Abstract
In seasonally breeding vertebrates, extrinsic factors like photoperiod and temperature are major determinants, controlling the annual reproductive cycle. In teleosts, kisspeptin, which occurs in two molecular forms: kisspeptin1 (Kiss1) and kisspetin2 (Kiss2), has been reported to alter gonadotropin (Lh and Fsh) secretion but its effect on gonadotropin-releasing hormone (Gnrh) secretion is not unequivocally proved. In the catfish Heteropneustes fossilis, we isolated and characterized kiss2 and gnrh2 cDNAs and the present work reports effects of altered photo-thermal conditions and melatonin (MT, a pineal hormone) on their expressions in the brain. The exposure of the catfish to long photoperiod (LP, 16 h light) at normal temperature (NT) or high temperature (HT, 28 °C) at normal photoperiod (NP) for 14 or 28 days stimulated both kiss2 and gnrh2 expression in both gonad resting and preparatory phases with the combination of LP + HT eliciting maximal effects. Short photoperiod (SP, 8 h light) under NT or HT altered the gene expression according to the reproductive phase and temperature. MT that mediates photo-thermal signals to the brain inhibited brain kiss2 and gnrh2 gene expression in the NP + HT, LP + NT, and SP + NT groups. The altered photo-thermal conditions elicited changes in steroidogenic pathway as evident from changes in plasma E2, progesterone, and testosterone levels. The results show that brain kiss2-gnrh2 signaling is involved in photo-thermal-mediated mechanisms controlling reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In teleosts, as in other vertebrates, gametogenesis and spawning are regulated by hormones of the brain-pituitary-gonadal (BPG) endocrine axis in a hierarchical manner. The primary regulators of the BPG axis are kisspeptin (Kiss), gonadotropin-releasing hormone (Gnrh), and Gnrh-inhibiting hormone (Gnih) in the brain, gonadotropins (Gth: Lh and Fsh) in the pituitary, and steroid hormones in the gonads (Zohar et al. 2010; Chang and Pemberton 2018; Ohga et al. 2018; Somoza et al. 2020; Muñoz-Cueto et al. 2020). Kisspeptin-10 with its cognate receptor GPR54 was first identified in mammals as the upstream regulator of Gnrh. Mutations of the receptor led to reproductive failures (Roa et al. 2008). The early studies have generated great interest in the search for the orthologs in non-mammalian vertebrates and an interesting evolutionary history of kisspeptins was presented (Lee et al. 2009; Pasquier et al. 2012). Accordingly, teleosts have retained differentially one or two paralogs (kiss1 or kiss2) that code for Kiss1 or Kiss2, respectively. The distribution of the two forms varies in teleosts: there are species with both Kiss1 and Kiss2, and species with Kiss2 only. The two forms have distinct anatomical distribution and functions, as shown in the zebrafish brain: Kiss1 is located in the habenular nucleus and Kiss2 in hypothalamus with projections into the pituitary (Servili et al. 2011). While the Kiss system is indispensable for reproductive functions in mammals, its importance is debated in teleosts (Tang et al. 2015; Nakajo et al. 2018) and the documented studies report species variations in its reproductive role depending on acute or chronic treatments or the nature of the interactive systems (Ohga et al. 2018).
Gnrh occurs in multiple forms: Gnrh1 and/or Gnrh3 in the forebrain and Gnrh2 in the midbrain (Chang and Pemberton 2018; Somoza et al. 2020; Muñoz-Cueto et al. 2020). In addition to the hypophysiotropic role, Gnrh serves as a neuromodulator in the control of sexual and feeding behaviors (Okubo and Nagahama 2008; Zohar et al. 2010; Kang et al. 2011). The midbrain Gnrh2 mRNA or the peptide is expressed in the pituitary of teleosts to varying extents (Kim et al. 1995; Guzman et al. 2009; Xia et al. 2014), suggesting a hypophysiotropic role as well. In the African catfish, for example, Gnrh2 fibers were traced along with Gnrh1 fibers in the pituitary and showed a higher in vitro LH-releasing capacity compared with that of catfish Gnrh1 (Schulz et al. 1993).
Most teleosts are seasonal breeders and exhibit distinct annual or multiple reproductive cycles, associated with cyclic changes in photoperiod and temperature (Borg 2009; Miranda et al. 2009; Martins et al. 2015). The role of the extrinsic factors regulating the sexual cycle varies between species living in temperate and tropical regions in order to maximize the survival of off-springs (Tian et al. 2019). In temperate zone fish like salmon, fall in photoperiod and temperature are cues responsible for sexual maturation (short day breeders). In tropical and subtropical fishes like carp and catfish which are long day breeders, rising photoperiod and temperature are cues that initiate and maintain gonadal maturation (Sundararaj 1981). The physiological responses of the BPG axis to natural (cyclic) or artificial changes in environmental photoperiod and temperature have been extensively documented in teleosts and the information have been updated with newer methodological approaches and techniques. The effects of photoperiod and temperature alterations on expression of the reproductive hormone genes such as kiss, gpr54, gnrh, gnrh receptor genes, and gonadotropin subunit genes have been reported in different fish species (Martinez-Chavez et al. 2008; Shin et al. 2014; Kim et al. 2014; Chi et al. 2017; Shao et al. 2019).
