Abstract
This experiment was aimed to determine the possible beneficial effects of dietary ascorbic acid (AA) on hematological indices, immune responses, and antioxidative capacity of Oncorhynchus mykiss treated with antibiotic oxytetracycline (OTC). A total of 150 fish were divided evenly among five experimental groups (30 fish of each, in 3 replicates) receiving diets containing OTC (0 and 100 mg per kg fish weight) and AA (100, 200, 400, and 800 mg per kg fish diet) for 28 days. Treatments include group A or control (100 mg AA without OTC), group B (100 mg AA with OTC), group C (200 mg AA with OTC), group D (400 mg AA with OTC), and group E (800 mg AA with OTC). The results obtained showed that the hematological indices (red blood cells, white blood cells, hematocrit, hemoglobin, and neutrophils), immunological parameters (plasma lysozyme, plasma complement, and skin mucus alkaline phosphatase activities), and antioxidant enzymes activities (superoxide dismutase and catalase) were significantly decreased by OTC in O. mykiss fed control diet (P < 0.05). The results also revealed that OTC significantly increased the activity of biochemical enzymes (aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase) in the plasma of O. mykiss fed control diet (P < 0.05). However, in comparison to the control diet, feeding fish with higher amounts of AA (400 and 800 mg/kg diet) significantly restored the hematological, immunological, and antioxidative responses in OTC-treated groups (p < 0.05). These findings show that the dietary supplementation of AA at 400 or 800 mg/kg diet is beneficial in relieving O. mykiss from OTC-induced oxidative stress and immunosuppression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the previous decade, the outbreak of infectious diseases has emerged as a major limiting factor for aquaculture expansion which causes massive mortality and economic losses (Shalini et al. 2019). An important strategy used to address this problem is the administration of antibiotics (Rico et al. 2013). Tetracyclines are a common class of antibiotics which are widely used in aquaculture. Oxytetracycline (OTC) is a well-known tetracycline antibiotic that is produced from Streptomyces rimosus fungi (Rodrigues et al. 2017a; FAO 1990). OTC is commonly used as an effective treatment against bacterial pathogens of fishes, such as Aeromonas salmonicida, Aeromonas hydrophila, Lactococcus garvieae, Vibrio anguillarum, and Pseudomonas sp. (Leal et al. 2019; Nakano et al. 2018). More utilization of OTC when compared with other antibiotics is due to its low-cost, legal availability, high efficiency, and non-specific selectivity in the treatment of bacterial infection (Pês et al. 2018; Lee et al. 2020; Rodrigues et al. 2017a). OTC can be applied via injection, immersion and oral administration, but is commonly administered through diet in the amount of approximately 75 mg per kg of fish biomass (Yonar 2012).
However, the administration of antibiotic drugs may pose several side effects such as the occurrence of antibiotic resistance in bacterial pathogens (Lunden et al. 1998), residue in fish tissues (Harikrishnan et al. 2009), and reduction of fish health (Shalini et al. 2019). For instance, OTC is found to induce oxidative stress and liver malfunctioning. OTC can increase the generation of reactive oxygen species (ROS) in the fish body, which cause peroxidation of liver cell membrane lipids resulting in an increased leakage of intracellular enzymes to the blood (Nakano et al. 2018). In addition, negative effects of OTC on immune response, antioxidant defense, hematological parameters, nephrotoxicity, genotoxicity, and histological changes in fish species have been previously documented (Rodrigues et al. 2017b; Hoseini and Yousefi 2019; Reda et al. 2013; Yonar et al. 2011; Mathew and Ambili 2017). As a result, OTC may increase the fish susceptibility to secondary pathogens and also to environmental stressors. Hence, there is a necessity for the discovery of methods to alleviate the harmful effects of OTC on fishes.
Vitamin C or L-ascorbic acid (AA) is an important micro-nutrient for fishes, playing a key role in the growth performance and physiological process (Lin and Shiau 2005). It is also a potent natural antioxidant that plays a vital role in scavenging ROS to protect the fish from oxidative stress (Bae et al. 2012; Hajirezaee et al. 2020). Moreover, AA has an important role in hematology (Sandnes et al. 1990), collagen formation (Hunter et al. 1979), reproduction (Emata et al. 2000; Cavalli et al. 2003), immune response (Misra et al. 2007; Roosta et al. 2014; Zhou et al. 2012), and recovery from exposure to toxicant and environmental stressors (Saha and Kaviraj 2009; Kim et al. 2017; Wahli et al. 2003). Dietary supplementation of AA can provide normal growth of fish at low doses; however, a higher dose of AA is required to increase the stress tolerance in fish (Vani et al. 2011).
Nevertheless, up to date, there is no data available on the role of dietary AA on OTC-induced stress in fish. Therefore, the current study was designed to assess the possible role of dietary AA in mitigating the adverse effects of OTC in terms of hematological parameters, skin mucus and blood immune parameters, and antioxidant capacity of rainbow trout (Oncorhynchus mykiss).
Materials and methods
Chemicals
Vitamin C (L-ascorbyl-2-monophosphate) and oxytetracycline were purchased from Sigma-Aldrich Co. (Darmstadt, Germany) and Rooyandarou Co. (Tehran, Iran), respectively. All the other chemicals were provided by Merck Chemical Co. (Darmstadt, Germany).
Experimental fish
Healthy juveniles of Oncorhynchus mykiss (43 ± 2.6 g) were purchased from a local fish farm and were transported to the wet lab in a fiberglass-aerated tank. They were acclimated to lab conditions for 14 days in 1000-L fiberglass tanks filled with tap water under a natural 12-h photoperiod. During the adaptation period, rainbow trout were fed three times a day with a commercial diet containing 43% crude protein, 14% crude fat, 4% crude fiber, and 11% ash. The water physicochemical parameters were regularly monitored, and water temperature range from 13.64 to 15.47 °C, pH from 7.2 to 7.5, and oxygen levels from 7.1 to 7.6 mg L−1. Excess feed and fecal waste were siphoned out daily and a quarter portion of water in the tank is replaced with clean water.
