (protocol #28 from 15.02. 2018). All authors agree to participate in this paper.
Consent for publication
All authors agree to submit the paper for publication in the Journal of Fish Physiology and Biochemistry.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soldatov, A.A., Kukhareva, T.A., Rychkova, V.N. et al. Cellular composition of the black scorpionfish (Scorpaena porcus, L 1758) blood and head kidney under short-time acute exposure to hypoxia. Fish Physiol Biochem 48, 1209–1220 (2022). https://doi.org/10.1007/s10695-022-01115-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-022-01115-y