In seasonally breeding mammals like hamsters, Kiss1 mediates the photoperiodic control of reproduction via the secretion of pineal melatonin (Revel et al. 2007; Scott et al. 2019). In fish, the pineal organ secretes melatonin and provides a crucial endocrine signal to influence the BPG axis for reproduction (Joy and Agha 1991; Falcón et al. 2010; Migaud et al. 2010). Few studies in fish also indicate that the Kiss system seems to be the target of melatonin in the photoperiodic pathway of reproduction (Servili et al. 2013; Alvarado et al. 2015).
The catfish Heteropneustes fossilis is a freshwater subtropical species living at bottom of ponds, lakes, streams, and other natural water bodies. It comes to the surface of water to gulp in air (air-breathing habit). It is a seasonal breeder with a distinct annual reproductive cycle, synchronized with the photoperiod and temperature cycles (Sundararaj 1981; Joy 1999). Gonadal recrudescence starts with the increase in photoperiod and temperature (March to June), spawning occurs during monsoon rainfall (July–August), and a prolonged gonadal quiescence sets in (September to February) until the next breeding cycle. The species is iteroparous and attains puberty in the first year of development. Therefore, gonadal recrudescence alternates with quiescence and the breeding cycle commences with the activation of the BPG endocrine machinery each time. The catfish has been studied extensively for seasonal changes in reproductive parameters like gonado-somatic index (GSI), gonad histology and plasma steroid hormone profiles (Sundararaj 1981; Lamba et al. 1983), plasma gonadotropin (Lh) levels (Tharakan and Joy 1996), and pituitary gonadotropin (Gth) subunit mRNA levels (Acharjee et al. 2015). A later study has determined the effects of altered photoperiod and temperature as key factors responsible for the seasonal changes (Acharjee et al. 2017). Recently, gnrh2 and kiss2 cDNAs were isolated and characterized in this species (Chaube et al. 2019; Chaube et al. 2020). The gnrh2 and kiss2 mRNAs were highly expressed in the brain, followed by gonads and showed seasonal patterns with low levels in the gonad resting phase, increments during gonad recrudescence and a decline after spawning. The seasonal patterns could be driven by the cyclic changes in the environmental photoperiod and temperature. Since melatonin mediates the effects of photoperiod and temperature on the BPG axis, exogenously administered melatonin could modify the effects of altered photo-thermal conditions on the gene expression. To verify these hypotheses, the present study was undertaken in two phases of the reproductive cycle: gonad inactive (resting) and early gonad active (preparatory). Our efforts to isolate kiss1 and gnrh1 genes by molecular cloning or retrieving from transcriptome database of the catfish were not yet successful. Hence, we focused the experiments on kiss2 and gnrh2 gene expression.
Materials and methods
Chemicals and reagents
The following molecular biology kits and reagents were used: RNeasy lipid tissue mini kit (Qiagen; www.qiagen.com), Revert-Aid H minus first strand complementary cDNA synthesis kit (Fermentas; www.thermofisher.com), DNase I RNase-free (Ambion Inc.; www.thermofisher.com), RNAlater (Ambion Inc.), 2X PCR master mix (Fermentas), and 2X VeriQuest SYBR Green qPCR Master Mix (Affymetrix Inc.; www.affymetrix.com). The primers were synthesized by Integrated DNA Technologies (www.idtdna.com). Melatonin and MS222 were purchased from Sigma-Aldrich (New Delhi, India). ELISA kits for estradiol-17β, testosterone, and progesterone were obtained from Labor Diagnostika Nord GmbH & Co. KG (www.ldn.de). Other chemicals were of molecular biology grade from Merck, India (www.merck.com).
Experimental location and facilities
The experiments were conducted at the Department of Zoology, Banaras Hindu University, Varanasi, and the fish were maintained in the aquarium facility of the animal house. The experiments were performed in accordance with the guidelines of the Banaras Hindu University for experimentation in animals and all care was taken to prevent cruelty of any kind.
