Abstract
This study aimed at assessing the effects of the dietary tryptophan (Trp) supplementation on growth and feed utilization, brain serotonin content, and expression of selected liver genes (involved in the liver serotonin pathway, protein synthesis degradation, and antioxidant activity) in zebrafish. A growth trial was conducted with zebrafish juveniles fed five experimental isoproteic (40%DM) and isolipidic (8%DM) fishmeal-based diets containing graded levels of Trp: a Trp-non-supplemented diet (diet Trp0, with 0.22% Trp) and four Trp-supplemented diets containing 2–16 times higher Trp content (diets Trp2, Trp4, Trp8, and Trp16 with 0.40, 0.91, 2.02, and 3.34% Trp, respectively). Diets were tested in quadruplicate, with fish being fed twice a day, 6 days a week for 6 weeks to apparent visual satiation. At the end of the trial, growth performance and feed utilization were assessed, and fish from all experimental groups were sampled for whole-body composition analysis. In addition, fish fed low (Trp0), medium (Trp4), and high (Trp16) Trp diets were also sampled for analysis of brain serotonin content and liver gene expression. Tested tryptophan levels did not influence growth performance nor feed intake. However, values of energy and nitrogen retention as well as body energy content indicate a better feed utilization with diets containing around 0.9% and 2.0% DM Trp. Brain serotonin content increased with increasing dietary tryptophan levels. In addition, regarding liver genes, dietary treatment had a modulatory effect on the expression of Htr1aa and Htr2cl1 genes (encoding for serotonin receptors), TPH1a gene (encoding for tryptophan hydroxylase, the rate-limiting enzyme in the synthesis of serotonin from tryptophan), TOR gene (involved in protein synthesis), and Keap1 gene (involved in antioxidant responses).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tryptophan (Trp) is an essential amino acid (EAA), which needs to be supplied through diet in appropriate amounts (NRC, 2011). Trp is not only used for protein accretion but is also involved in several physiological and behavioral functions (revised by Hoseini et al., 2019).
This amino acid is the only precursor for the synthesis of the neurotransmitter serotonin (5-HT), which occurs through hydroxylation of the indole ring of Trp followed by decarboxylation (Katzung et al., 2012), in the brain and peripheral organs (Höglund et al., 2019; Hoseini et al., 2019). In turn, serotonin can be converted in the hormone melatonin, primarily produced in the pineal gland. In certain brain areas, this pathway follows a dose–response mechanism dependent on the availability of the substrate, and therefore dietary Trp can be a limiting factor for the synthesis of brain serotonin and of melatonin (Höglund et al., 2019).
In fish, serotonin has been reported to be deeply involved in stress reaction, social dominance, sexual behavior, and reduction of aggressiveness (Höglund et al., 2005, 2017, 2019; Hoseini et al., 2019; Lillesaar, 2011; Papoutsoglou et al., 2005a; Tejpal et al., 2009; Winberg and Thörnqvist, 2016), while melatonin has been indicated as conditioning growth, metabolism, and digestive capacity, as well as enhancing humoral innate immunity and protection against oxidative stress (Cuesta et al., 2008; Falcón et al., 2010; Lepage et al., 2005; López-Olmeda et al., 2006). Thus, through its pivotal role in the biosynthesis of these two compounds, Trp can act as a modulator of important behavioral and physiological responses with implications on growth, welfare, and health status of farmed fish.
In fact, reports have shown that the availability of dietary Trp has a modulatory effect on behavior, neuroendocrine stress responses, and other physiological responses in teleosts. For example, dietary Trp deficiency has been shown to lower 5-HT levels leading to several behavioral disorders (Herrero et al., 2007; Johnston et al., 1990; Le Floc’h et al., 2011). On the other hand, supplementation of diets with Trp, by increasing the serotonergic activity at the hypothalamic-pituitary-interrenal axis level, can affect neuroendocrine stress responses, suppressing the release of adrenocorticotropic hormone and cortisol in stressed fish (Höglund et al., 2005, 2017, 2019; Hoseini et al., 2019; Tejpal et al., 2009; Winberg et al., 2001; Wolkers et al., 2012); accordingly, supplementing fish diets with Trp has been used as a strategy to mitigate stress associated to aquaculture practices, and Trp requirements may effectively increase in stressful conditions (Costas et al., 2008; Tejpal et al., 2009). Additional positive effects of dietary Trp supplementation include promotion of growth, immune response, antioxidant capacity, and sexual maturation (Akiyama et al., 1996; Azeredo et al., 2017; Hoseini et al., 2019), while dietary Trp deficiency has been associated with growth retardation and skeletal deformities (Hoseini et al., 2019).
Several inconsistencies have been found regarding dietary Trp requirements in fish, which may be related to its functions aside protein synthesis (Wu, 2009) as well as regarding the effects of dietary Trp supplementation. This work aimed at a better understanding on this subject by assessing the effects of a wide range of dietary Trp in zebrafish at different levels: growth and feed utilization, brain serotonin content, and expression of liver genes involved in the liver serotonin pathway (to understand effects of Trp supplementation on peripheral serotonin), protein synthesis/degradation pathways, and modulation of antioxidant activity.
Materials and methods
Fish
The growth trial was conducted using laboratory-reared zebrafish (Danio rerio) juveniles, starting at 52 days post fertilization (dpf), with 65.9-mg average body weight and 17.7-mm fork length. Up to 52 dpf, during the larval and post larval period, these fish were reared in a recirculating water system (Charlon and Bergot, 1984) at 28 ± 1 °C and 14-h light/10-h darkness, fed a commercial microparticulate diet (Gemma Micro 75, Skretting: 59% protein, 14% lipids) supplemented weekly with Artemia nauplii (INVE; 56% protein, 15% lipid). The experiment was carried out in compliance with the European Union directive 2010/63/EU on the protection of animals used for scientific purposes.
