Abstract
In this investigation, we examined the influence of alpha-melanocyte stimulating hormone (α-MSH), a proopiomelanocortin-derived peptide, along the hypothalamic-pituitary-gonad axis in a cichlid fish Oreochromis mossambicus. Administration of α-MSH (40 µg/0.1 ml saline) for 22 days did not affect the number of stage I (previtellogenic) follicles but caused significant reduction in the mean numbers of previtellogenic (stages II and III), vitellogenic (stage IV) and preovulatory (stage V) follicles compared to those of controls. While the gonadosomatic index was significantly lower, the rate of follicular atresia in stages II, III and IV remained significantly higher in α-MSH-treated fish compared to the controls. Furthermore, the mean percent area of gonadotropin-releasing hormone-immunoreactive (GnRH-ir) fibres and luteinizing hormone-immunoreactive (LH-ir) cells were significantly reduced in the proximal pars distalis of the pituitary gland in α-MSH-treated fish compared with the controls. Together, our findings suggest for the first time that the treatment of α-MSH blocks the follicular developmental process during the ovarian cycle, possibly through the inhibition of GnRH-LH pathway in teleosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alpha-melanocyte-stimulating hormone (α-MSH) is a 13 amino acid long neuropeptide, which is derived from the posttranslational modifications of the protein precursor pro-opiomelanocortin (POMC). Originally, α-MSH is shown to play a role in melanin-inducing activity in frogs, but now, this peptide is implicated in regulation of several physiological functions including reproduction in vertebrates (Newman et al. 1985; Filadelfi and Castrucci 1994; Vaudry et al. 1999). α-MSH downregulates the cytokines resulting in immunosuppression (Luger et al. 2003) and plays a role in the regulation of energy homeostasis (Cone 2005), sexual behaviour (Thody and Wilson 1983; Cragnolini et al. 2000; Caquineau et al. 2006) and luteinizing hormone (LH) secretion (Newman et al. 1985) in mammals. In addition, products of the POMC (α-MSH and β-endorphin) have either direct or indirect effects on feeding and metabolism, as well as on the secretion of gonadotropin-releasing hormone (GnRH) and LH (Roa and Herbison 2012). However, in female rats, the influence of α-MSH appears to be dependent on estrous state. For example, in females with a low level of receptivity, α-MSH stimulates lordosis behaviour; however, this effect is inhibited in receptive females (Thody and Wilson 1983). In addition to the brain and pituitary gland, α-MSH is also expressed in the skin and gut in vertebrates (Thody et al. 1983; Catania et al. 2000). Despite these studies, our understanding on the relationship between α-MSH and reproduction is still opaque.
Among fish, as a main factor in the hypothalamic-pituitary-gonad (HPG) axis, GnRH from the hypothalamus acts on the pituitary gland to control the release of the gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which in turn regulate the gonadal activities (Swanson et al. 2003). The ovarian cycle includes the oogonial proliferation, primary oocyte growth followed by vitellogenesis and then final maturation and ovulation of oocytes in fish (Guraya 1986). The vitellogenic follicular growth involves the synthesis of vitellogenin in the liver under the influence of estradiol, and transportation and accumulation of vitellogenin in the oocytes resulting in the appearance of large yolky granules (Kwon et al. 1993). The final oocyte maturation and ovulation are controlled by LH, which stimulates the production of maturation inducing steroid in fish (Lubzens et al. 2010). However, the functioning of the HPG axis is complex and the knowledge on the interplay between this axis and other neurohormones is still fragmentary. For example, modulation of the endocrine stress axis is known to potentially interfere with the HPG axis (Chabbi and Ganesh 2014) and an interaction among corticotrophin releasing hormone (CRH), thyrotrophin releasing hormone (TRH) and α-MSH during stress is implicated in fish (Rotllant et al. 2000; Flik et al. 2006). Furthermore, the brain distribution of α-MSH-immunoreactive cells/fibres is demonstrated in different groups of fish (Vallarino et al. 1988, 1992; Pandolfi et al. 2003; Kasper et al. 2006; Amiya et al. 2008; Kumbar and Ganesh 2021). In addition, mRNA of α-MSH-receptors are also detected in the brain of goldfish (Ikari et al. 2018). However, the involvement of α-MSH in reproduction is understudied in fish.