Experimental animals and husbandry
H. fossilis is an annual breeder and the reproductive cycle can be divided into five phases: resting (November–January), preparatory (February–April), prespawning (May–June), spawning (July–August), and post-spawning (September–October) phases. Adult female catfish (40–50 g) were collected from local fish markets in Varanasi in the first week of December (resting phase) and in the first week of March (preparatory phase). The fish were maintained in 60-l experimental tanks (size 1 × 1 m × 0.2 m) under natural photoperiod and temperature (10.5 L: 13.5D, 10 ± 2 °C and 12.5 L: 11.5 D, 18 ± 2 °C) for a week to overcome stress due to transportation. They were fed daily with goat liver ad libitum. The resting phase represents the quiescent state of gonads under natural conditions; the ovaries have only small follicles with a GSI of 0.74% and large germinal vesicle (GV) containing a prominent nucleolus. Cytoplasm is strongly positive to hematoxylin staining. The gonads respond when exposed to high photoperiod and temperature. The preparatory phase marks the initiation of gonadal recrudescence in response to the increase in natural photoperiod and ambient temperature. The ovaries contain large previtellogenic follicles with the oocytes in perinucleolar stage and cortical alveoli may appear peripherally. Yolk nucleus will appear at this stage. The cytoplasm is less hematoxylin-positive and develops eosin affinity. The resting and preparative phases were selected in the experiments because the former represented the natural non-responsive stage and the latter the early responsive stage of the ovary.
Experimental treatments
Effects of altered photoperiod and temperature
In each phase, acclimated fish were divided into seven groups of 15 each and exposed to the following photoperiod and temperature treatments:
Groups | Resting phase | Preparatory phase |
1. Initial control (IC) group. The fish were sacrificed at the start of the experiment after acclimatization. 2. Normal photoperiod + normal temperature (NP + NT, parallel control). | 10.5 L:13.5 D, 10 ± 2 °C | 12.5 L:11.5 D, 18 ± 2 °C |
3. NP + high temperature (HT). Water temperature was raised by an aquarium heater with thermostatic control. | 10.5 L:13.5 D, 28 ± 2 °C | 10.5 L:13.5 D, 28 ± 2 °C |
4. Long photoperiod (LP) + NT. Light source was a 80-W fluorescent rod fixed to the ceiling of the animal house from 06 to 22 h | 16 L: 8 D, 10 ± 2 °C | 16 L: 8 D, 18 ± 2 °C |
5. LP + HT | 16 L: 8 D, 28 ± 2 °C | 16 L: 8 D, 28 ± 2 °C |
6. Short photoperiod (SP) + NT. Light was on from 10 to 18 h | 8 L: 16 D, 10 ± 2 °C | 8 L: 16 D, 18 ± 2 °C |
7. SP + HT | 8 L: 16 D, 28 ± 2 °C | 8 L: 16 D, 28 ± 2 °C |
The fish were fed with goat liver twice daily and maintained for 14 or 28 days. After the experiment, the fish were anesthetized with MS222, weighed, and blood was collected by caudal vein puncture. Blood was centrifuged at 2800g for 15 min at 4 °C to separate plasma and the plasma was stored at − 80 °C for steroid assays. After the blood collection, the fish were sacrificed between 0900 and 1100 h by decapitation and brains were dissected out and weighed. The tissues were snap-frozen in liquid nitrogen and stored in RNAlater at − 80 °C for qPCR assays.
Effects of melatonin
In the second experiment, in each phase, acclimatized female catfish were maintained for 14 days under NP + HT, LP + NT, and SP + NT conditions separately, as described above. Melatonin (dissolved in fish saline, 0.65% NaCl) was injected intraperitoneally in a dose of 25 μg/fish on alternate days for the next 14 days (7 injections) to half the fish. One batch of 5 fish was kept as untreated (NP + NT) group and the remaining fish were given the vehicle. Five fish from each group were sacrificed on day 28. Brains were dissected out and snap-frozen in liquid nitrogen and stored in RNAlater at − 80 °C for qPCR assays.
qPCR assay
About 100 mg of each tissue was used for the extraction of total RNA using the RNeasy mini kit (Qiagen). RNA purity was checked by calculating A260/A280 ratio and samples giving a ratio above 2.0 were only used. Genomic DNA contamination in RNA was checked using non-reverse transcribed samples as templates. In addition, the samples were treated with DNAse I before proceeding for the first strand cDNA synthesis. Five micrograms of total RNA was reverse transcribed using random hexamer primers and Revert Aid M-MuLV reverse transcriptase in a 20-μL reaction volume (first strand cDNA synthesis kit, Fermentas), using the manufacturer’s protocol. The primer specificity was confirmed by dissociation curve analysis. Quantitative PCR assays were performed in triplicate for different samples using specific primers (see the table below) and VeriQuest TM SYBR Green qPCR master mix with ROX (Affymetrix, Inc. Cleveland, OH, USA) in a ABI Prism 7500 thermal cycler (Applied Biosystems, Foster, CA, USA) at 95 °C (15 s), 60 °C (1 min) for 40 cycles. The primer efficiencies were as follows: gnrh2—100.32%, kiss2—99.91%, and β-actin—109.67%. Each sample was run in a final volume of 20 μL containing 1 μL of cDNA, 10 pM of each primer, and 10 μL of SYBR Green PCR master mix. Specificity of amplicons was verified by melting curve analysis (60–95 °C) after 40 PCR cycles. As controls, the assays were performed without templates and reverse transcriptase. No amplification was observed in the control studies. Cycle threshold (Ct) values were obtained from the exponential phase of PCR amplification and target gene (gnrh2) expression was normalized against the β-actin gene expression to generate 2−ΔΔCt values to quantify the target gene abundance (Livak and Schmittgen 2001). As an internal standard, catfish β-actin was used in the qPCR assay and its expression was stable in previous validation studies in the catfish (Chaube et al. 2017).