Experimental diets
Five experimental isoproteic (40%DM) and isolipidic (8%DM) diets were formulated to contain graded levels of Trp: a Trp-non-supplemented diet (diet Trp0, with 0.19% Trp) and four Trp-supplemented diets containing 2–16 times higher Trp content (diets Trp2, Trp4, Trp8, and Trp16 with 0.38, 0.76, 1.52, and 3.04% Trp, respectively). These Trp levels were selected to get a broad enough range that could include both deficient and excessive Trp levels regarding Trp requirements (0.35%DM, Tang et al., 2013) for another cyprinid, the common carp, as there are no reference values for zebrafish. Trp was included in the experimental diets at the expense of a mixture of non-essential amino acids. In order to avoid leaching and to delay absorption from the digestive tract, crystalline amino acids were coated with agar before mixing with other ingredients. For diet production, grounded ingredients were mixed thoroughly, and the cod liver oil and water were added to form a moist blend. This blend was then pelletized using a grinder and pellets were dried at 60 °C for 24 h. Afterwards, pellets were crushed and sieved through a battery of sieves to obtain particles of 400–600 and 600–1000 µm of diameter. Diet formulation and proximate composition are presented in Table 1.
Growth trial
The growth trial was performed in a recirculating water system containing twenty 10-L plastic tanks (experimental units). Twenty homogenous groups of twenty fish were randomly distributed into the experimental units and each experimental diet was assigned to quadruplicate groups.
Fish were fed by hand to apparent visual satiation, twice a day, 6 days a week, during a 6-week period. Utmost care was taken to assure that all feed supplied was consumed. Each food container was numbered, to avoid any bias during feeding. Food particle size was adjusted to mouth opening as fish grew: 400–600 µm from 52 to 67 dpf and 600–1000 µm from 68 to 94 dpf.
At the beginning of the trial and every 2 weeks, fish in each tank were bulk weighed following 1 day of feed deprivation; also, at the same periods, feed containers were weighed to calculate food consumption. Fish weighing was taken with fish placed inside a beaker with system water.
During the entire trial, the water temperature was regulated at 28 ± 1 °C, and the photoperiod to 14-h light/10-h darkness; nitrogenous compounds were kept at residual levels.
Sampling
At the beginning of the trial, 100 fish from the initial stock were euthanized with excess of phenoxyethanol and frozen at − 20 °C until analysis of body composition was performed. Similarly, at the end of the trial, 12 fish from each tank were euthanized using the aforementioned method and kept at − 20° C until body composition analysis was performed.
Additionally, three fish from each replicate of diets Trp0, Trp4, and Trp16 (low, medium, and high Trp diets) were euthanized by submersion in ice-cold water; after the opercular movement ceased, the head was removed to extract the brain. Brains were kept in perchloric acid for 2 h at 4 °C and then frozen in the perchloric acid at − 80 °C until processing for determination of serotonin level. From these three fish, the liver was also collected and kept in RNAlater for 24 h at 4 °C and then stored at − 80 °C until gene expression analysis.
Chemical analysis
Chemical composition of diets and whole fish were analyzed using the standard methodology: dry matter by drying at 105 °C until constant weight; crude protein by using the Kjeldahl method, following an acidic digestion and distillation (Tecator System, Högamäs, Sweden; extraction unit model 1015 and 1026, respectively); ash by incinerating samples for 16 h at 450 °C; gross energy through direct combustion using an adiabatic bomb calorimeter. Diet lipid content was determined in a SoxTec system (Velp Scientífica SER 148/6 Solvent Extractor) using petroleum ether extraction.
Dietary amino acid content was analyzed in duplicate using high-performance liquid chromatography (HPLC), according to the Pico Tag method. Samples were hydrolyzed for 23 h with 6 N hydrochloric acid at 110 °C under N2 atmosphere and derivatized with phenylisothiocyanate reagent (PITC, Waters WAT088120) before separation by gradient exchange chromatography at 46 °C (Waters auto sample model 717 plus, Waters binary pump model 1525, Waters dual absorbance detector model 2487). For an internal standard, norleucine was used. Chromatographic peaks obtained were identified, integrated, and quantified by comparison to known amino acid standard, using Waters Breeze software package (Pierce NC10180). Tryptophan content was measured using a spectrophotometric method as described by DeVries et al., 1980. Dietary amino acid composition is shown in Table 2.
Assessment of brain serotonin levels
Brain serotonin levels were determined by high-performance liquid chromatography with electrochemical detection (HPLC-ECD) as described by Soares-da-Silva et al. (1995). For this, samples were kept in perchloric acid at 0.2 M for 2 h at 4 °C and then frozen in the acid at − 80 °C until processing; aliquots of the resulting solution were centrifuged at 2700 rpm for 2 min at 4 °C using Costar Spin-X microfilter tubes.
Fifty microliters of the eluate was directly injected into the HPLC-ECD system. 3,4-Hydroxybenzylamine (DHBA) was used as an internal standard. Lower limit for detection of 5-HT ranges from 350 to 1000 fmol. The system is composed of a pump (Gilson model 302) connected to a Manometric module (Gilson model 802C) and a stainless-steel 5-µm ODS column (Biophase Bioanalytical Systems) with 25-cm length; samples were injected by means of an automatic sample injector (Gilson model 231) connected to a Gilson dilutor (model 401). Electrochemical detection was performed with a glassy carbon electrode, an Ag/AgCl reference electrode, and an amperometric detector (Gilson model 141), operated at 0.75 V. The procedure was monitored with Gilson 712 HPLC software.
Gene expression in liver
Three reference genes and twelve genes of interest were assessed in zebrafish liver (Supplementary Table 1). The selected reference genes selected were Elongation factor 1-alpha (Ef1), Actin, beta 1 (Actb1), and Ribosomal protein L8 (Rpl8). The selected genes of interest were (1) genes encoding isoforms of tryptophan hydroxylase (TPH), the rate-limiting enzyme in serotonin synthesis—TPH1a, TPH1b, TPH2 (variants TPH2a and TPH2b); (2) serotonin receptor encoding genes—5-hydroxytryptamine receptor 1A a (Htr1aa) and 5-hydroxytryptamine receptor 2C, G-protein-coupled-like 1 (Htr2cl1); (3) the serotonin transporter encoding gene—Solute carrier family 6 member 4a (Slc6a4a); (4) genes involved in protein synthesis/degradation pathways—Insulin Growth–Like Factor 1 (IGF1), Target of rapamycin (TOR), and Ubiquitin-conjugating Enzyme 2H (UBE2H); and (5) genes involved in the modulation of antioxidant activity—Nuclear factor (erythroid-derived-2)-like-2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1).
Primers for UBE2H, TOR, Nrf2, Keap1, and reference genes were obtained from literature available for zebrafish (Supplementary Table 1). For the remaining genes of interest, the primers were designed using gene sequences available in GenBank, using the Pick Primers tool from the National Centre of Biotechnology Information (NCBI).