The tilapia Oreochromis mossambicus exhibits short-ovarian cycles throughout the year. In non-mouthbrooding condition (manually stripped), this fish shows previtellogenic (1–12 days), vitellogenic (13–18 days) and prespawning (19–24 days) phases during the ovarian cycle (Ganesh 2014). The ovary shows the presence of preovulatory follicles with large yolky granules only during the prespawning phase. This fish also exhibits mouthbrooding and intermittent parental care for the offspring, which lasts for 40–42 days (Smith and Haley 1988). Previous study on this fish revealed the presence of α-MSH-immunoreactive cells in the brain and pituitary gland (Kumbar and Ganesh 2021). However, the functional significance of this peptide along the HPG axis is largely unknown. Therefore, the aim of the present study is to elucidate the influence of α-MSH on the HPG axis in the tilapia. In this study, we have used immunofluorescence technique to detect the GnRH-immunoreactive (GnRH-ir) fibres and LH–immunoreactive (LH-ir) cells in the pituitary gland and follicular kinetics of the ovary to assess the functional status of the HPG axis.
Materials and methods
Animals
Sexually mature O. mossambicus were collected from ponds in and around Dharwad District, Karnataka (75°01′E, 15°27′N), transported to the laboratory and were reared in freshwater tanks measuring 92 × 92 × 92 cm under the natural conditions (photoperiod, 11.57 ± 0.5 h; dissolved oxygen 8.90 ± 0.24 mg/L; pH, 9.10 ± 0.16; water temperature, 28.60 ± 0.34 °C). Fish weighing between 35 and 42 g were acclimatized to 75-l freshwater aquaria (size, 92 × 30 × 46 cm; length × width × height) for a month. Fish were stocked at the sex ratio of five females plus two males in each aquarium. The aquaria were aerated and the fish were fed ad libitum with commercial food pellets (Taiyo pet feed, Chennai, India), twice a day.
Experimental procedure
Prior to the commencement of the experiment, the mouthbrooding fish were identified based on the appearance of the gular bulge. The eggs from the mouth of twenty fish were removed carefully and used for experimentation. The stripped fish (n = 20) were divided into two groups, each with two replicates (n = 5 in each replicate; n = 10 per group). The fish in first group received 100 µL saline/fish/day, whereas those in second group were administered with 40 µg α-MSH (M4135, Sigma-Aldrich, USA)/100 µL saline/fish/day. The dose for α-MSH was determined based on the pilot studies. All injections were given through intraperitoneal (i.p.) route for 22 days. The fish were euthanized 24 h after the last injection, following anaesthetization with 2-phenoxy ethanol (1:1500). The experimental procedures were approved by an IAEC (No. 639/GO/Re/S/02/CPCSEA).
Histology and morphometry of the ovary
The gonado-somatic index (GSI) was calculated using the formula: Gonadal weight/body weight × 100. The ovaries were immersed for 24 h in Bouin’s fixative and processed for the histology. Paraffin embedded serial sections (5 µm thick) were cut using a microtome (RM2125 RTS, Leica Microsystems, Wetzlar, Germany) and stained with haematoxylin and eosin. The follicles at different stages of development (I–V) were quantified as described earlier for this species (Ganesh 2014). Briefly, the previtellogenic follicles at stages I (0.01–0.04 mm), II (0.05–0.14 mm) and III (0.15–0.34 mm) were identified based on the chromatin nucleoli, perinucleoli and cortical alveoli, respectively, whereas the stages IV (vitellogenic; 0.35–0.80 mm) and V (early maturation; > 0.80 mm) follicles were recognized by the presence of large yolk granules. The follicles in stages I, II, III, IV and V were identified based on their size and counted in 9th, 25th, 60th, 120th and 300th sections of the ovary, respectively. The stage I follicles were counted under 10 × objective, whereas other follicles were counted under 4 × objective. The atretic follicles in different stages were identified based on their degenerative profile and their number was expressed as percent occurrence ± SE.