Primers | Primer sequences (5′-3′) | Expected amplicon size | |
gnrh2 | Forward | GTTCAGCACAGACGAGGCA | 145 bp |
Reverse | CTGATGTGTTCCTCCAGGGCA | ||
kiss2 | Forward | CAGACAGACCAGGATGTCCA | 165 bp |
Reverse | AGATCGGGATCGAGGAAAAT | ||
β-actin | Forward | TGGCCGTGACCTGACTGAC | 157 bp |
Reverse | CCTGCTCAAAGTCAAGAGCGAC | ||
Steroid assay
Plasma samples were extracted with 3 volumes of diethyl ether, thrice. The ether phase was collected and dried under N2 gas and stored at − 20 °C. Estradiol-17β (E2), testosterone (T), and progesterone (P4) were assayed by specific ELISA kits (Labor Diagnostika Nord GmbH & Co. KG, Germany). Standards and samples were processed according to the manufacturer’s instructions. Absorbance was taken at 450 nM using a Multiscan Microplate Reader (Thermo Electron Corporation, USA). The details of the steroid assays and validation were previously described (Acharjee et al. 2017).
Gonado-somatic index
Gonad weight was expressed in 100-g body weight in percentage.
Statistical analysis
The Statistical Package for the Social Sciences software program (version 10.0; SPSS) was used for the analyses. Data were presented as mean ± SEM and checked for homogeneity and normality. In the Levene’s test, the error variances of the dependent variables were equal across groups (P < 0.05) and the data were homogeneous. The data followed normal distribution in the Kolmogorov–Smirnov test. The data were analyzed by a two-way ANOVA (P < 0.001), taking treatment and duration, and photoperiod, temperature, and melatonin as independent variables. Multiple group comparisons were done by Dunett’s test (P < 0.05). Gene expression data were presented as mean of Log RQ ± SEM. The control sample was taken as the calibrator to give a relative ratio of the mRNA expression. The gene expression data were analyzed by the Pearson correlation test to determine the relationship between kiss2 and gnrh2 responses.
Results
Effects of altered photoperiod and temperature on brain kiss2 and gnrh2 expression
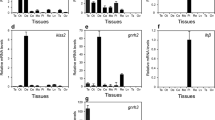
In the resting phase, the photo-thermal treatments produced overall significant effects on kiss2 (Fig. 1A; F14 = 14.18, F28 = 21.16, F14x28 = 18.65) and gnrh2 (Fig. 1B; F14 = 18.05, F28 = 17.11, 14.08) mRNA levels (two-way ANOVA, P < 0.001). In the NP + NT (parallel control, PC) group, the kiss2 expression increased significantly over the IC group on day 28 (Dunett’s test, P < 0.05). Similarly, the expression increased significantly in the LP + NT, NP + HT, and LP + HT groups with the highest increase in the LP + HT group. In the SP + NT group, the expression was not affected on day 14 compared with the PC group but was lowered on day 28. In the SP + HT group, the expression was increased on day 14 but decreased on day 28 compared with the control group. A significant duration (14 days vs. 28 days) effect was noticed in all groups with the expression higher on day 28 except in the LP + NT and SP + NT groups. The gnrh2 expression showed similar changes, like the kiss2 expression, in the LP + NT and LP + HT groups. In the NP + HT group, there was no change on day 14. In the SP + NT and SP + HT groups, the expression decreased compared with the NP + NT group. The increase in the gnrh2 expression showed significant duration effect except in the NP + NT, SP + NT, and SP + HT groups (P < 0.05).
Effects of altered photoperiod and temperature on brain kiss2 (A) and gnrh2 (B) expression levels in the female catfish H. fossilis in the resting phase. Data were expressed as mean ± SEM and analyzed by two-way ANOVA (P < 0.001), followed by Dunett’s test (P < 0.05). The groups with different letters are significantly different and those with the same letters are not significant. IC, initial control; NP, normal photoperiod, LP, long photoperiod; SP, short photoperiod; NT, normal temperature; HT, high temperature. NS, not significant between 12- and 28-day groups
In the preparatory phase, the expression of kiss2 and gnrh2 was higher than that in the resting phase. The mRNA levels showed significant changes to photo-thermal alterations (kiss2: Fig. 2A; F14 = 11.08, F28 = 14.17, F14x28 = 18.12; gnrh2: Fig. 2B; F14 = 20.08, F28 = 23.14, F14x28 = 14.62; two-way ANOVA, P < 0.001). The kiss2 expression increased significantly in the PC group without any significant duration effect (Dunett’s test, P < 0.05). The mRNA levels increased in the LP + NT, NP + HT, and LP + HT groups with significantly higher expression in the LP + HT group, compared with the control groups. A significant duration effect was noticed in these groups. In the SP groups, the expression was unchanged in the 14-day SP + NT group but increased in the 28-day SP + NT and SP + HT groups. The gnrh2 expression showed similar patterns of changes when exposed to different photo-thermal conditions (P < 0.05). A significant duration effect was noticed in all groups except the LP + HT group.