RNA extraction and cDNA synthesis
RNA was extracted from pools of 3 livers using the Illustra RNAspin Mini RNA Isolation Kit (GE Healthcare). Six pools were evaluated for each diet tested. A digestion of genomic DNA was subsequently carried out using deoxyribonuclease I Amplification Grade. Quantification of RNA was performed with 2 µL of eluate using Take3 micro-volume plates in a BioTek microplate spectrophotometer, according to the micro-volume quantification method. Then, 1 µg of RNA was used to synthesize cDNA, with the qScript cDNA Synthesis Kit (Quanta BioSciences, Inc.).
Quantitative real-time polymerase chain reaction (qPCR)
In order to evaluate gene expression, reverse transcriptase quantitative real-time polymerase chain reaction (qPCR) was performed in an Eppendorf Mastercycler realplex 4 (Eppendorf, Hamburg, Germany). The qPCR assays were performed with a final volume of 20 µL, using 10 µL of Sybr® Green SuperMix (Quanta BioSciences, Inc.), 4 µL of water, 2 µL of F primer, 2 µL of R primer, and 2 µL of cDNA. Reactions were performed in duplicate as follows: 2 min of denaturation at 94 °C; 40 cycles of denaturation for 30 s at 94 °C, 30 s at 54 °C for annealing (for reference genes, TOR, UBE2H, Htr1aa and Htc2cl1, Slc6a4a, Keap1, Nrf2, TPH1a, TPH1b), at 52 °C (TPH2a, TPH2b), at 59 °C (IGF1), and 30 s at 72 °C for elongation. A final elongation was also performed for 10 min at 72 °C. A blank sample was done for each gene to identify possible nonspecific products.
Standard linear curves were made for all primers, to determine the efficiency of each primer; eight dilutions were used from 0.5 to 50 ng/µL, from an initial mixture of cDNA. Quantification of gene expression was done with normalization by reference genes using the NormFinder algorithm (Urbatzka et al., 2013). Relative expression was calculated using the overall mean of all samples as a calibrator and accounting for efficiency, using the mathematical template of Plaffl (Plaffl, 2001).
Statistical analysis
Data collected during the experimental trial (initial and final fish weight, feed consumption, chemical composition of diets, and initial and final fish body chemical composition) were used to determine growth performance and feed utilization indices, including weight gain (WG), feed efficiency (FE), protein efficiency ratio (PER), daily growth index (DGI), nitrogen intake (NI), nitrogen retention (NR), energy intake (EI), and energy retention (ER). Data are presented as means and pooled standard error of the mean (SEM). Polynomial orthogonal contrast analysis was performed to determine whether the data followed linear, quadratic, and/or cubic responses to the dietary graded levels of Trp; significance was accepted when p < 0.05. This statistical analysis was performed using IBM SPSS Statistics 25.0 software package for Windows.
Gene expression analysis was performed by means of one-way analysis of variance (ANOVA); significance was accepted when p < 0.05. Linear contrasts were also performed to ANOVA when applicable. Data were transformed when needed to meet ANOVA requirements. When ANOVA requirements could not be met, a non-parametric test (Kruskal-Wallis) was performed to investigate possible differences between dietary treatments. Differences between the expression levels of genes within diets were sought using the t-test for paired samples or the non-parametric Wilcoxon test for paired samples. Correlations between gene expressions were calculated using the Pearson’s correlation coefficient.
Discriminant analysis was used to integrate all gene expression data. The dependent variable (categorical variable) entered in the model described the three tested diets: non-supplemented, moderately supplemented, and highly supplemented diets for Trp0, Trp4, and Trp16, respectively. Predictors entered in the model were the twelve assessed genes and two-by-two combinations, to investigate possible interactive responses elicited by the dietary Trp levels. One-way ANOVA followed by Tukey HSD test was used to investigate homogenous groups and describe the contribution of each significant predictor retained in the final model to group discrimination.
Results
Growth performance
Diets were well accepted by fish and mortality was less than 5%, similar in all experimental groups. Growth performance, feed intake, feed efficiency, and protein efficiency ratio were not statistically different among experimental diets (Table 3). Nevertheless, daily nitrogen retention showed a quadratic tendency with a maximum for the diet Trp4. Similarly, nitrogen retention expressed in percentage of nitrogen intake showed a quadratic response, attaining a peak with diet Trp8. Energy retention expressed as percentage of energy intake also showed a quadratic response to dietary treatment, with a peak for the diet Trp8. Regarding whole-body composition, dietary treatment only affected gross energy, for which a quadratic response was found, with a peak for the diet Trp8 (Table 4).
Brain serotonin levels
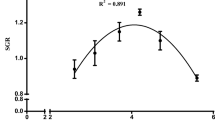
No significant differences were observed in brain serotonin levels between treatments. However, contrasts indicate a linear tendency, as brain 5-HT levels increase with increasing dietary Trp levels (Fig. 1).
Gene expression
According to NormFinder results, the most stable gene for single use was Actb1 (stability value: 0.017). However, the combination between Actb1 and Rpl8 (stability value: 0.013) was the most stable for multiple reference genes. Therefore, the normalization factor of Actb1 + Rpl8 was employed to normalize the expression of all genes.
Figure 2 illustrates the expression levels obtained for serotonin receptor genes Htr2cl1 and Htr1aa, and for the serotonin transporter Slc6a4a. Regarding serotonin receptor genes, Htr2cl1 showed lower expression in fish fed diet Trp4, while Htr1aa showed lower expression in fish fed the diet Trp0 than the fish fed the other two diets. No difference was detected in the expression of the serotonin transporter gene (Slc6a4a).
Figure 3 presents the levels of gene expression for Nrf2 and its repressor Keap1. A significant linear tendency of increase in expression with the increase of dietary Trp was encountered for Keap1. No significant differences among diets were found for Nrf2 expression levels. Because Keap1-Nrf2 inhibitory complex is recognized as the major regulator of cellular protective responses against electrophilic stress and oxidative stress caused by reactive oxygen species (ROS), possible differences in the expression levels of these two genes within diet were also sought. The statistical analysis showed the levels of Nrf2 were similar to those of Keap1 in fish fed diets for Trp0 and Trp4; however, the level of Nrf2 was 29% lower than Keap1 in fish fed the diet Trp16, suggesting a repressive effect of Keap1 over Nrf2.
IGF1, TOR, and UBE2H expression levels are presented in Fig. 4. No significant differences were found for the expression levels of UBE2H and IGF1 genes among dietary treatments. However, a significant linear tendency was encountered for TOR gene, with its expression levels increasing with the increase of dietary Trp.