Experimental diets
Five experimental diets were provided by mixing a basal diet (Table 1) with varying amounts of OTC (0 and 100 mg/kg body weight) and AA (100, 200, 400 and 800 mg/kg diet). Briefly, feed ingredients were ground into powder with multi-function pulvetizer, and then were passed through a 1.0-mm sieve. Grounded ingredients were mixed well with the OTC, AA, and oils, and then distilled water was added into the mixture to form a stiff dough. The dough was mixed for 30 min and passed through grinder, dried for 48 h at 25 °C, and finally stored at − 18 °C until use (Kim et al. 2017). Because it was floating diet, feeding was done slowly to make sure the experimental diet was eaten immediately by the fish, and the OTC and AA were not released to the water before eating by fish.
Experimental design
After acclimatization, a total number of 150 rainbow trout were randomly distributed into five experimental groups (A, B, C, D, and E) in triplicates (10 fish per tank) and maintained throughout an experimental period of 28 days. The diet used for all groups (B, C, D, and E) were contained OTC (100 mg/kg body weight) except group A (control). The diet used for groups A and B were supplemented by the normal dose of AA (100 mg/kg feed), while the diets used for groups C, D, and E were prepared by supplementing different high doses of AA (200, 400, and 800 mg/kg, respectively). During the experiment, O. mikiss were fed with one of these diets at 2% of biomass and three times (8:00, 13:00, and 19:00) daily.
Sampling
After 28 days of feeding, three fish were randomly harvested from each replicate and immediately anesthetized with powdered clove (200 ppm). Blood samples were drawn from the lateral tail vein using sterile syringes and become two parts. One part was kept in tubes containing heparin for analysis of blood parameters and the second sample was centrifuged at 1500 × g for 5 min to separate plasma (supernatant). The plasma was removed and maintained at − 70 °C until analysis (Yangthong et al. 2016).
Skin mucus was collected according to Shaluei et al. (2017). Briefly, three fish were randomly harvested from each replicate and anaesthetized using clove powder. Each harvested fish was transferred into a separate plastic bag containing 10 ml NaCl (50 mM). The bag was shaken well for 1 min and the skin mucus was immediately poured into 15-ml sterile tubes and centrifuged at 1500 × g for 4 min. The upper layer was collected and maintained at − 80 °C until use.
Analyses
Measurement of blood parameters
Total number of erythrocytes (RBC) and leucocytes (WBC) were determined using a Neubauer chamber by dilution of blood samples in the Natt and Herrick solution (Natt and Herrick 1952). Blood hematocrit (Ht) was measured via micro hematocrit centrifuge at 10,500 × g for 5 min (Baba et al. 2015). The hemoglobin amount (Hb) was estimated spectrophotometrically at 540 nm using cyanmethemoglobin method (Harikrishnan et al. 2009). The mean cell volume (MCV), the mean cell hemoglobin (MCH), and the mean cell hemoglobin concentration (MCHC) were determined from the total RBC counts, Hb concentrations, and Ht values according to the available formulae (Azaza et al. 2020). Neutrophils, lymphocytes, and monocyte numbers were quantified by Giemsa-stained blood smears under light microcopy (Safari and Sarkheil 2018).
Plasma and skin mucus immunology
The total protein and albumin contents of plasma samples were assayed using quantitative detection kits (Pars Azmun Co, Iran). Plasma total protein content was measured by Piotrowski’s assay at 540 nm. Plasma albumin content was assayed using bromocresol green dye at 630 nm (Mohammadi et al. 2020a, b). Plasma globulin content was measured by subtracting amount of albumin from total protein (Kumar et al. 2005).
The total immunoglobulin content (Total Ig) of plasma and mucus samples was quantified according to Siwicki and Anderson (1993). The sample was mixed with 12% polyethylene glycol, incubated at 25 °C for 2 h, centrifuged at 3000 × g for 15 min, and then the supernatant removed and the remaining protein was measured after its subtraction from total protein content.
Lysozyme activity of plasma and mucus sample was measured according to method of Ellis (1990) using lyophilized Micrococcus lysodeikticus as target in phosphate buffer (pH 6.2). One unit of enzymatic activity was equal to the amount of lysozyme causing a decrease in absorbance of 0.001 at 490 nm.
The method of Yano (1992) involving the use of rabbit red blood cells (RaRBC) as substrate was used to determine the alternative complement activity (ACH50) in plasma. The volume of plasma producing 50% hemolysis of RaRBC was monitored spectrophotometrically at 414 nm.
Protease activity in mucus was measured following Guardiola et al. (2014) using the azocasein hydolysis assay. One unit of protease activity was considered as nanogram of the azo-dye released during 60 s at 37 °C.
Plasma antioxidant enzymes
Blood superoxide dismutase (SOD) and catalase (CAT) activities were assayed spectrophotometrically similar to the method of Yonar et al. (2011). SOD activity was measured by checking the amount of enzyme required to prevent the reduction of nitroblue tetrazolium, which determined at 560 nm. The CAT enzyme activity determination was based on measuring the rate constant of hydrogen peroxide decomposition by this enzyme. The conversion of H2O2 to H2O, and O2 was measured at 240 nm.
Plasma malondialdehyde content (MDA) was measured spectrophotometrically at 548 nm according to Placer et al. (1966) using the thiobarbituric acid reaction. The MDA activity was expressed as the nanomol of enzyme per milliliter of plasma.
Biochemical parameters
The activities of plasma alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were measured spectrophotometrically using quantitative analyses kits (Pars Azmun Co, Iran). ALT activity was expressed as the amount of enzyme needed to form one molecule of pyruvate, which is determined at 340 nm. AST activity was defined as the amount of enzyme needed to form one molecule of glutamate, which is determined at 340 nm. ALP activity was expressed as the amount of enzyme needed to make one micromole of p-nitrophenol, which is determined at 405 nm.
Statistical analysis
The normality of acquired data was determined by Kolmogorov–Smirnov test and the differences between the means were examined via one-way analysis of variance (ANOVA), followed by Duncan test. Differences between mean values were considered to be significant when the confidence level was 95% (P < 0.05). Results are defined as mean ± standard deviation for each group. All the statistical analyses were done using the SPSS computer software program (Version 24).