Effects of altered photoperiod and temperature on brain kiss2 (A) and gnrh2 (B) expression levels in the female catfish H. fossilis in the preparatory phase. Data were expressed as mean ± SEM and analyzed by two-way ANOVA (P < 0.001), followed by Dunett’s test (P < 0.05). The groups with different letters are significantly different and those with the same letters are not significant. IC, initial control; NP, normal photoperiod, LP, long photoperiod; SP, short photoperiod; NT, normal temperature; HT, high temperature. NS, not significant between 12- and 28-day groups
A significant positive correlation was obtained between kiss2 and gnrh2 expression after photo-thermal treatments for both 14 and 28 days of exposure in the resting and preparatory phases (Fig. 3, Table 1).
Effects of melatonin on brain kiss2 and gnrh2 mRNA levels
The administration of melatonin produced overall significant effects on kiss2 and gnrh2 mRNA expression in the resting phase (Fkiss2 = 18.02, Fgnrh2 = 28.18; Fkiss2xgnrh2 = 31.62; Fig. 4A; two-way ANOVA, P < 0.001). Melatonin decreased the kiss2 mRNA levels by about half a fold in the NP + HT group and by about 1.5 folds in the LP + NT group (Dunett’s test, P < 0.05). In the SP + NT + Mel group, the kiss2 expression was upregulated by about half a fold. There was a significant decrease in the gnrh2 mRNA levels in the NP + HT + Mel group in comparison with the NP + HT + Veh group (P < 0.05). Melatonin decreased the gnrh2 expression significantly in the LP group but increased it in the SP group.
Effects of melatonin (mel) on brain kiss2 and gnrh2 expression levels in the female catfish H. fossilis in the resting (A) and preparatory (B) phases. Data were expressed as mean ± SEM and analyzed by two-way ANOVA (P < 0.001), followed by Dunett’s test (P < 0.05). The groups with different letters are significantly different, and those with the same letters are not significant. IC, initial control; NP, normal photoperiod, LP, long photoperiod; SP, short photoperiod; NT, normal temperature; HT, high temperature. NS, not significant between 12- and 28-day groups
In the preparatory phase, the melatonin treatment produced overall significant effects on kiss2 and gnrh2 expression levels (F = 32.12, F = 35.24, Fkiss2xgnrh2 = 41.06; Fig. 4B; two-way ANOVA, P < 0.001). The kiss2 mRNA expression was decreased significantly in the NP + HT + Mel, LP + NT + Mel, and SP + NT + Mel groups (Dunett’s test, P < 0.05). The gnrh2 mRNA level was decreased to about 40–50% in the NP + HT + Mel group in comparison with the NP + HT + Veh group. In the LP and SP groups, melatonin decreased the mRNA levels significantly, but the inhibition was moderate unlike the NP + HT + Mel group.
A highly significant positive correlation was obtained between kiss2 and gnrh2 expression after melatonin treatments in the preparatory phase (P < 0.001) but the coefficient of correlation in the resting phase was significant at P < 0.01 level (Fig. 5, Table 1).
Effects of altered photo-thermal conditions on GSI
The GSI showed overall significant effects when the catfish were exposed to different photo-thermal treatments in the resting (F14 = 15.10, F28 = 18.25, F14x 28 = 16.04) and preparatory (F14 = 21.08, F28 = 23.14, F14x 28 = 26.51) phases (Fig.6; two-way ANOVA, P < 0.001). In the resting phase, the GSI increased significantly in the PC, LP + NT, NP + HT, and LP + HT groups with the highest increase in the LP + HT group (Dunett’s test, P < 0.05). A duration effect was also noticed with higher significant increases on day 28. In the SP + NT group, the GSI decreased without any significant duration effect. In the SP + HT group, a mild increase was noticed on day 14. In the preparatory phase, a significant increase in the GSI was noticed only in the 28-day PC group compared with the IC group. The GSI increased significantly in the LP + NT, NP + HT, and LP + HT groups with the highest increase in the LP + HT group. The increase showed significant differences between the treatment duration. In the SP groups, the increase was seen in the 28-day SP + NT group and at either duration in the SP + HT group.