Figure 5 shows the expression levels for two isoforms of tryptophan hydroxylase 1 (TPH1a and TPH1b) as well as the two variants of tryptophan hydroxylase 2 (TPH2a and TPH2b). No significant differences among diets were observed for the Trp-degrading enzymes TPH1b, TPH2a, and TPH2b. A significant negative linear tendency was encountered for TPH1a, showing that increasing levels of Trp decreased its expression in the liver.
The integrated analysis of genes and their two-by-two interaction was done with discriminant analysis. Wilk’s lambda value was used as the criterion for choosing the best subset of predictors. The best resulting subset, consisting of two interaction predictors Keap1.UBE2H and Htr2cl1.Slc6a4a, was able to discriminate all tested diets, showing 100% of discrimination capacity. The two corresponding discriminant functions are statistically significant, with chi-square p-value less than 0.01 (degrees of freedom equal to 4 and 1, respectively), explaining 100% of the between-group variability (85.5% and 14.5%, respectively).
Analysis of homogenous subsets produced by the Tukey HSD test showed how each predictor discriminated dietary treatments (Table 5). The graphic representation of the two selected interaction predictors (Keap1.UBE2H and Htr2cl1.Slc6a4a) is presented in Fig. 6. As evidenced, fish fed Trp0 and Trp4 diets, but mainly Trp0, showed lower expression of Keap1 and UBE2H genes than fish fed diet Trp16 (Fig. 6a). Moreover, fish fed Trp16 and Trp0 diets exhibited, respectively, high and moderate levels of Htr2cl1 and Slc6a4a gene expression; comparatively, fish fed Trp4 exhibited low level of Htr2cl1 gene expression and low to moderate levels of Slc6a4a gene expression (Fig. 6b).
Discussion
Amino acids are needed to promote optimum fish growth, as growth is dependent on protein synthesis and deposition. Trp is an essential amino acid, and therefore it must be supplied through diet in appropriate amounts. Hoseini et al. (2019) reviewed Trp deficiency signs in teleosts and reported impairment of growth performance and skeletal deformities. On the other hand, dietary Trp levels above an optimum may depress fish growth performance as well (Ahmed and Khan, 2005; Ahmed, 2012; Murthy and Varghese, 1997; Papoutsoglou et al., 2005b; Pianesso et al., 2015; Zaminhan et al., 2018), probably due to disproportional absorption related to amino acid imbalance. In the present study, no changes were observed neither on growth performance nor on skeletal deformities in response to dietary Trp levels, which indicates that all tested levels would be above deficiency threshold for zebrafish. However, the quadratic response found for body energy content, as well as for energy and nitrogen retention in relation to dietary Trp levels, suggests a better feed utilization with diets containing Trp levels around 0.9% (diet Trp4) and 2% (diet Trp8) of DM. Considering that peripheral serotonin seems to play an important role in improving nutrient absorption and assimilation as well as the storage of body energy, as indicated by studies in mammals (Yabut et al., 2019), these results are worthy of further investigation.
Amino acid supplementation can influence expression of genes related to protein turnover and growth regulation (Rolland et al., 2015). Studies have reported a correlation between growth hormone/insulin growth–like factor hormone system (GH/IGF) and growth, especially between IGF1 and growth (Beckman, 2011; Rolland et al., 2015). Metabolic active tissues, such as the liver, have higher protein synthesis, mostly because the liver plays an important role in amino acid metabolism as well as synthesis and exportation of proteins (Fraser and Rogers, 2007). Indeed, the liver is the main site of expression of IGF1 (Rolland et al., 2015). Gaylord et al. (2005) studied Trp requirements for hybrid striped bass juveniles and reported that deficiency in Trp leads to a reduction in plasma IGF1 levels and growth, while Trp supplementation can increase IGF1 levels and growth. Zhao et al. (2019) also showed that supplementation of Trp up to 4.2 g Trp kg−1 diet increased liver expression of IGF1 and IGF2 in hybrid catfish. Contrarily, our results showed no effects of Trp supplementation neither on growth nor on liver expression of IGF1, reinforcing the suggestion that even the level of Trp in the control diet would be above the deficiency threshold for this species.
The GH/IGF system has been reported as a regulator of protein synthesis through the TOR pathway. TOR plays an important role in protein synthesis, as it is activated by an amino acid pool (Tang et al., 2013). A downregulation of TOR expression related to increasing dietary Trp was reported in the hepatopancreas, intestine, and muscle of Jian carp (Tang et al., 2013), and in the intestine of grass carp (Wen et al., 2014). However, differently, an upregulation of TOR expression related to increasing Trp was found in the liver of hybrid catfish (Zhao et al., 2019) and in gills of grass carp (Jiang et al., 2015). Our findings are in line with these latter results, as we got a positive linear relationship between dietary Trp levels and TOR expression levels in the liver. This could be attributed to the important role in protein synthesis played by the liver, as previously mentioned.
Protein degradation is important in the assessment of protein turnover. Degradation of proteins is achieved through different pathways controlled by IGFs. The ubiquitin proteosomase pathway (UPP) is one of these degradation pathways, degrading intracellular short-lived or abnormal proteins. Protein degradation is important for several reasons, firstly as a way to control protein turnover and eliminate non-functional or denatured proteins (Fraser and Rogers, 2007; Rolland et al., 2015). The UPP involves the use of specific enzymes, including the ubiquitin conjugating enzyme-E2 H encoded by the UBE2H gene. In this study, Trp supplementation did not affect expression levels of UBE2H. Moreover, there was a similar expression of UBE2H and TOR genes in each dietary treatment, probably meaning a balance between protein synthesis and degradation (i.e., protein turnover), which agrees with the lack of differences in growth.
Tryptophan hydroxylase (TPH), the rate-limiting enzyme in serotonin synthesis, occurs under several isoforms encoded by paralogous genes. Four isoforms have been identified in the zebrafish brain, expressed in different brain areas: TPH1a, TPH1b, TPH2, and TPH3 (Höglund et al., 2019; Horzmann and Freeman, 2016; Lillesaar, 2011). In particular, the expression of TPH2 has been detected in brain neurons and is a marker of CNS serotonergic activity. This TPH2 is not saturated by brain Trp levels, and therefore variations in Trp availability affect directly brain serotonin production (Fernstrom, 2016; Höglund et al., 2007, 2019). Thus, increased Trp intake, leading to increased Trp plasma concentration and Trp uptake to the brain, will increase brain serotonin level, as observed in the present study with zebrafish and previous studies with other fish species (Basic et al., 2013; Herrero et al., 2007; Höglund et al., 2007, 2019; Hseu et al., 2003; Lepage et al., 2002, 2003).