GnRH and LH immunofluorescence labelling
The fish were subjected to transcardial perfusion with 20 ml of chilled phosphate buffered saline (PBS, pH 7.4) followed by 20 ml of chilled 4% paraformaldehyde. After dissection, the brains with intact pituitary glands were again kept in the same fixative for 24 h. Following a rinse in PBS, the tissues were cryoprotected in chilled 30% sucrose solution overnight. Frozen sections of the brain through the pituitary gland were cut (14 µm thick) using a cryostat (CM1510S; Leica Microsystems, Wetzlar, Germany). The sections on poly-L-lysine-coated slides were processed in a moist chamber at room temperature using immunofluorescence procedure as described earlier (Vijayalaxmi et al. 2020). For the immunolabelling of GnRH, polyclonal rabbit anti-GnRH antibody (1: 2000; kind gift of Dr. Ishwar Parhar, Monash University, Malaysia) was employed, whereas rabbit polyclonal human LHβ antiserum (1:8000; NHPP, Harbor-UCLA Medical Centre, CA, USA) was used to label LH secreting cells in the proximal pars distalis (PPD) of the pituitary gland. The sections were incubated overnight at 4 °C. The sections were rinsed three times (10 min each) in PBS and incubated for 2 h with Alexa Fluor 488 or Texas Red–conjugated anti-rabbit, goat IgG (1: 200; Sigma-Aldrich, USA; Vector laboratories Inc, USA) at room temperature in the dark. Entire incubation protocol was carried out in a humidified chamber. The sections were washed again three times in PBS (10 min each) and mounted using an anti-fade mountant, vectashield (Vector laboratories Inc, USA).
The following control procedures were employed to check the specificity of the antibodies: (1) omission of the primary antibody (GnRH or LH) and its replacement with 2% BSA or goat serum; (2) preabsorption of diluted GnRH or LH antibody with GnRH or LH peptide (Sigma-Aldrich, USA) 24 h prior to the incubation, respectively; and (3) omission of the secondary antibody. These procedures resulted in lack of immunostaining, affirming the specificity of the primary antibodies. The photomicrography was done using a fluorescent microscope (BX53, Olympus, Japan). The intensity and the percent area of GnRH or LH immunoreactivities were evaluated in Alexa flour 488/Texas Red–labelled sections using ImageJ, version 1.46 (NIH, Bethesda, MD, USA). The detailed procedure for the same is described previously (Bhat and Ganesh 2020). The pixel intensities and area of immunoreaction were measured for immunoreactive cells or fibres/section in the pituitary gland. These values from each experimental group (n = 10) were expressed as the mean intensity (Arbitrary units) or percent immunoreactive area/section ± SE.
Statistical analysis
The data were tested for normality and equal variance. Once these tests were passed, the mean values of different parameters were subjected to Student t-test using SigmaStat 3.5 software. The significant differences were evaluated statistically at the level of P < 0.05.
Results
The GSI showed a significant (P < 0.05) decrease in α-MSH-treated fish compared to the controls group (Fig. 1). The ovary showed follicles in different stages of development from stages I–V in both experimental groups (Fig. 2A and B). No significant difference was observed in the mean number of stage I follicles, whereas the numbers of follicles belonging to stages II–V were significantly (P < 0.05) lower in α-MSH-treated fish compared with the controls group (Fig. 3A–E). No incidence of follicular atresia was noticed in stages I and V. However, a significant (P < 0.05) increase in the stage II–IV follicular atresia was found in α-MSH-treated fish compared to those of controls (Fig. 4).