Results
Hematological parameters
The hematological parameters of rainbow trout are presented in Table 2. The RBC, WBC, Hb, Hct, and neutrophils values were significantly changed with the incorporation of OTC in the diet of rainbow trout (P < 0.05). The highest numbers of RBC and WBC were recorded in group A (100 mg AA without OTC) while the group B (100 mg AA with OTC) showed the lowest values. Feeding fish in the E group (800 mg AA with OTC) significantly increased these parameters as compared to the B group (P < 0.05). Hemoglobin, hematocrit, and neutrophils values were significantly lower in group B than in group A (control) and the group which were fed with 800 mg/kg diet of AA (group E) recorded significantly increased Hct value as compared to group B (P < 0.05). There was no significant alteration in the MCV, lymphocyte and monocyte values between the five groups.
Biochemical enzymes
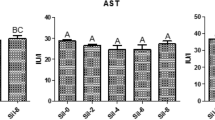
As shown in Fig. 1, the plasma AST, ALT, and ALP activities were significantly elevated by the addition of OTC to the diet of fish (P < 0.05). The highest activities of these enzymes were recorded in the group B. Fish in the group E exhibited significantly decreased ALT and ALP activities compared with those in the group B. Dietary supplementation with different levels of AA did not cause significant changes in the AST activity (P > 0.05).
Activity of AST (A), ALT (B), and ALP (C) in blood of rainbow trout fed with experimental diets after 28 days. The presence of letters indicates significant differences between treatments (P < 0.05) for n = 3. A—diet contained 100 mg AA without OTC; B—diet contained 100 mg AA with OTC; C—diet contained 200 mg AA with OTC; D—diet contained 400 mg AA with OTC; E—diet contained 800 mg AA with OTC
Plasma immune responses
The measurements of the humoral immunity parameters are presented in Table 3. The lysozyme and complement activities were significantly affected with the incorporation of OTC in the diet (P < 0.05). The highest values of these indicators were revealed in the group A whereas the lowest were in the group B. Dietary supplementation of AA significantly improved the lysozyme and complement activities in the group D (400 mg AA/kg diet). There were no significant changes in the total immunoglobulin, total protein, albumin, and globulin levels between the study treatments (P > 0.05).
Mucus immune responses
The measurements of the mucus immunity parameters are presented in Table 4. The mucus lysozyme, alkaline phosphatase, and protease activities in the group B (diet contained 100 mg AA with OTC) decreased significantly compared to the control group (P < 0.05). The lowest activities of lysozyme and protease were observed in group B and dietary supplementation with additional amounts of AA had no significant change for these parameters (P > 0.05). The lowest alkaline phosphatase activity was observed in group B. The dietary supplementation with AA significantly improved alkaline phosphatase activity in the group E (800 mg AA/kg diet) (P < 0.05). The total immunoglobulin content did not significantly vary among the different study groups (P > 0.05).
Antioxidant responses
As seen in Fig. 2, the activities of SOD and CAT were significantly altered by the incorporation of OTC in the diet of fish (P < 0.05). The lowest SOD and CAT activities were observed in group B and the groups which were supplemented with higher levels of AA (groups D and E) showed significantly increased SOD and CAT activities. The blood level of MDA found to be significantly increased upon OTC administration in group B compared to control group (P < 0.05). The highest activity of MDA was recorded in group B and dietary supplementation with additional levels of AA (200, 400, and 800 mg/kg diet) significantly decreased the level of blood MDA compared to the group B.
Activity of SOD (A), CAT (B) and MDA (C) in blood of rainbow trout fed with experimental diets after 28 days. The presence of letters indicates significant differences between treatments (P < 0.05) for n = 3. A—diet contained 100 mg AA without OTC; B—diet contained 100 mg AA with OTC; C—diet contained 200 mg AA with OTC; D—diet contained 400 mg AA with OTC; E—diet contained 800 mg AA with OTC
Discussion
Vitamin C or L-ascorbic acid (AA) is a water-soluble micronutrient needed for multiple physiological process of aquatic animals (Shahkar et al. 2015). Previous studies showed the anti-stress (Misra et al. 2007), antioxidant (Wan et al. 2014), immuno-stimulatory (Zhou et al. 2012), and growth promoting (Liang et al. 2017) properties of AA in fishes. Considering the beneficial effects of AA, the current study evaluated the possible role of dietary AA in the reduction of OTC-induced stress in O. mykiss.
The measurement of hematological indicators is an important tool to monitor the physiological and the pathological alterations in fish (Burgos-Aceves et al. 2019; Javanmardi et al. 2020a, b). In the existing experiment, the RBC number and the Ht and Hb values were significantly declined by the inclusion of OTC in the diet of O. mykiss fed with low dose of AA (100 mg/kg diet). These results suggested that OTC has caused anemia condition in O. mykiss, which may be a result of erythropoiesis inhibition and also increased erythrocyte lysis (Ramesh et al. 2018; Yonar et al. 2020). Similarly, significant reductions in RBC, Ht, and Hb values were observed by Omoregie and Oyebanji (2002) in Oreochromis niloticus fed diets incorporated with OTC. However, in our study, administration of AA at 800 mg/kg diet markedly restored the RBC and Ht values in fish treated with OTC, which were comparable with control group. This may be due to the anti-oxidative properties of AA which prolongs the life span of erythrocytes by its potent ROS scavenging activity (Nayak et al. 2007). Our finding agrees with the observation of Affonso et al. (2007), who suggested that feeding with higher levels of AA significantly increased the RBC and Ht values in Brycon amazonicus.