There is a general recognition of the inhibitory effect of increased serotonergic activity on feed intake in fish (De Pedro et al., 1998; Øverli et al. 1998; Ortega et al., 2005; Rubio et al., 2006; Ruibal et al., 2002), as in other vertebrates, which contributes to the so-called stress-induced anorexia (Höglund et al., 2007). Contradictory results have however been observed regarding the effect of Trp dietary supplementation on feed intake (Höglund et al., 2007). In fact, contradictions may be only apparent as this effect seems to be context-dependent (Höglund et al., 2007; Martins et al., 2013), for instance, stimulating feed intake in stressed fish (thus mitigating stress-induced anorexia) but not in unstressed fish. In our study, despite the up to16-fold increase in Trp content between diets and the increase this caused in brain serotonin level, there was no change in feed intake between fish fed different Trp levels. Considering that crowding, a major stress source in cultured fish, was prevented by keeping fish density below the maximum recommended for the species (Aleström et al., 2020) and no other stressor was present, our results on feed intake are in line with those observed in other species in non-stressful conditions (Höglund et al., 2007).
Outside the brain, TPH1 is primarily expressed in non-neuronal cells of peripheral organs that are able to synthesize serotonin, such as the gut, kidney, and liver (Chen et al., 2017; Yabut et al., 2019; Yang et al., 2017), among others. In addition, THP2 is also expressed peripherally, especially in neurons of the enteric nervous system (Matthes and Bader, 2018; Yabut et al., 2019); moreover, in rats and humans, its expression was detected in several other organs as well, including the liver, simultaneously with the expression of TPH1 (Chen et al., 2017; Park et al., 2021). In line with this, we detected the isoforms TPH1a, TPH1b, and TPH2 (including variants TPH2a and TPH2b) expressed in zebrafish liver. Serotonergic nerve fibers from the autonomic nervous system have been identified in the human liver (Ruddell et al., 2008); assuming that serotonergic innervation also exists in zebrafish liver, it is likely that TPH2 would be synthesized in these nerve fibers, similarly to what happens in fibers of the enteric nervous system, while TPH1 would be produced in hepatic cells. In general, following intestinal uptake, Trp is used for protein synthesis and serotonin/melatonin synthesis, and the excess is catabolized. The liver is a major site of Trp catabolism via the kynurenine pathway (Chen et al., 2017; Hoseini et al., 2019; Palego et al., 2016), though some Trp is converted to serotonin in this organ through the action of TPH1. To our knowledge, there is no information about the effect of dietary supplementation with tryptophan on the serotonin pathway in the liver. Our results, showing an inverse correlation between TPH1a expression and dietary level of Trp, suggest a downregulation of TPH1a expression in response to increasing Trp concentration, probably as a mean of controlling serotonin production by the liver.
Serotonin coming from peripheral organs will be freely soluble in the circulating plasma (Hoseini et al., 2019) and can exert regulatory effects in many of these organs in mammals (Yabut et al., 2019) and fish (Caamaño-Tubío et al., 2007) through binding to target receptors. This is the case of the liver, where different serotonin receptors have been identified in several hepatic tissue cells, and in which serotonin regulates blood flow, innervation, regeneration ability, or lipogenesis in humans (Ruddell et al., 2008; Yabut et al., 2019). On the other hand, circulating serotonin is also internalized in cells of some organs through a specific membrane transporter and catabolized to 5-hydroxyindoleacetic acid (5-HIAA), which is eliminated by the kidney. In fish, the liver and gills seem to be the major sites of circulating serotonin breakdown, making an important contribution to control peripheral circulating levels of this compound and thus preventing harmful levels that can affect the normal functions of peripheral organs (Caamaño-Tubío et al., 2007). Our study sought to understand how different dietary levels of tryptophan can affect the expression of genes involved in the synthesis of some serotonin receptors and the serotonin transporter in the liver. Our results show a modulatory effect of the Trp dietary level on the expression of both serotonin receptor genes examined, but no effect on the serotonin transporter gene. The implications these effects may have are not possible to predict, since studies in mammals report that the liver has several other serotonin receptors and that all these receptors may be located in different liver cells (Ruddell et al., 2008; Yabut et al., 2019), and also because there are no studies on fish on this subject. In fact, most studies on the effects of serotonin in fish concern brain serotonin and very little importance has been given to potential effects of peripheral serotonin on the physiology of peripheral organs and how these effects can be modulated.
Serotonin and melatonin play an important role in antioxidant responses. Moreover, it was suggested that tryptophan itself can have antioxidant action, as it modulates antioxidant enzyme activity. Some studies reported that metabolites from Trp degradation can act as ROS scavengers and modulate antioxidant enzymes (Hoseini et al., 2019). The Nrf2 gene regulates cellular antioxidant response. At a resting state, Nrf2 and Keap1 combine in the cytoplasm and Keap1 promotes proteosomal degradation of Nrf2; after stimuli, Keap1 and Nrf2 uncouple, and Nrf2 can be transferred to the nucleus. Trp can activate the expression of antioxidant enzymes, through the inhibition of Nrf2 degradation, allowing Nrf2 translocation into the nucleus. Keap1 acts as a repressor of Nrf2 (Zheng et al., 2016). Wen et al. (2014) showed that Trp supplementation increased Nrf2 gene expression as it decreased Keap1 gene expression, and thus Nrf2 gene expression was negatively correlated to Keap1 expression in the intestine. The same study reported that antioxidant enzyme mRNA expression levels increased with Trp supplementation. The majority of Trp is degraded through the kynurenine pathway and this pathway produces a metabolite (3-hydroxyanthranilic acid) by TDO and kynurenase action. This metabolite can induce dissociation between Keap1 and Nrf2, promoting the translocation of Nrf2 to the nucleus and consequent expression (Xu et al., 2018). In rat liver, melatonin upregulated Nrf2 expression (Jung et al., 2010), a similar effect to that observed in hybrid catfish intestine due to Trp dietary supplementation (Zhao et al., 2019). In hybrid catfish intestine, Nrf2 was upregulated with the maximum of expression at 4.2 g Trp kg−1 of diet, while Keap1 expression hit the lowest value for that same dietary treatment, highlighting the negative correlation in the expression of these two genes. Contrarily, our results show that supplementation of Trp induced the expression of Keap1, increasing it linearly, and lowered Nrf2 expression for the highest supplemented diet (Trp16), which sustains the negative correlation of these genes. A possible explanation is that tested Trp levels were not enough to suppress Keap1, compromising the expression of Nrf2, or that no stimuli occurred that needed antioxidant responses.