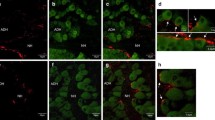
Photomicrographs of transverse sections of the ovary (A and B) and pituitary gland (P) through the proximal pars distalis (PPD) region showing GnRH-ir fibres (arrows, C and E) and LH-ir cells (arrow heads) in Oreochromis mossambicus. Note the increased follicular atresia in fish treated with α-MSH (B) compared to the controls (A), whereas GnRH-ir fibre density and LH-ir content is decreased in the pituitary gland of α-MSH-treated fish (E and F) compared to the controls (C and D) respectively. I, II, III, IV and V, stages of follicular development; AF, atretic follicle; HHT, hypothalamo-hypophyseal tract. Scale bar, 100 µm. C and E, Alexa Fluor 488 labelled; D and F, Texas Red labelled. A and B, Haematoxylin and Eosin
In the pituitary gland, GnRH-ir fibres were detected in the hypothalamo-hypophyseal tract (HHT) as well as throughout the PPD in controls and α-MSH-treated fish (Fig. 2C and E). While the intensity of GnRH immunoreaction did not significantly differ between the two groups, the percent area occupied by GnRH-ir fibres was significantly decreased in α-MSH-treated fish compared with the controls (Fig. 5A). In the PPD region, LH-ir content was also detected (Fig. 2D and F). Although the intensity of immunoreactivity was not significantly different, there was a significant decrease in the percent area of LH-immunoreactivity in α-MSH-treated fish compared with the controls group (Fig. 5B).
Discussion
To date, studies on the influence of POMC peptides on reproductive functions in fish were confined only to adrenocorticotrophic hormone (ACTH) and β-endorphin (Alsop et al. 2009; Chabbi and Ganesh 2013; Ganesh and Chabbi 2013; Ganesh 2021). This is the first study reporting the inhibitory effect of another POMC peptide α-MSH along the HPG axis in fish. In the present study, treatment of 40 µg α-MSH for 22 days resulted in a significant reduction in the GSI concomitant with the suppression of follicular development compared to that of controls. In the tilapia, an increase in the GSI coincides with the increased number of fully ripened follicles (stage V) during the prespawning phase (day 23) compared to other phases of the ovarian cycle (Chabbi and Ganesh 2012). Therefore, a significant decrease in the GSI following α-MSH treatment may be mainly due to the decreased number of stage V follicles.
We may recall that treatment of 4 µg β-endorphin (Chabbi and Ganesh 2013) resulted in the complete blockade of the stage V follicles in the Mozambique tilapia, whereas administration of 40 µg α-MSH (tenfold high dose compared to that of β-endorphin) in the present study did not fully block the stage V follicular development as shown by the presence of a few stage V follicles in the ovary. In the previous study, 4 µg β-endorphin exerted either stimulatory effect or no significant inhibitory effect on follicular stages I–IV, whereas in the present study, except the stage I, the mean numbers of follicles in other stages (II–V) were significantly lower following α-MSH treatment. These results indicate that treatment of α-MSH does not block early follicular recruitment but inhibits the follicular development at later stages.
In the present study, significant decrease in the numbers of stage II–IV follicles in α-MSH-treated fish coincide with significantly high rate of atresia in these follicles. These results suggest that, regardless of the follicular recruitment at stage I, the significant loss of healthy follicles at stages II–IV seem to be due to the demise of these follicles in α-MSH-treated fish. Therefore, it is unlikely that the progression of stage V follicular development was blocked, but rather the healthy follicles available for the recruitment from the stage IV to V were decreased due to α-MSH treatment.
Our current knowledge on the relationship between α-MSH and GnRH is mainly confined to mammals. The POMC neurons were shown to synapse with GnRH neurons (Naftolin et al. 1996) and α-MSH (Mezey et al. 1985) neurons were labelled in the medial preoptic nucleus (MPO), wherein GnRH neurons are also located. Furthermore, MC4R receptors are implicated in the regulation of HPG axis as shown by the fact that GnRH neurons express MC4R receptors (Israel et al. 2012). Studies on electrophysiological recordings of GnRH neurons have shown that α-MSH increases the cell firing in a majority of GnRH neurons (approximately 70%) through the postsynaptic activation of both MC3R and MC4R receptors, whereas small population of GnRH neurons were excited or inhibited by cocaine and amphetamine-regulated transcript, POMC-related peptide β-endorphin and neuropeptide Y (NPY) in mice (Roa and Herbison 2012). Indeed, administration of melanocortin receptor agonist, Melanotan II augmented the GnRH pulse generator activity in goats and this effect was attenuated by estradiol (Matsuyama et al. 2005). These studies suggest a stimulatory role for α-MSH on GnRH neurons in mammals. In teleosts, the median eminence is absent and the secretory products from the hypothalamus are directly released into the pituitary gland through the HHT (Holmes and Ball 1974). In the present study, although the intensity of GnRH-immunolabelling remained unchanged, the percent area occupied by GnRH-ir fibres in the pituitary gland was significantly reduced in α-MSH-treated fish compared to the controls. Since the hypophysiotrophic neurons of GnRH are located in the POA in teleosts similar to that of mammals (Mezey et al. 1985; Shahjahan et al. 2014; Ganesh 2021), a significant decrease in the percent area of GnRH-ir fibres labelled in the pituitary gland in the present study suggests that α-MSH treatment may inhibit the release of GnRH in the hypothalamus.