The study of white blood cell (WBC) profile can provide useful information regarding the general health status of fish. The white blood cells and differential leukocytes number are useful tools to evaluate the immune response in fish (De Pedro et al. 2005; Vali et al. 2020). In this study, OTC administration significantly decreased the WBC and neutrophils values with no significant effect on monocytes and lymphocytes. The reduction in the WBC and neutrophils values may indicate immunosuppressive effects of OTC and subsequently increased fish’s susceptibility to infectious diseases. These obtained results are in accordance with the finding of Dobšíková et al. (2013), who observed that OTC decreases the WBC value in Cyprinus carpio. On contrary, a significant increase in WBC value was recorded with the supplementation of AA in groups D and E (400 and 800 mg/kg diet respectively) compared to group B, which indicates that the immune system has been restored. Similarly, Misra et al. (2007) suggested that higher levels of dietary AA could induce a significant elevation in WBC count in Labeo rohita.
The innate immunity in fish includes two parts of mucosal and humoral immunity which both defense lines play a vital role in the fight against pathogens (Harikrishnan et al. 2012; Hoseinifar et al. 2016). The presence of various factors such as lysozymes, complement, and other lytic components in plasma prevents/or kills microorganisms (Alexander and Ingram 1992). The result of the current study revealed that OTC administration significantly decreased the activities of lysozyme and complement in the plasma of fish fed low dose of AA, suggesting that innate immunity of fish was suppressed following treatment with OTC antibiotic. This OTC-induced immunosuppression is attributable to its high tissue penetration capacity, making OTC capable of interfering with immune system organs, such as the liver and kidney (Yonar et al. 2011). Our results are in accordance with Hoseini and Yousefi (2019) that indicated OTC treatment in rainbow trout significantly decreased lysozyme and complement activities. The results of our experiment also revealed that the plasma lysozyme and complement activities were significantly increased in the D group (fed 400 mg AA/kg diet) compared with those in the group B (fed 100 mg AA per kg diet), which may indicate an improved immune response in fish. In line with our results, several studies showed that dietary AA elevated the innate immune responses in fishes (Li and Lovell, 1985; Hardie et al. 1991; Dunier et al. 1995).
The skin mucus includes various factors such as lysozyme, protease, immunoglobulins, and lectins that play a vital role in the primary defense and protection of fish against pathogens (Hoseinifar et al. 2015; Mohammadi et al. 2020a, b). The results of the present experiment revealed that the mucus lysozyme, protease, and alkaline phosphatase activities markedly decreased with the incorporation of OTC in the diet of rainbow trout fed with low dose of AA (100 mg/kg diet), which may indicate a weakened ability to cope with pathogens. While many experiments have focused on the immunosuppressive effects of OTC on the humoral immunity of fish, these effects in mucosal immunity have not been investigated. Our results also showed that the mucus alkaline phosphatase activity of fish treated with OTC increased significantly in the group E (800 mg AA/kg diet), which may be attributed to an increased mucosal immune response by the AA supplementation at higher doses. Similar to our results, Roosta et al. (2014) observed an increase in the activity of mucus alkaline phosphatase in Rutilus rutilus caspicus following dietary supplementation with high levels of AA.
This is well established that increased reactive oxygen species (ROS) levels will lead to oxidative stress, which may negatively affect the fish and crustaceans’ health and cause structural damage in tissue cells (Akbary and Aminikhoei 2018; Tavabe et al. 2020; Khan et al. 2021). Antioxidant defense system includes some pivotal enzymes such as superoxide dismutase (SOD) and catalase (CAT) that tend to prevent ROS formation (Yonar et al. 2014). In our study, a significant decline in the activity of SOD and CAT as well as a significant increase in the level of MDA was observed in the plasma of fish in the group B (100 mg AA with OTC) compared to the control group (100 mg AA without OTC). These recorded changes in SOD and CAT activities can be attributed to an excessive formation of ROS in the fish body, which resulted in the high consumption of these enzymes during elimination of ROS (Rahman et al. 2020). Moreover, the increase in MDA level similarly shows this situation, knowing that MDA is the main product of lipid peroxidation (Yonar 2018). Previously, similar results were obtained from dietary administration of OTC in O. mykiss by Nazeri et al. (2017). On the other hand, feeding fish in the D and E treatments (400 and 800 mg/kg diet, respectively) significantly reversed the activity of SOD and CAT enzymes and as well as the MDA level in the fish plasma, indicating the ameliorative effects of dietary AA on OTC-induced oxidative damage. This can be due to the high anti-oxidative capacity of AA which makes it capable of neutralizing ROS and reduction of oxidative stress (Rouhier et al. 2008; Verma et al. 2007). This is in agreement with Wan et al. (2014) who found that higher levels of vitamin C could effectively suppress oxidative stress induced by the high levels of pH.
The measurement of the activity of biochemical enzymes (AST, ALT, and ALP) in the plasma can provide useful information regarding the health condition of fish and crustaceans’ liver tissue (Nakano et al. 2018; Javanmardi et al. 2020a, b). In the current study, our results showed significantly increased plasma AST, ALT, and ALP activities in OTC-treated fish (group B) compared to the group A (control). This increase may be linked to the OTC-induced oxidative stress, which affect the permeability of hepatocyte through oxidative damage resulting in leakage of these enzymes to the fish blood (Yonar 2012; Banaee et al. 2011). In line with our findings, the increased activities of biochemical enzymes (AST and ALT) in fish treated with OTC have been previously observed also in Oncorhynchus kisutch (Nakano et al. 2018). The results of present study also showed that feeding fish in the group E (diet contained 800 mg AA) significantly decreased these parameters as compared to the B group (diet contained 100 mg AA). These changes may indicate that dietary supplementation with AA helped to reduce the OTC-induced liver damage, resulting in the decreased leakage of these enzymes from liver to the blood. In similar study conducted by Nazeri et al. (2017), feeding with different levels of rutin (a flavonoid) significantly alleviated the activities of plasma enzymes in OTC-treated Oncorhynchus mykiss.