Multivariate analysis fully discriminated tested diets based on the expression levels of four of the twelve measured genes. These genes belong to the signaling pathway of serotonergic transmission (serotonin receptor, Htr2cl1 and serotonin transporter, Slc6a4a), protein degradation from the UPP (UBE2H), and antioxidant mechanisms (Keap1). A high discrimination was achieved as a result from potential interactions or concomitant responses. The two-by-two interaction predictors provided further mechanistic knowledge on liver effects of Trp supplementation. On the one hand, these results sustain the relationship between serotonin receptor and transporter. On the other hand, present results highlight a crosstalk between the UBE2H and Keap1 pathways. Indeed, according to literature, Keap1 targets Nrf2 for ubiquination and subsequent degradation by the proteosome; however, this occurs associated with an ubiquitin ligase E3 complex, with at least a conjugating enzyme (Zhang and Hannick, 2003; Plafker et al., 2010). Keap1 is an adaptor for an ubiquitin ligase (Fuse and Kobayashi, 2017). Zebrafish has a similar Keap1-Nrf2 system to mammals, with this system being highly conserved (Fuse and Kobayashi, 2017). Present results show that Keap1 and ubiquitin conjugating enzyme E2 (UBE2H) are highly correlated. Moreover, expression levels of Keap1 increased with increasing expression of UBE2H, showing a higher expression of these genes with Trp supplementation. However, further investigation should be conducted to fully understand the regulation of the Keap1-Nrf2 system. Anyway, according to our results, the expression levels of Keap1, UBE2H, Htr2cl1, and Slc6a4a appear to be a useful tool to assess dietary Trp supplementation.
Conclusion
The results of this study indicate that under normal, non-stressful husbandry conditions, supplementation of the diet with tryptophan has no effect on growth and feed intake in zebrafish, although intermediate levels of supplementation appear to promote better feed utilization. As previously observed in other species, there was a linear increase in brain serotonin level with increasing levels of dietary tryptophan. In addition, the level of tryptophan in the diet had a modulatory effect on the expression of some liver genes, with relevance for serotonin receptor encoding genes and the tryptophan hydroxylase (the rate-limiting enzyme in serotonin synthesis from tryptophan) encoding gene.
Data availability
Not applicable.
Code availability
Not applicable.
References
Ahmed I (2012) Dietary amino acid L-tryptophan requirement of fingerling Indian catfish, Heteropneustes fossilis (Bloch), estimated by growth and heamato-biochemical parameters. Fish Physiol Biochem 38:1195–1209
Ahmed I, Khan MA (2005) Dietary tryptophan requirement of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Aquac Res 36:687–695
Akiyama T, Shiraishi M, Yamamoto T, Unuma T (1996) Effect of Dietary Tryptophan on Maturation of Ayu Plecoglossus altivelis Fish Sci 62:776–782
Aleström P, D’Angelo L, Midtlyng PJ, Schorderet DF, Schulte-Merker S, Sohm F, Warner S (2020) Zebrafish: housing and husbandry recommendations. Lab Anim 54:213–224
Azeredo R, Machado M, Guardiola FA, Cerezuela R, Afonso A, Peres H, Oliva Teles A, Esteban MA, Costas B (2017) Local immune response of two mucosal surfaces of the European seabass, Dicentrarchus labrax, fed tryptophan-or methionine supplemented diets. Fish Shellfish Immunol 70:76–86
Basic D, Schjolden J, Krogdahl A, von Krogh K, Hillestad M, Winberg S (2013) Changes in regional brain monoaminergic activity and temporary down-regulation in stress response from dietary supplementation with L-tryptophan in Atlantic cod (Gadus morhua). Br J Nutr 109:2166–2174
Beckman BR (2011) Perspectives on concordant and discordant relations between insulin-like growth factor 1 (IGF1) and growth in fishes. Gen Comp Endocrinol 170:233–252
Caamaño-Tubío RI, Pérez J, Ferreiro S, Aldegunde M (2007) Peripheral serotonina dynamics in the rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 145:245–255
Charlon N, Bergot P (1984) Rearing system for feeding fish larvae on dry diets. Trial with carp (Cyprinus carpio L.) larvae. Aquaculture 41:1–9
Chen Y, Xu H, Zhu M, Liu K, Lin B, Lio R, Chen C, Li M (2017) Stress inhibits tryptophan hydroxylase expression in a rat model of depression. Oncotarget 8:63247–63257
Costas B, Aragão C, Mancera JM, Dinis MT, Conceição LE (2008) High stock density induces crowding stress and affects amino acid metabolism in Senegalese sole Solea senegalensis (Kaup, 1858) juveniles. Aquac Res 39:1–9
Cuesta A, Cerezuela R, Esteban MA, Meseguer J (2008) In vivo actions of melatonin on the innate immune parameters in the teleost fish gilthead seabream. J Pineal Res 45:70–78
De Pedro N, Pinillos ML, Valenciano AI, Alonso-Bedate M, Delgado MJ (1998) Inhibitory effect of serotonin on feeding behavior in goldfish: involvement of CRF. Peptides 19:505–511
DeVries JW, Koski CM, Egberg DC, Larson PA (1980) Comparison between a spectrophotometric and a high-pressure liquid chromatography method for determining tryptophan in food products. J Agric Food Chem 28:896–898
Falcón J, Migaud H, Muñoz-Cueto JA, Carrillo M (2010) Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol 165:469–482
Fernstrom JD (2016) A perspective on the safety of supplemental tryptophan based on its metabolic fates. J Nutr 146(Suppl):2601S-2608S
Fraser KP, Rogers AD (2007) Protein metabolism in marine animals: the underlying mechanism of growth. Adv Mar Biol 52:267–362
FuseY, Kobayashi M (2017) Conservation of the Keap1-Nrf2 system: an evolutionary journey through stressful space and time. Molecules 22:436