In addition to the above mechanism, α-MSH can also act the level of the pituitary gland; however, its effect on LH secretion appears to be equivocal. For example, α-MSH treatment (2.5 mg) resulted in the release of LH from the pituitary in men and normal women during the luteal phase or in women with the amenorrhea (Reid et al. 1984; Limone et al. 1997). Similar stimulatory effect on LH was also observed following treatment of α-MSH agonist Melanotan II in ewes (Backholer et al. 2009). Moreover, α-MSH treatment stimulated the sexual receptivity as well as lordosis behaviour in female rats (Cragnolini et al. 2000). On the other hand, LH secretion was either decreased or unaffected following α-MSH treatment in rats (Khorram et al. 1984; Scimonelli and Celis 1990). In the present study, although the intensity of immunolabelling of LH was not significantly different, the area of LH-ir content was significantly decreased in the pituitaries of α-MSH-treated fish compared to the controls. These results are suggestive of decreased synthesis/secretion of LH in the pituitary gland due to α-MSH treatment. Since the inhibition of GnRH following α-MSH treatment is evident in the present study, it is more likely that the suppression of LH is due to the blockade of release of the hypothalamic GnRH into the pituitary gland. However, the possibility of direct effect of α-MSH treatment on the pituitary gland cannot be ruled out. A separate experimental protocol is required to confirm this possibility. Additionally, whether the effect of α-MSH on LH depends on the steroid hormone feedback deserves further studies in fish. For example, in non-estrous female rat, α-MSH was shown to stimulate LH-dependent behaviour — lordosis, whereas this behaviour was inhibited in estrous females (Thody and Wilson 1983) and the response of LH might depend on steroid hormone levels (Celis 1985).
The release of α-MSH is also influenced by many neurohormones, particularly biogenic amines in fish. For example, exposure to acid stress resulted in D1-like dopamine receptor expression in the pituitary α-MSH cells in the Mozambique tilapia (Lamers et al. 1997), whereas a stimulatory influence of serotonin on α-MSH synthesis and release from the pituitary gland was demonstrated in the eel Anguilla anguilla (Olivereau 1978). Similar role for these amines in α-MSH-induced HPG axis function cannot be ruled out in the tilapia, but this possibility merits further investigation.
In conclusion, the results of the present study reveals for the first time that α-MSH can potentially block the follicular development process. The inhibition of the ovarian activity appears to be mediated through the suppression of GnRH release into the pituitary gland, and concomitant reduction in LH secretion in teleosts.
Data availability
Not applicable.
Code availability (software application or custom code)
Not applicable.