In conclusion, the current results showed that the dietary administration of OTC in rainbow trout significantly affected hematological profile, innate immune response, and antioxidant capacity of O. mykiss. The low dose of dietary AA was not capable of alleviating the OTC-induced stress. However, supplementation of fish with higher levels of AA found to restore the suppressed immune response as evidenced by increased activities of plasma lysozyme and complement, skin mucus alkaline phosphatase, and augmented WBC and neutrophils values. Moreover, attenuation of OTC-induced oxidative stress by increased activity of anti-oxidative enzymes (SOD and CAT) and decreased activity of plasma biochemical enzymes (AST, ALT and ALP) was also found in fish fed with higher levels of AA. Hence, dietary administration of AA at higher levels could be an effective strategy to decrease the negative effects of OTC on O. mykiss.
Data availability
All data and materials used in the experiment are available and achievable upon request to the corresponding author Sina.javanmardi@ut.ac.ir.
Code availability
Not applicable.
References
Affonso EG, da Costa SE, Tavares-Dias M, de Menezes GC, de Carvalho CSM, Nunes ÉDSS, Ituassú DR, Roubach R, Ono EA, Fim JDI, Marcon JL (2007) Effect of high levels of dietary vitamin C on the blood responses of matrinxã (Brycon amazonicus). Comp Biochem Physiol A 147:383–388. https://doi.org/10.1016/j.cbpa.2007.01.004
Akbary P, Aminikhoei Z (2018) Effect of polysaccharides extracts of algae Ulva rigida on growth, antioxidant, immune response and resistance of shrimp, Litopenaeus vannamei against Photobacterium damselae. Aquacult Res 49:2503–2510. https://doi.org/10.1111/are.13710
Alexander JB, Ingram GA (1992) Noncellular nonspecific defence mechanisms of fish. Annu Rev Fish Dis 2:249–279. https://doi.org/10.1016/0959-8030(92)90066-7
Azaza MS, Saidi SA, Dhraief MN, El-Feki A (2020) Growth performance, nutrient digestibility, hematological parameters, and hepatic oxidative stress response in juvenile Nile Tilapia, Oreochromis niloticus, fed carbohydrates of different complexities. Animals 10:1913. https://doi.org/10.3390/ani10101913
Baba E, Uluköy G, Öntaş C (2015) Effects of feed supplemented with Lentinula edodes mushroom extract on the immune response of rainbow trout, Oncorhynchus mykiss, and disease resistance against Lactococcus garvieae. Aquaculture 448:476–482. https://doi.org/10.1016/j.aquaculture.2015.04.031
Bae JY, Park GH, Yoo KY, Lee JY, Kim DJ, Bai SC (2012) Re-evaluation of the optimum dietary vitamin C requirement in juvenile eel, Anguilla japonica by using L-Ascorbyl-2-monophosphate. Asian-Aust J Anim Sci 25:98–103. https://doi.org/10.5713/ajas.2011.11201
Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K (2011) Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss). Pestic Biochemand Phys 99:1–6. https://doi.org/10.1016/j.pestbp.2010.09.001
Burgos-Aceves MA, Lionetti L, Faggio C (2019) Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci Total Environ 670:1170–1183. https://doi.org/10.1016/j.scitotenv.2019.03.275
Cavalli RO, Batista FM, Lavens P, Sorgeloos P, Nelis HJ, De Leenheer AP (2003) Effect of dietary supplementation of vitamins C and E on maternal performance and larval quality of the prawn Macrobrachium rosenbergii. Aquaculture 227:131–146. https://doi.org/10.1016/S0044-8486(03)00499-X
Dabrowski K, Lee KJ, Guz L, Verlhac V, Gabaudan J (2004) Effects of dietary ascorbic acid on oxygen stress (hypoxia or hyperoxia), growth and tissue vitamin concentrations in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 233:383–392. https://doi.org/10.1016/j.aquaculture.2003.09.047
De Pedro N, Guijarro AI, López-Patiño MA, Martínez-Álvarez R, Delgado MJ (2005) Daily and seasonal variations in haematological and blood biochemical parameters in the tench, Tinca tinca Linnaeus, 1758. Aquac Res 36:1185–1196. https://doi.org/10.1111/j.1365-2109.2005.01338.x
Dobšíková R, Blahová J, Mikulíková I, Modrá H, Prášková E, Svobodová Z, Škorič M, Jarkovský J, Siwicki AK (2013) The effect of oyster mushroom β-1.3/1.6-D-glucan and oxytetracycline antibiotic on biometrical, haematological, biochemical, and immunological indices, and histopathological changes in common carp (Cyprinus carpio L.). Fish Shellfish Immun 35:1813–1823. https://doi.org/10.1016/j.fsi.2013.09.006
Dunier M, Vergnet C, Siwicki AK, Verlhac V (1995) Effect of lindane exposure on rainbow trout (Oncorhynchus mykiss) immunity: IV. Prevention of nonspecific and specific immunosuppression by dietary vitamin C (ascorbate-2-polyphosphate). Ecotox Environ Saf 30:259–268. https://doi.org/10.1006/eesa.1995.1029
Ellis AE (1990) Lysozyme assays. In: Stolen JS, Fletcher TC, Anderson DP, Robersen BS, Van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publications, Fair Haven, pp 101–104
Emata AC, Borlongan IG, Damaso JP (2000) Dietary vitamin C and E supplementation and reproduction of milkfish Chanos chanos Forsskal. Aquac Res 31:557–564. https://doi.org/10.1046/j.1365-2109.2000.00467.x
FAO (1990). Oxytetracycline. Residues of some veterinary drugs in animals and foods. Food and Agriculture Organization of the United Nations, Rome 131 p.