Gaylord TG, Rawles SD, Davis KB (2005) Dietary tryptophan requirement of hybrid striped bass (Morone chrysops x M. saxatilis) Aquac Nutr 11:367–374
Herrero M, Martínez F, Míguez J, Madrid J (2007) Response of plasma and gastrointestinal melatonin, plasma cortisol and activity rhythms of European sea bass (Dicentrarchus labrax) to dietary supplementation with tryptophan and melatonin. J Comp Physiol B 177:319–326
Höglund E, Bakke MJ, Øverli Ø, Winberg S, Nilsson GE (2005) Suppression of aggressive behavior in juvenile Atlantic cod (Gadus morhua) by L-tryptophan supplementation. Aquaculture 249:525–531
Höglund E, Sørensen C, Bakke MJ, Nilsson GE, Øverli Ø (2007) Attenuation of stress-induced anorexia in brown trout (Salmo trutta) by pre-treatment with dietary L-tryptophan. Br J Nutr 97:786–789
Höglund E, Øverli Ø, Andersson MÅ, Silva P, Laursen DC, Moltesen MM, Krogdahl Å, Schjolden J, Winberg S, Vindas MA, Mayer I, Hillestad M (2017) Dietary L-tryptophan leaves a lasting impression on the brain and stress response. Br J Nutr 117:1351–1357
Höglund E, Øverli Ø, Winberg S (2019) Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front Endocrinol 10:158
Horzmann KA, Freeman JL (2016) Zebrafish get connected: investigating neurotransmission targets and alterations in chemical toxicity. Toxics 4:19
Hoseini SM, Pérez-Jiménez A, Costas B, Azeredo R, Gesto M (2019) Physiological roles of tryptophan in teleosts: current knowledge and perspectives for future studies. Rev Aquac 11:3–24
Hseu JR, Lu FI, Su HM (2003) Effect of exogenous tryptophan on cannibalism survival and growth in juvenile grouper, Ephinephelus coioides. Aquaculture 218:251–263
Jiang W-D, Wen H-L, Liu Y, Jiang J, Kuang S-Y, Wu P, Zhao J, Tang L, Tang W-N, Zhang YA, Zhou X-Q, Feng L (2015) The tight junction protein transcript abundance changes and oxidative damage by tryptophan deficiency or excess are related to the modulation of the signaling molecules, NF-rB, p65, TOR, caspase-(3,8,9) and Nrf2 mRNA levels, in the gill of young grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol 46:168–180
Johnston WL, Atkinson JL, Hilton JW, Were KE (1990) Effect of dietary tryptophan on plasma and brain tryptophan, brain serotonin and brain 5-hydroxyindoleacetic acid in rainbow trout. J Nutr Biochem 1:49–54
Jung KH, Hong SW, Zheng HM, Lee HS, Lee H, Lee DH (2010) Melatonin ameliorates cerulein-induced pancreatitis by the modulation of nuclear erythroid 2-related factor 2 and nuclear factor-kappaB in rats. Pineal Res 48:239–250
Katzung BG, Masters SB, Trevor A (2012) Basic and clinical pharmacology, 12th edn. The McGraw-Hill Companies, United States, pp 297–303
Le Floc’h N, Otten W, Merlot E, (2011) Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 41:1195–1205
Lepage O, Tottmar O, Winberg S (2002) Elevated dietary intake of L-tryptophan counteracts the stress-induced elevation of plasma cortisol in rainbow trout (Oncorhynchus mykiss). J Exp Biol 205:3679–3687
Lepage O, Vilchez IM, Pottinger TG, Winberg S (2003) Timecourse of the effect of dietary L-tryptophan on plasma cortisol levels in rainbow trout Oncorhynchus mykiss.J Exp Biol 206: 3589–3599
Lepage O, Larson ET, Mayer L, Winberg S (2005) Serotonin, but not melatonin plays a role in shaping dominant-subordinate relationships and aggression in rainbow trout. Horm Behav 48:33–242
Lillesaar C (2011) The serotonergic system in fish. J Chem Neuroanat 41:294–308
López-Olmeda JF, Bayarri MJ, Rol de Lama MA, Madrid JA, Sánchez-Vázquez FJ (2006) Effects of melatonin administration on oxidative stress and daily locomotor activity patterns in goldfish. J Physiol Biochem 62:17–25
Martins CIM, Silva PIM, Costas B, Larser BK, Santos GA, Conceição LEC, Dias J, Øverli Ø, Höglund E, Schrama JW (2013) The effect of tryptophan supplemented diets on brain serotonerfic activity and plasma cortisol under undisturbed and stressed conditions in grouped-housed Nile tilapia Oreochromis niloticus. Aquaculture 400–401:129–134
Matthes S, Bader M (2018) Peripheral serotonin synthesis as a new drug target. Trends Pharmacol Sci 39:560–572
Murthy HS, Varghese TJ (1997) Dietary tryptophan requirement for growth of rohu, Labeo rohita. J Appl Aquac 7:71–79
NRC (National Research Council) (2011) Nutrient requirements of fish and shrimp. The National Academies Press, Washington, D.C., USA, p 360
Ortega VA, Renner KJ, Bernier NJ (2005) Appetite-suppressing effects of ammonia exposure in rainbow trout associated with regional and temporal activation of brain monoaminergic and CRF systems. J Exp Biol 208:1855–1866
Øverli Ø, Winberg S, Damsgard B, Jobling M (1998) Food intake and spontaneous swimming activity in Arctic char (Salvelinus alpinus): role of brain serotonergic activity and social interactions. Can J Zool 76:1366–1370
Palego L, Betti L, Rossi A, Giannaccini G (2016) Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J Amino Acids 8952520
Papoutsoglou SE, Karakatsouli N, Koustas P (2005a) Effects of dietary L-tryptophan and lighting conditions on growth performance of European sea bass (Dicentrarchus labrax) juveniles reared in a recirculating water system. J Appl Ichthyol 21:520–524
Papoutsoglou SE, Karakatsouli N, Chiras G (2005b) Dietary l-tryptophan and tank colour effects on growth performance of rainbow trout (Oncorhynchus mykiss) juveniles reared in a recirculating water system. Aquac Eng 32:277–284
Park S, Kim Y, Lee JY, Kim H, Lee S, Oh C-MA (2021) Systems biology approach to investigating the interaction between serotonin synthesis by tryptophan hydroxylase and the metabolic homeostasis. Int J Mol Sci 22:2452