References
Alsop D, Ings JS, Vijayan MM (2009) Adrenocorticotropic hormone suppresses gonadotropin-stimulated estradiol release from zebrafish ovarian follicles. PLoS ONE 4(7):e6463. https://doi.org/10.1371/journal.pone.0006463
Amiya N, Amano M, Oka Y, Iigo M, Takahashi A, Yamamori K (2008) Interaction of orexin/hypocretin-like immunoreactive neurons with melanin-concentrating hormone and α-melanocyte-stimulating hormone neurons in brain of a pleuronectiform fish, barfin flounder. Fish Sci 74:1040–1046. https://doi.org/10.1111/j.1444-2906.2008.01622.x
Backholer K, Smith J, Clarke IJ (2009) Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology 150:5488–5497. https://doi.org/10.1210/en.2009-0604
Bhat SK, Ganesh CB (2020) Domperidone treatment attenuates stress-induced suppression of reproduction in viviparous mosquitofish Gambusia affinis. J Fish Biol 96:37–48. https://doi.org/10.1111/jfb.14183
Caquineau C, Len G, Guan XM, Jiang M, Van der Ploeg L, Douglas AJ (2006) Effects of alpha-melanocyte-stimulating hormone on magnocellular oxytocin neurones and their activation at intromission in male rats. J Neuroendocrinol 18:685–691. https://doi.org/10.1111/j.1365-2826.2006.01465.x
Catania A, Cutuli M, Garofalo L, Carlin A, Airaghi L, Barcellini W, Lipton, JM (2000) The Neuropeptide α-MSH in Host Defense. Ann N Y Acad Sci 917:227–231. https://doi.org/10.1111/j.1749-6632.2000.tb05387.x
Chabbi A, Ganesh CB (2012) Stress-induced inhibition of recruitment of ovarian follicles for vitellogenic growth and interruption of spawning cycle in the fish Oreochromis mossambicus. Fish Physiol Biochem 38:1521–1532. https://doi.org/10.1007/s10695-012-9643-z
Chabbi A, Ganesh CB (2013) β-endorphin-induced inhibition of vitellogenic follicular growth in the fish Oreochromis mossambicus evidence for opioidergic mediation of ovarian stress response. J Exp Zool A Ecol Genet Physiol 319:156–165. https://doi.org/10.1002/jez.1781
Chabbi A, Ganesh CB (2014) Glucocorticoid synthesis inhibitor metyrapone blocks stress-induced suppression along luteinizing hormone secreting cells–ovary axis in the fish Oreochromis mossambicus. J Exp Zool A Ecol Genet Physiol 321:125–134. https://doi.org/10.1002/jez.1842
Celis ME (1985) Release of LH in response to alpha-MSH administration. Acta Physiol Pharmacol Latinoam 35:281–290
Cone RD (2005) Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578
Cragnolini A, Scimonelli T, Celis M, Schiöth H (2000) The role of melanocotin receptors in sexual behavior in female rats. Neuropeptides 34:211–215. https://doi.org/10.1054/npep.2000.0815
Filadelfi AM, Castrucci AM (1994) Melatonin desensitizing effects on the in vitro responses to MCH, alpha-MSH, isoproterenol and melatonin in pigment cells of a fish (S. marmoratus), a toad (B. ictericus), a frog (R. pipiens) and a lizard (A. carolinensis), exposed to varying photoperiodic regimens. Comp Biochem Physiol A 109:1027–1037. https://doi.org/10.1016/0300-9629(94)90252-64
Flik G, Klaren PH, Van den Burg EH, Metz JR, Huising MO (2006) CRF and stress in fish. Gen Comp Endocrinol 146:36–44. https://doi.org/10.1016/j.ygcen.2005.11.005
Ganesh CB (2014) Follicular development status and profile of 17β estradiol and cortisol levels during the spawning cycle in Oreochromis mossambicus (Peters). Ind J Fish 61:45–51
Ganesh CB (2021) The stress—reproductive axis in fish: the involvement of functional neuroanatomical systems in the brain. J Chem Neuroanat 112:101904. https://doi.org/10.1016/j.jchemneu.2020.101904
Ganesh CB, Chabbi A (2013) Naltrexone attenuates stress-induced suppression of LH secretion in the pituitary gland in the cichlid fish Oreochromis mossambicus evidence for the opioidergic mediation of reproductive stress response. Fish Physiol Biochem 39:627–636. https://doi.org/10.1007/s10695-012-9725-y