Guardiola FA, Cuesta A, Abellán E, Meseguer J, Esteban MA (2014) Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immun 40:24–31. https://doi.org/10.1016/j.fsi.2014.06.018
Hajirezaee S, Mohammadi G, Naserabad SS (2020) The protective effects of vitamin C on common carp (Cyprinus carpio) exposed to titanium oxide nanoparticles (TiO2-NPs). Aquaculture 518:734734. https://doi.org/10.1016/j.aquaculture.2019.734734
Hardie LJ, Fletcher TC, Secombes CJ (1991) The effect of dietary vitamin C on the immune response of the Atlantic salmon (Salmo salar L.). Aquaculture 95:201–214. https://doi.org/10.1016/0044-8486(91)90087-N
Harikrishnan R, Balasundaram C, Heo MS (2009) Effect of chemotherapy, vaccines and immunostimulants on innate immunity of goldfish infected with Aeromonas hydrophila. Dis Aquat Organ 88:45–54. https://doi.org/10.3354/dao02143
Harikrishnan R, Kim JS, Balasundaram C, Heo MS (2012) Immunomodulatory effects of chitin and chitosan enriched diets in Epinephelus bruneus against Vibrio alginolyticus infection. Aquaculture 326:46–52. https://doi.org/10.1016/j.aquaculture.2011.11.034
Hoseini SM, Yousefi M (2019) Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline-induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquacult Nutr 25:298–309. https://doi.org/10.1111/anu.12853
Hoseinifar SH, Roosta Z, Hajimoradloo A, Vakili F (2015) The effects of Lactobacillus acidophilus as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail (Xiphophorus helleri). Fish Shellfish Immun 42:533–538. https://doi.org/10.1016/j.fsi.2014.12.003
Hoseinifar SH, Zoheiri F, Dadar M, Rufchaei R, Ringø E (2016) Dietary galactooligosaccharide elicits positive effects on non-specific immune parameters and growth performance in Caspian white fish (Rutilus frisii kutum) fry. Fish Shellfish Immun 56:467–472. https://doi.org/10.1016/j.fsi.2016.08.001
Hunter B, Magarelli PC Jr, Lightner DV, Colvin LB (1979) Ascorbic acid-dependent collagen formation in penaeid shrimp. Comp Biochem Physiol B 64:381–385. https://doi.org/10.1016/0305-0491(79)90286-4
Javanmardi S, Rezaei Tavabe K, Rosentrater KA, Solgi M, Bahadori R (2020a) Effects of different levels of vitamin B 6 in tank water on the Nile tilapia (Oreochromis niloticus): growth performance, blood biochemical parameters, intestine and liver histology, and intestinal enzyme activity. Fish Physiol Biochem 46:1909–1920. https://doi.org/10.1007/s10695-020-00840-6
Javanmardi S, RezaeiTavabe K, Moradi S, Abed-Elmdoust A (2020) The effects of dietary levels of the sea cucumber (Bohadschia ocellata Jaeger, 1833) meal on growth performance, blood biochemical parameters, digestive enzymes activity and body composition of Pacific white shrimp (Penaeus vannamei Boone, 1931) juveniles. Iran J Fish Sci 19:2366–2383. https://doi.org/10.22092/ijfs.2020.122330
Khan GB, Akhtar N, Khan MF, Ullah Z, Tabassum S, Tedesse Z (2021). Toxicological impact of zinc nano particles on tilapia fish (Oreochromis mossambicus). Saudi Journal of Biological Sciences. https://doi.org/10.1016/j.sjbs.2021.09.044
Kim JH, Park HJ, Kang JC (2017) Alterations in growth performance and stress responses in juvenile rockfish, Sebastes schlegelii, exposed to dietary chromium with varying levels of dietary ascorbic acid supplementation. Chemosphere 189:672–678. https://doi.org/10.1016/j.chemosphere.2017.09.071
Kumar S, Sahu NP, Pal AK, Choudhury D, Yengkokpam S, Mukherjee SC (2005) Effect of dietary carbohydrate on haematology, respiratory burst activity and histological changes in L. rohita juveniles. Fish Shellfish Immun 19:331–344. https://doi.org/10.1016/j.fsi.2005.03.001
Leal JF, Santos EB, Esteves VI (2019) Oxytetracycline in intensive aquaculture: water quality during and after its administration, environmental fate, toxicity and bacterial resistance. Rev Aquacult 11:1176–1194. https://doi.org/10.1111/raq.12286
Lee PT, Liao ZH, Huang HT, Chuang CY, Nan FH (2020) β-glucan alleviates the immunosuppressive effects of oxytetracycline on the non-specific immune responses and resistance against Vibrio alginolyticus infection in Epinephelus fuscoguttatus× Epinephelus lanceolatus hybrids. Fish Shellfish Immun 100:267–475. https://doi.org/10.1016/j.fsi.2020.03.046
Li Y, Lovell RT (1985) Elevated levels of dietary ascorbic acid increase immune responses in channel catfish. J Nutr 115:123–131. https://doi.org/10.1093/jn/115.1.123
Liang XP, Li Y, Hou YM, Qiu H, Zhou QC (2017) Effect of dietary vitamin C on the growth performance, antioxidant ability and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco Richardson). Aquac Res 48:149–160. https://doi.org/10.1111/are.12869
Lin MF, Shiau SY (2005) Dietary L-ascorbic acid affects growth, nonspecific immune responses and disease resistance in juvenile grouper, Epinephelus malabaricus. Aquaculture 244:215–221. https://doi.org/10.1016/j.aquaculture.2004.10.026
Lunden T, Miettinen S, Lönnström LG, Lilius EM, Bylund G (1998) Influence of oxytetracycline and oxolinic acid on the immune response of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immun 8:217–230. https://doi.org/10.1006/fsim.1998.0142
Mathew AR, Ambili TR (2017). Haematological, enzymological and biochemical effects of antibiotic oxytetracycline in a fresh water fish Labeo rohita. Devagiri Journal of Science 3:94–109. http://devagirijournals.com/uploads/pack/66_avila.admin_14185008181053.pdf
Misra CK, Das BK, Mukherjee SC, Pradhan J (2007) Effects of dietary vitamin C on immunity, growth and survival of Indian major carp Labeo rohita, fingerlings. Aquacult Nutr 13:35–44. https://doi.org/10.1111/j.1365-2095.2007.00451.x