Effects of altered photoperiod and temperature on gonadosomatic index (GSI, g%) of the female catfish H. fossilis in the resting (A) and preparatory (B) phases. Data were expressed as mean ± SEM and analyzed by two-way ANOVA (P < 0.001), followed by Dunett’s test (P < 0.05). The groups with different letters are significantly different, and those with the same letters are not significant. IC, initial control; NP, normal photoperiod, LP, long photoperiod; SP, short photoperiod; NT, normal temperature; HT, high temperature. NS, not significant between 12- and 28-day groups
Effects of altered photo-thermal conditions on plasma steroid hormone levels
Plasma steroid hormone profiles showed overall significant changes (two-way ANOVA, P < 0.001) in the catfish exposed to different photo-thermal treatments: E2 (Fig. 7; resting phase, F14 = 12.62, F28 = 15.02, F14x 2 8 = 13.24; preparatory phase, F14 = 17.10, F28 = 19.24, F14x 28 = 15.12), testosterone (Fig. 8; resting phase, F14 = 18.07, F28 = 22.03, F14x 28 = 20.18; preparatory phase, F14 = 21.68, F28 = 24.05, F14x 28 = 23.47), and progesterone (Fig. 9; resting phase, F14 = 21.18, F28 = 20.08, F14x 28 = 18.07; preparatory phase, F14 = 25.04, F28 = 27.12, F14x 28 = 20.24).
Effects of altered photoperiod and temperature on plasma estradiol-17β (E2) level in the female catfish H. fossilis in the resting (A) and preparatory (B) phases. Data were expressed as mean ± SEM and analyzed by two-way ANOVA (P < 0.001), followed by Dunett’s test (P < 0.05). The groups with different letters are significantly different, and those with the same letters are not significant. IC, initial control; NP, normal photoperiod, LP, long photoperiod; SP, short photoperiod; NT, normal temperature; HT, high temperature. NS, not significant between 12- and 28-day groups
Effects of altered photoperiod and temperature treatments on plasma testosterone (T) level in the female catfish H. fossilis in the resting (A) and preparatory (B) phases. Data were expressed as mean ± SEM and analyzed by two-way ANOVA (P < 0.001), followed by Dunett’s test (P < 0.05). The groups with different letters are significantly different, and those with the same letters are not significant. IC, initial control; NP, normal photoperiod, LP, long photoperiod; SP, short photoperiod; NT, normal temperature; HT, high temperature. NS, not significant between 12- and 28-day groups
Effects of altered photoperiod and temperature treatments on plasma progesterone (P4) level in the female catfish H. fossilis in the resting (A) and preparatory (B) phases. Data were expressed as mean ± SEM and analyzed by two-way ANOVA (P < 0.001), followed by Dunett’s test (P < 0.05). The groups with different letters are significantly different, and those with the same letters are not significant. IC, initial control; NP, normal photoperiod, LP, long photoperiod; SP, short photoperiod; NT, normal temperature; HT, high temperature. NS, not significant between 12- and 28-day groups
Plasma E2 level increased significantly only on day 28 in the PC (NP + NT) groups in both resting and preparatory phases compared with the IC groups (Dunett’s test, P < 0.05). In the resting phase, E2 increased significantly on day 14 in the LP + NT group and at either duration in the NP + HT and LP + HT groups. The increase was greater in the LP + HT group and a significant duration effect was noticed in all these groups with a higher increase on day 14. In the preparatory phase, a significant increase in the steroid level was noticed in the NP + HT and LP + HT groups; the increase was not significant in the LP + NT group. In all these groups, a duration effect with higher levels on day 28 was seen. The E2 level decreased in the SP + NT group on day 14 but increased on day 14 in the SP + HT group compared with the PC group in the resting phase. The duration effect was found only in the SP + NT group. In the preparatory phase, a significant decrease was found in the SP + NT and SP + HT groups with a significant duration effect.
In the resting phase, plasma testosterone (T) level increased in the LP + NT group without any duration effect but decreased in the NP + HT group (Dunett’s test, P < 0.05). In the LP + HT group, the T level was unchanged on day 14 but increased on day 28 compared with the PC group. In the SP + NT and SP + HT groups, a decrease was seen on day 14 but with no significant change on day 28 compared with the PC group. In the preparatory phase, a significant duration effect was found in all groups with higher levels on day 28. The T level increased significantly only in the LP + HT group on day 14 and on day 28 in the LP + NT, NP + HT, and LP + HT groups. The steroid level decreased in the SP groups on day 14 but increased on day 28.
In the resting phase, plasma P4 level showed a significant increase at both the time points in the LP + NT, NP + HT, and LP + HT groups and a significant decrease in the SP groups (Dunett’s test, P < 0.05). The increase was the highest in the LP + HT group and insignificantly different between the LP + NT and NP + HT groups. A duration effect was seen in all the groups except the SP + NT group. In the preparatory phase, the P4 level increased significantly in the LP + HT group but did not vary in the LP + NT and NP + HT groups. A significant decrease was noticed in the SP groups.