Guraya SS (1986) The cell and molecular biology of fish oogenesis. In: Saver HW (ed) Monographs in developmental biology. Karger, New York, pp 1–223
Holmes R, Ball J (1974) The pituitary gland—a comparative account. Cambridge University Press, Cambridge
Ikari T, Kobayashi Y, Kitani Y, Sekiguchi T, Endo M, Kambegawa A, Asahina K, Hattori A, Tabuchi Y, Amornsakun T, Mizusawa K, Takahashi A, Suzuki N (2018) α-Melanocyte-stimulating hormone directly increases the plasma calcitonin level and involves calcium metabolism in goldfish. Int Aquat Res 10:283–292. https://doi.org/10.1007/s40071-018-0206-5
Israel DD, Sheffer-Babila S, de Luca C, Jo YH, Liu SM, Xia Q, Spergel DJ, Dun SL, Dun NJ, Chua SC Jr (2012) Effects of leptin and melanocortin signaling interactions on pubertal development and reproduction. Endocrinology 153(5):2408–2419. https://doi.org/10.1210/en.2011-1822
Kasper RS, Shved N, Takahashi A, Reinecke M, Eppler E (2006) A systematic immunohistochemical survey of the distribution patterns of GH prolactin somatolactin beta-TSH, beta-FSH, beta-LH, ACTH and alpha-MSH in the adenohypophysis of Oreochromis niloticus, the Nile tilapia. Cell Tissue Res 325:303–313. https://doi.org/10.1007/s00441-005-0119-7
Khorram O, DePalatis LR, McCann SM (1984) The effect and possible mode of action of alpha-melanocyte-stimulating hormone on gonadotropin release in the ovariectomized rat: an in vivo and in vitro analysis. Endocrinology 114(1):227–233. https://doi.org/10.1210/endo-114-1-227
Kumbar J, Ganesh CB (2021) Alpha-melanocyte stimulating hormone immunoreactivity in the brain of the cichlid fish Oreochromis mossambicus. Neuropeptides 87:102128. https://doi.org/10.1016/j.npep.2021.102128
Kwon HC, Hayashi S, Mugiya Y (1993) Vitellogenin induction by estradiol-17b in primary hepatocyte culture in the rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol 104B:381–396
Lamers AE, Ter Brugge PJ, Flik G, Wendelaar Bonga SE (1997) Acid stress induces a D1-like dopamine receptor in pituitary MSH cells of Oreochromis mossambicus. Am J Physiol 273:387–392
Limone P, Calvelli P, Altare F, Ajmone-Catt P, Lima T, Molinatti GM (1997) Evidence for an interaction between alpha-MSH and opioids in the regulation of gonadotropin secretion in man. J Endocrinol Invest 20:207–210. https://doi.org/10.1007/BF03346904
Lubzens E, Young G, Bobe J, Cerda J (2010) Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165:367–389
Luger TA, Scholzen TE, Brzoska T, Bohm M (2003) New insights into the functions of alpha-MSH and related peptides in the immune system. Ann N Y Acad Sci 994:133–140
Matsuyama S, Ohkura S, Sakurai K, Tsukamura H, Maeda K, Okamura H (2005) Activation of melanocortin receptors accelerates the gonadotropin-releasing hormone pulse generator activity in goats. Neurosci Lett 383:289–294. https://doi.org/10.1016/j.neulet.2005.04.026
Mezey E, Kiss JZ, Mueller GP, Eskay R, O’Donohue TL, Palkovits M (1985) Distribution of the pro-opiomelanocortin derived peptides, adrenocorticotrope hormone, alpha-melanocyte-stimulating hormone and beta-endorphin (ACTH, alpha-MSH, beta-END) in the rat hypothalamus. Brain Res 328:341–347
Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J (1996) Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology 63:149–155
Newman CB, Wardlaw SL, Frantz AG (1985) Suppression of basal and stress-induced prolactin release and stimulation of luteinizing hormone secretion by α-melanocyte-stimulating hormone. Life Sci 36:1661–1668. https://doi.org/10.1016/0024-3205(85)90369-8
Olivereau M (1978) Serotonin and MSH secretion: effect of parachlorophenylalanine on the pituitary cytology of the eel. Cell Tissue Res 191:83–92. https://doi.org/10.1007/BF00223217