Mohammadi G, Rafiee G, El Basuini MF, Abdel-Latif HM, Dawood MA (2020a) The growth performance, antioxidant capacity, immunological responses, and the resistance against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus) fed Pistacia vera hulls derived polysaccharide. Fish Shellfish Immun 106:36–43. https://doi.org/10.1016/j.fsi.2020.07.064
Mohammadi G, Rashidian G, Hoseinifar SH, Naserabad SS, Van Doan H (2020b) Ginger (Zingiber officinale) extract affects growth performance, body composition, haematology, serum and mucosal immune parameters in common carp (Cyprinus carpio). Fish Shellfish Immun 99:267–273. https://doi.org/10.1016/j.fsi.2020.01.032
Nakano T, Hayashi S, Nagamine N (2018) Effect of excessive doses of oxytetracycline on stress-related biomarker expression in coho salmon. Environ Sci Pollut Res 25:7121–7128. https://doi.org/10.1007/s11356-015-4898-4
Natt MP, Herrick CA (1952) A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poult Sci 31:735–738. https://doi.org/10.3382/ps.0310735
Nayak SK, Swain P, Mukherjee SC (2007) Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (Ham.). Fish Shellfish Immun 23:892–896. https://doi.org/10.1016/j.fsi.2007.02.008
Nazeri S, Farhangi M, Modarres S (2017) The effect of different dietary inclusion levels of rutin (a flavonoid) on some liver enzyme activities and oxidative stress indices in rainbow trout, Oncorhynchus mykiss (Walbaum) exposed to Oxytetracycline. Aquac Res 48:4356–4362. https://doi.org/10.1111/are.13257
Omoregie E, Oyebanji SM (2002) Oxytetracycline-Induced Blood Disorder in Juvenile Nile Tilapia Oreochromis niloticus (Trewavas). J World Aquac Soc 33:377–382. https://doi.org/10.1111/j.1749-7345.2002.tb00514.x
Pês TS, Saccol EM, Londero ÉP, Bressan CA, Ourique GM, Rizzetti TM, Prestes OD, Zanella R, Baldisserotto B, Pavanato MA (2018) Protective effect of quercetin against oxidative stress induced by oxytetracycline in muscle of silver catfish. Aquaculture 484:120–125. https://doi.org/10.1016/j.aquaculture.2017.10.043
Placer ZA, Cushman L, Johnson B (1966) Estimation of product of lipid peroxidation (malonyldialdehyde) in biochemical system. Anal Biochem 16:359–364. https://doi.org/10.1016/0003-2697(66)90167-9
Rahman ANA, Mohamed AAR, Mohammed HH, Elseddawy NM, Salem GA, El-Ghareeb WR (2020) The ameliorative role of geranium (Pelargonium graveolens) essential oil against hepato-renal toxicity, immunosuppression, and oxidative stress of profenofos in common carp, Cyprinus carpio (L.). Aquaculture 517:734777. https://doi.org/10.1016/j.aquaculture.2019.734777
Ramesh M, Thilagavathi T, Rathika R, Poopal RK (2018) Antioxidant status, biochemical, and hematological responses in a cultivable fish Cirrhinus mrigala exposed to an aquaculture antibiotic sulfamethazine. Aquaculture 491:10–19. https://doi.org/10.1016/j.aquaculture.2018.02.046
Reda RM, Ibrahim RE, Ahmed ENG, El-Bouhy ZM (2013) Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egypt J Aquat Res 39:241–248. https://doi.org/10.1016/j.ejar.2013.12.001
Rico A, Phu TM, Satapornvanit K, Min J, Shahabuddin AM, Henriksson PJ, Van den Brink PJ (2013) Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 412:231–243. https://doi.org/10.1016/j.aquaculture.2013.07.028
Rodrigues S, Antunes SC, Correia AT, Nunes B (2017a) Rainbow trout (Oncorhynchus mykiss) pro-oxidant and genotoxic responses following acute and chronic exposure to the antibiotic oxytetracycline. Ecotoxicology 26:104–117. https://doi.org/10.1007/s10646-016-1746-3
Rodrigues S, Antunes SC, Nunes B, Correia AT (2017b) Histological alterations in gills and liver of rainbow trout (Oncorhynchus mykiss) after exposure to the antibiotic oxytetracycline. Environ Toxicol Pharmacol 53:164–176. https://doi.org/10.1016/j.etap.2017.05.012
Roosta Z, Hajimoradloo A, Ghorbani R, Hoseinifar SH (2014) The effects of dietary vitamin C on mucosal immune responses and growth performance in Caspian roach (Rutilus rutilus caspicus) fry. Fish Physiol Biochem 40:1601–1607. https://doi.org/10.1007/s10695-014-9951-6
Rouhier N, Lemaire SD, Jacquot JP (2008) The role of glutathione in photosynthetic organisms: emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol 59:143–166. https://doi.org/10.1146/annurev.arplant.59.032607.092811.PMID18444899
Safari O, Sarkheil M (2018) Dietary administration of eryngii mushroom (Pleurotus eryngii) powder on haemato-immunological responses, bactericidal activity of skin mucus and growth performance of koi carp fingerlings (Cyprinus carpio koi). Fish Shellfish Immun 80:505–513. https://doi.org/10.1016/j.fsi.2018.06.046
Saha S, Kaviraj A (2009) Effects of cypermethrin on some biochemical parameters and its amelioration through dietary supplementation of ascorbic acid in freshwater catfish Heteropneustes fossilis. Chemosphere 74:1254–1259. https://doi.org/10.1016/j.chemosphere.2008.10.056
Sandnes K, Hansen T, Killie JA, Waagbø R (1990) Ascorbate-2-sulfate as a dietary vitamin C source for Atlantic salmon (Salmo salar): 1. Growth, bioactivity, haematology and humoral immune response. Fish Physiol Biochem 8:419–427. https://doi.org/10.1007/BF00003398
Shahkar E, Yun H, Kim DJ, Kim SK, Lee BI, Bai SC (2015) Effects of dietary vitamin C levels on tissue ascorbic acid concentration, hematology, non-specific immune response and gonad histology in broodstock Japanese eel, Anguilla japonica. Aquaculture 438:115–121. https://doi.org/10.1016/j.aquaculture.2015.01.001
Shalini KS, Yengkhom O, Subramani PA, Michael RD (2019) Polysaccharide fraction from the Indian mistletoe, Dendrophthoe falcata (Lf) Ettingsh enhances innate immunity and disease resistance in Oreochromis niloticus (Linn.). Fish Shellfish Immun 88:407–414. https://doi.org/10.1016/j.fsi.2019.03.008