Pianesso D, Neto R, da Silva LP, Goulart FR, Adorian TJ, Mombach PI, Loureiro BB, Dalcin MO, Rotili DA, Lazzari R (2015) Determination of tryptophan requirements for juvenile silver catfish (Rhamdia quelen) and its effects on growth performance, plasma and hepatic metabolites and digestive enzymes activity. Anim Feed Sci Technol 210:172–183
Plaffl MW (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Plafker KS, Nguyen L, Barneche M, Mirza S, Crawford D, Plafker SM (2010) The ubiquitin-conjugating enzyme UbcM2 can regulate the stability and activity of the antioxidant transcription factor Nrf2. J Biol Chem 285:23064–23074
Rolland M, Dalsgaard J, Holm J, Gómez-Requeni P, Skov PV (2015) Dietary methionine level affects growth performance and hepatic gene expression of GH-IGF system and protein turnover regulators in rainbow trout (Oncorhynchus mykiss) fed plant protein-based diets. Comp Biochem Physiol B 181:33–41
Rubio VC, Sanchez-Vazquez FJ, Madrid JA (2006) Oral serotonin administration affects the quantity and the quality of macronutrients selection in European sea bass Dicentrarchus labrax L. Physiol Behav 87:7–15
Ruddell RG, Mann DA, Ramm GA (2008) The function of serotonin within the liver. J Hepatol 48:666–675
Ruibal C, Soengas JL, Aldegunde M (2002) Brain serotonin and the control of food intake in rainbow trout (Oncorhynchus mykiss): effects of changes in plasma glucose levels. J Comp Physiol A 188:479–484
Soares-da-Silva P, Pestana M, Vieira-Coelho MA, Fernandes MH, Albino-Teixeira A (1995) Assessment of renal dopaminergic system activity in the nitric oxide-deprived hypertensive rat model. Br J Pharmacol 114:1403–1413
Tang L, Feng L, Sun C-Y, Chen G-F, Jiang W-D, Hu K, Liu Y, Jiang J, Li S-H, Kuang S-Y, Zhou X-Q (2013) Effect of tryptophan on growth, intestinal enzyme activities and TOR gene expression in juvenile Jian carp (Cyprinus carpio var. Jian): studies in vivo and in vitro. Aquaculture 412:23–33
Tejpal CS, Pal AK, Sahu NP, Ashish Kumar J, Muthappa NA, Vidva S, Rajan MG (2009) Dietary supplementation of L-tryptophan mitigates crowding stress and augments the growth in Cirrhinus mrigala fingerlings. Aquaculture 293:272–277
Urbatzka R, Galante-Oliveira S, Castro LF, Cunha I (2013) Normalization strategies for gene expression studies by real-time PCR in a marine fish species, Scophthalmus maximus. Mar Genomics 10:17–25
Wen HI, Feng L, Jiang WD, Liu Y, Jiang J, Li SH, Tang L, Zhang YG, Kuang SY, Zhou X (2014) Dietary tryptophan modulates intestinal immune response, barrier function, antioxidant status and gene expression of TOR and Nrf2 in young grass carp (Ctenopharyngofon idella) Fish Shellfish Immunol 40:275–287
Winberg S, Øverli Ø, Lepage O (2001) Suppression of aggression in rainbow trout (Oncorhynchus mykiss) by dietary L-tryptophan. J Exp Biol 204:3867–3876
Winberg S, Thörnqvist P-O (2016) Role of brain serotonin in modulating fish behavior. Curr Zool 62:317–323
Wolkers CPB, Serra M, Hoshiba MA, Urbinati EC (2012) Dietary L-tryptophan alters aggression in juvenile matrinxa Brycon amazonicus. Fish Physiol Biochem 38:819–827
Wu G (2009) Amino acids: metabolism, functions and nutrition. Amino Acids 37:1–17
Xu K., Liu G., Fu C. (2018) The tryptophan pathway targeting antioxidant capacity in the placenta. Oxid. Med. Cell. Longev. ID: 1054797
Yang Q, Yan C, Yin C, Gong Z (2017) Serotonin activated hepatic stellate cells contribute to sex disparity in hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol 3:484–499
Yabut JM, Crane JD, Green AE, Keating DJ, Khan WI, Steinberg GR (2019) Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule. Endocr Rev 40:1092–1107
Zaminhan M, Michelato M, Furuya VRB, Boscolo WR, Araújo FE, Cruz TP, Urbich AV, Furuya WM (2018) Total and available tryptophan requirement of Nile tilapia, Oreochromis niloticus, fingerlings. Aquac Nutr 24:1553–1562
Zhang DD, Hannick M (2003) Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23:8137–8151
Zhao Y, Wu X-Y, Xu S-X, Cien J-Y, Xiang K-W, Feng L, Liu Y, Jiang W-D, Wu P, Zhao J, Zhou X-Q, Jiang J (2019) Dietary tryptophan affects growth performance, digestive and absorptive enzyme activities, intestinal antioxidant capacity and appetite and GH-IGF axis-related gene expression of hybrid catfish (Pelteobagrus vachelli♀ x Leiocassis longirostris♂). Fish Physiol Biochem 45:1627–1647
Zheng J-L, Zeng L, Shen B, Xu M-Y, Zhu AY, Wu C-W (2016) Antioxidant defenses at transcriptional and enzymatic levels and gene expression of Nrf2-Keap1 signaling molecules in response to acute zinc exposure in the spleen of the large yellow croaker Pseudosciaena crocea. Fish Shellfish Immunol 52:1–8
Funding
This work was funded by Programa Operacional Mar2020, Portugal 2020 under the project InovFeed (ref. MAR-02.01.01-FEAMP-0111).
Author information
Authors and Affiliations
Contributions
C.T., H.P., and A.P.C. conceived the study and designed the trial. C.T. and A.P.C. carried out the experiments. C.T., P.R., P.S., and L.F. carried out the chemical, biochemical, and genomic analyses. C.T., H.P., L.G., and L.O.T. analyzed the data. C.T., A.P.C., and L.G. wrote and L.O.T. revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The experiment was carried out in compliance with the European Union directive 2010/63/EU on the protection of animals used for scientific purposes.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teixeira, C., Rodrigues, P., Serrão, P. et al. Dietary tryptophan supplementation does not affect growth but increases brain serotonin level and modulates the expression of some liver genes in zebrafish (Danio rerio). Fish Physiol Biochem 47, 1541–1558 (2021). https://doi.org/10.1007/s10695-021-00994-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-021-00994-x