Discussion
The alterations in photo-thermal conditions produced parallel changes in the expression of kiss2 and gnrh2 mRNA levels in the catfish brain suggesting involvement of the two systems in the photo-thermal control of reproduction. The Pearson correlation analysis showed a significant and positive correlation of the mRNA levels in both gonad-inactive (resting) and gonad-responsive (preparatory) phases. The photo-thermal responses were higher in the preparatory phase pointing to a greater sensitivity of the systems in this phase. The exposure to LP or HT alone stimulated the expression but the combination exerted greater responses in both phases. An overall duration effect (14 vs. 28 days) was evident from the significant interaction between these variables. The SP treatment produced varied responses on the expression depending on season, exposure time, and temperature. These variations can be due to the fact that higher temperature can modify the photoperiodic effect in this species under a minimal photoperiod (Sundararaj 1981). The results show that cyclic changes in the environmental photoperiod and temperature are responsible for the seasonal changes in the mRNA expression (Chaube et al. 2019; Chaube et al. 2020).
Studies in teleosts show that the Kiss and Gnrh2 systems have responded variedly to photo-thermal changes. An integrated stimulation of the BPG axis was demonstrated after exposure to different photoperiods in adult Odontesthes bonariensis (pejerrey) females (Miranda et al. 2009). Pejerrey females exposed to a long photoperiod (16 h) at low (12 °C) or high (20 °C) temperature showed a significantly higher expression of salmon gnrh, pejerrey gnrh, gnrh2, fshβ, lhβ, and gpα, and high GSIs and plasma E2 levels compared with those held at the short photoperiod (8 h light and 12 °C temperature) in the winter (resting) conditions. In contrast, in stripped/white bass hybrid females exposed to a short (7 L:17D) or long (16 L: 8D) day regimes for 4 weeks, brain kiss2 transcript levels, but not kiss 1 transcript levels, increased by 2.5 folds, in the short photoperiod group in comparison with the long photoperiod group (Zmora et al. 2011). In this fish, the expression of brain gnrh1 increased in the short photoperiod group in pubertal (May trial) but not in recrudescent females (October trial). In goldfish (Carassius auratus) exposed to 10 L:14D (short day), 12 L:12D (control), and 14 L:10D (long day) for 2, 4, and 6 months, time-dependent significant increases in hypothalamic mRNA levels of kiss1, gpr54, salmon gnrh and gnrh2, and pituitary fshβ, lhβ, and gthα, plasma levels of Fsh, Lh, and 17α-hydroxypregnenolone were reported in the 14 L:10D group, suggesting the importance of day length in sexual maturation (Shin et al. 2014). In the three spine stickleback Gasterosteus aculeatus, Shao et al. (2019) reported time-dependent variations in the response of kiss2, gpr54, gnrh, and Gth subunit mRNA levels to photoperiods. Cowan et al. (2012) reported no clear temporal pattern in the expression of kiss2 or kissr4 mRNAs in maturing population of cod Gadus morhua exposed to stimulated natural photoperiod or constant light; however, kiss2 expression was elevated in vitellogenic females and spermiating males. The varied responses reported in teleosts can be attributed to reproductive stage, temperature, length of exposure, and long or short day breeding habits.
The changes in the expression of brain kiss2 and gnrh2 can be related to changes in ovarian activity during the altered photo-thermal conditions. Concurrent with the upregulation of kiss2 and gnrh2 expression, the ovary was stimulated, as judged from increased steroidogenesis (increased plasma P4, E2 and T levels), and GSI in the LP-, HT-, and LP + HT-treated catfish. Short photoperiod exposure caused inhibition of ovarian activity. In measuring P4 and T levels along with E2, we monitored the entire steroidogenic pathway, which was activated by photo-thermal changes. Therefore, it is inferred that the changes in the expression of kiss2 and gnrh2 seem to have caused changes in Gth secretion pattern that controls ovarian activity. Acharjee et al. (2017) showed that exposure of the catfish in the preparatory phase to LP (16 h light) or HT (28 ± 2 °C), alone or in combination, stimulated pituitary fshβ, lhβ, and gpα expression, plasma levels of E2, P4, and T, and increased GSI and ovarian growth. The simultaneous increase in the expression of both kiss2 and gnrh2 mRNAs in the LP, HT, and LP + HT groups and inhibition in the SP groups suggest that both systems act in a functionally integrated manner. As we did not measure Gnrh1 expression in the catfish, a future study should address this aspect. Since Lh-stimulating action of Gnrh2 has been demonstrated in the African catfish Clarias gariepinus (Schulz et al. 1993), it is likely that Gnrh2 is a target of photo-thermal effects with a hypophysiotropic role.