Shaluei F, Nematollahi A, Naderi-Farsani HR, Rahimi R, Kaboutari Katadj J (2017) Effect of ethanolic extract of Zingiber officinale on growth performance and mucosal immune responses in rainbow trout (Oncorhynchus mykiss). Aquac Nutr 23:814–821. https://doi.org/10.1111/anu.12448
Siwicki AK, Anderson DP (1993) Non-specific defense mechanisms assay in fish. II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. In: Siwicki AK, Anderson DP, Waluga J (eds) Fish disease diagnosis and preventions methods. Olsztyn, Poland, pp 105–112
Tavabe KR, Kuchaksaraei BS, Javanmardi S (2020). Effects of ZnO nanoparticles on the Giant freshwater prawn (Macrobrachium rosenbergii, de Man, 1879): reproductive performance, larvae development, CHH concentrations and anti-oxidative enzymes activity. Anim Reprod Sci 106603https://doi.org/10.1016/j.anireprosci.2020.106603
Vali S, Mohammadi G, Tavabe KR, Moghadas F, Naserabad SS (2020) The effects of silver nanoparticles (Ag-NPs) sublethal concentrations on common carp (Cyprinus carpio): bioaccumulation, hematology, serum biochemistry and immunology, antioxidant enzymes, and skin mucosal responses. Ecotox Environ Saf 194:110353. https://doi.org/10.1016/j.ecoenv.2020.110353
Vani T, Saharan N, Mukherjee SC, Ranjan R, Kumar R, Brahmchari RK (2011) Deltamethrin induced alterations of hematological and biochemical parameters in fingerlings of Catla catla (Ham.) and their amelioration by dietary supplement of vitamin C. Pestic Biochem and Phys 101:16–20. https://doi.org/10.1016/j.pestbp.2011.05.007
Verma RS, Mehta A, Srivastava N (2007) In vivo chlorpyrifos induced oxidative stress: attenuation by antioxidant vitamins. Pestic Biochem and Phys 88:191–196. https://doi.org/10.1016/j.pestbp.2006.11.002
Wahli T, Verlhac V, Girling P, Gabaudan J, Aebischer C (2003) Influence of dietary vitamin C on the wound healing process in rainbow trout (Oncorhynchus mykiss). Aquaculture 225:371–386. https://doi.org/10.1016/S0044-8486(03)00302-8
Wan J, Ge X, Liu B, Xie J, Cui S, Zhou M, Xia S, Chen R (2014) Effect of dietary vitamin C on non-specific immunity and mRNA expression of three heat shock proteins (HSPs) in juvenile Megalobrama amblycephala under pH stress. Aquaculture 434:325–333. https://doi.org/10.1016/j.aquaculture.2014.08.043
Yangthong M, Hutadilok-Towatana N, Thawonsuwan J, Phromkunthong W (2016) An aqueous extract from Sargassum sp. enhances the immune response and resistance against Streptococcus iniae in the Asian sea bass (Lates calcarifer Bloch). J Appl Phycol 28:3587–3598. https://doi.org/10.1007/s10811-016-0859-7
Yano T (1992) Assays for haemolytic complement activity. In: Stolen JS, Fletcher TC, Anderson DP, Kaattari SL, Rowley AF (eds) Techniques in fish immunology. FITC2 SOS Publications, Fairhaven, pp 131–141
Yonar ME (2012) The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immun 32:994–1001. https://doi.org/10.1016/j.fsi.2012.02.012
Yonar ME (2018) Chlorpyrifos-induced biochemical changes in Cyprinus carpio: Ameliorative effect of curcumin. Ecotox Environ Saf 151:49–54. https://doi.org/10.1016/j.ecoenv.2017.12.065
Yonar ME, Yonar SM, Silici S (2011) Protective effect of propolis against oxidative stress and immunosuppression induced by oxytetracycline in rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immun 31:318–325. https://doi.org/10.1016/j.fsi.2011.05.019
Yonar SM, Ural MŞ, Silici S, Yonar ME (2014) Malathion-induced changes in the haematological profile, the immune response, and the oxidative/antioxidant status of Cyprinus carpio carpio: protective role of propolis. Ecotox Environ Saf 102:202–209. https://doi.org/10.1016/j.ecoenv.2014.01.007
Yonar SM, Yonar ME, Pala A, Sağlam N, Sakin F (2020) Effect of trichlorfon on some haematological and biochemical changes in Cyprinus carpio: the ameliorative effect of lycopene. Aquac Rep 16:100246. https://doi.org/10.1016/j.aqrep.2019.100246
Zhou Q, Wang L, Wang H, Xie F, Wang T (2012) Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish Shellfish Immun 32:969–975. https://doi.org/10.1016/j.fsi.2012.01.024
Acknowledgements
The authors would like to sincerely thank Mr. Mahan Motamedian, Mr. Ghasem Mohammadi, and Dr. Hadi Poorbagher for their technical assistance.
Funding
Current research was funded by the University of Tehran under grant number 26713.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Saeed Moradi, Sina Javanmardi, and Pooria Gholamzadeh. The first draft of the manuscript was written by Saeed Moradi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Supervision and funding acquisition: Kamran Rezaei Tavabe.
Corresponding author
Ethics declarations
Ethics approval
The trial protocol was approved by the Ethics Committee for the Animal Research, University of Tehran; none of the fish suffered starvation, trauma or electrical shock and all the fish were completely anesthetized before tissue sampling.
Consent to participate
All names in author list have been involved in various stages of experimentation or writing and agree with being a part of this work.
Consent for publication
All authors agree with submission of the paper for publication in the journal of Fish Physiology and Biochemistry.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moradi, S., Javanmardi, S., Gholamzadeh, P. et al. The ameliorative role of ascorbic acid against blood disorder, immunosuppression, and oxidative damage of oxytetracycline in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 48, 201–213 (2022). https://doi.org/10.1007/s10695-022-01045-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-022-01045-9