Pandolfi M, Cánepa MM, Ravaglia MA, Maggese MC, Paz DA, Vissio PG (2003) Melanin-concentrating hormone system in the brain and skin of the cichlid fish Cichlasoma dimerus: anatomical localization, ontogeny and distribution in comparison to alpha-melanocyte-stimulating hormone-expressing cells. Cell Tissue Res 311:61–69. https://doi.org/10.1007/s00441-002-0654-4
Reid RL, Ling N, Yen SS (1984) Gonadotropin-releasing activity of alpha-melanocyte-stimulating hormone in normal subjects and in subjects with hypothalamic-pituitary dysfunction. J Clin Endocrinol Metab 58:773–777. https://doi.org/10.1210/jcem-58-5-773
Roa J, Herbison AE (2012) Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology 153:5587–5599
Rotllant J, Balm P, Wendelaar-Bonga S, Pérez-Sánchez J, Tort L (2000) A drop in ambient temperature results in a transient reduction of interrenal ACTH responsiveness in the gilthead sea bream (Sparus aurata, L.). Fish Physiol Biochem 23:265–273. https://doi.org/10.1023/A:1007873811975
Scimonelli T, Celis ME (1990) A central action of alpha-melanocyte-stimulating hormone on serum levels of LH and prolactin in rats. J Endocrinol 124:127–132. https://doi.org/10.1677/joe.0.1240127
Shahjahan M, Kitahashi T, Parhar IS (2014) Central pathways integrating metabolism and reproduction in teleosts. Front Endocrinol (Lausanne) 5:25–36. https://doi.org/10.3389/fendo.2014.00036
Smith CJ, Haley SR (1988) Steroid profiles of the female Tilapia, Oreochromis mossambicus, and correlation with oocyte growth and mouthbrooding behavior. Gen Comp Endocrinol 69:88–98
Swanson P, Dickey JT, Campbell B (2003) Biochemistry and physiology of fish gonadotropins. Fish Physiol Biochem 28:53–55
Thody AJ, Ridley K, Penny RJ, Chalmers R, Fisher C, Shuster S (1983) MSH peptides are present in mammalian skin. Peptides 4:813–816
Thody AJ, Wilson CA (1983) Melanocyte stimulating hormone and the inhibition of sexual behaviour in the female rat. Physiol Behav 31:67–72. https://doi.org/10.1016/0031-9384(83)90097-5
Vallarino M, Delbende C, Jegou S, Vaudry H (1988) Alpha-melanocyte-stimulating hormone (alpha-MSH) in the brain of the cartilaginous fish. Immunohistochemical localization and biochemical characterization. Peptides 9:899–907. https://doi.org/10.1016/0196-9781(88)90139-8
Vallarino M, Tranchand Bunel D, Vaudry H (1992) Alpha-melanocyte-stimulating hormone (alpha-MSH) in the brain of the African lungfish Protopterus annectens immunohistochemical localization and biochemical characterization. J Comp Neurol 322:266–274. https://doi.org/10.1002/cne.903220212
Vaudry H, Chartrel N, Desrues L, Galas L, Kikuyama S, Mor A, Nicolas P, Tonon MC (1999) The pituitary-skin connection in amphibians reciprocal regulation of melanotrope cells and dermal melanocytes. Ann N Y Acad Sci 885:2041–2056. https://doi.org/10.1111/j.1749-6632.1999.tb08664.x
Vijayalaxmi, Sakharkar AJ, Ganesh CB (2020) Leucine-enkephalin-immunoreactive neurons in the brain of the cichlid fish Oreochromis mossambicus. Neuropeptides 81:101999. https://doi.org/10.1016/j.npep.2019.101999
Funding
This study was supported by a grant from Science and Engineering Research Board, Department of Science and Technology (No. EEQ/2017/414), New Delhi, Government of India.
Author information
Authors and Affiliations
Contributions
Jyoti Kumbar conducted the study, analysed the results and prepared the draft. CBG was involved in conceptualization, funding acquisition and review and editing of the paper.
Corresponding author
Ethics declarations
Ethics approval
The experimental procedures were approved by an IAEC (No. 639/GO/Re/S/02/CPCSEA).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumbar, J., Ganesh, C.B. The effect of α-MSH treatment on the hypothalamic-pituitary-gonad axis in the cichlid fish Oreochromis mossambicus. Fish Physiol Biochem 47, 1659–1668 (2021). https://doi.org/10.1007/s10695-021-01005-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-021-01005-9