Since melatonin is known to mediate the photoperiodic effects, we tested the effects of exogenously administered melatonin on kiss2 and gnrh2 expression. Melatonin elicits varied effects, stimulatory or inhibitory, on the activity of the BPG axis in teleosts and this is attributed to various factors like season, doses used, experimental design, age of the animal, and so on (Servili et al. 2013). In the catfish, melatonin inhibited GSI, vitellogenesis, plasma levels of Lh and E2, and hypothalamic serotonergic activity depending on season and photoperiod conditions (Joy and Agha 1991; Senthilkumaran and Joy 1995a). In the present study, melatonin treatment caused an inhibition of kiss2 and gnrh2 expression in the fish kept under NP + HT and LP + NT regimes at 14 and 28 days in both resting and preparatory phases. Under short photoperiod (SP + NT), the melatonin effects were different according to the season. In the resting phase, melatonin elicited a stimulatory effect on the mRNA levels but induced an inhibitory effect in the preparatory phase. This study further shows an overall highly significant positive correlation between kiss2 and gnrh2 expression in the preparatory phase and a lower interaction in the resting phase. Alvarado et al. (2015) reported a stimulatory effect of melatonin on kiss1 and kiss2 expression in European sea bass. In the same species, Servili et al. (2013) reported an inhibitory effect of melatonin on the nocturnal brain expression of gnrh, gnrh3, and gnrh receptor subtypes. The varied effects of melatonin on the Kiss and Gnrh systems are difficult to explain and have much to do with the environmental conditions, doses, and species in question. It has been shown that the effect of melatonin could be indirect by modifying neurotransmitters like serotonin. Melatonin inhibited hypothalamic serotonin (5-HT) content and turnover (activity) in H. fossilis (Senthilkumaran and Joy 1995a) and brain 5-HT, 5-HIAA, and 5-HIAA/5-HT in another catfish Mystus cavasius (Badruzzaman et al. 2020). Summing up, the present data and previous studies in the catfish support predominantly an inhibitory role of melatonin on the BPG axis during gonadal recrudescence.
The importance of the Kiss system in the activation of the BPG axis has been debated in teleosts and conflicting results, both stimulatory effect and none, were reported (Tena-Sempere et al. 2012). Studies in goldfish have highlighted the varied effects as due to gonadal stage and experimental modalities used (Li et al. 2009; Yang et al. 2010). Mutant lines generated for Kiss or its receptors in zebrafish and medaka showed that reproductive performance was not affected in the mutants (Tang et al. 2015; Nakajo et al. 2018). Gnrh neurons lack Kiss receptors in European sea bass (Escobar et al. 2013) and medaka (Nakajo et al. 2018) or Kiss action is mediated without the intervention of Gnrh, as in goldfish (Chang et al. 2012). According to Ohga et al. (2018), evidence for a direct functional relation between Kiss and Gnrh neurons may or may not exist and a direct interaction may not be the only way of defining Kiss action. Kiss system may influence the Gnrh neurons indirectly through interneurons. In European sea bass, the interneurons co-expressing Kiss2 receptors contained neuronal nitric oxide synthase, neuropeptide Y, tyrosine hydroxylase, and somatostatin neurons and localized proximate to the Gnrh neurons (Escobar et al. 2013). In the catfish, kiss2 transcripts were localized in neurons of nucleus preopticus (NPO), nucleus recessus posterioris (NRP), and nucleus recessus lateralis (NRL) in the hypothalamus (Chaube et al. 2020), which are either monoaminergic or are innervated by monoamines (Joy 1999). It was shown that photoperiod and temperature regulate differentially hypothalamic monoaminergic activity (Senthilkumaran and Joy 1995b). LP and HT increased noradrenergic and adrenergic activity that stimulated Lh secretion and suppressed dopaminergic activity that inhibited Lh secretion. Similarly, LP with or without changing the ambient temperature stimulated serotonergic activity that stimulated Lh secretion (Joy and Senthilkumaran 1995). Taken together, these observations indicate that the Kiss2 and Gnrh systems could interact via neurotransmitters, neuropeptides, and melatonin in the mediation of photoperiod and temperature signaling.
Kiss2 cells are distributed in the pituitary and act directly as a paracrine or autocrine factor in hormone secretion (Tena-Sempere et al. 2012; Zhang et al. 2018). In goldfish pituitaries cultured under different photoperiods (10 L, 12 L, or 14 L) for 5 days, the expression of fshβ, lhβ, and gthα was stimulated by a low dose (100 nM), but inhibited by a high dose (000 nM) of Kiss1 under 14 h light (Shin et al. 2014). In zebrafish, Kiss2 stimulated in vitro lhβ, fshβ, and prl1 expression (Song et al. 2020). In the pituitary of the catfish, kiss2 transcripts were highly expressed and localized in an un-identified cell population in the proximal pars distalis (Chaube et al. 2020). Thus, Kiss2 can act on the Gnrh system at the brain level or act in a paracrine manner at the pituitary level depending on species. Currently, research work is in progress in the catfish to define the functions of Kiss2.
In conclusion, brain kiss2 and gnrh2 expression is sensitive to photo-thermal changes. Long photoperiod and high temperature elicit a stimulatory effect with the combination yielding maximal effect and short photoperiod has a depressing role on the expression. Melatonin treatment altered the gene expression pattern in the brain according to reproductive stage and temperature conditions. The evidence shows that Kiss2 and Gnrh2 are part of the photo-thermal mechanisms controlling reproduction in the catfish.
References
Acharjee A, Chaube R, Joy KP (2015) Molecular cloning and characterization of the gonadotropin subunits GPα, FSHβ, and LHβ genes in the stinging catfish Heteropneustes fossilis: phylogeny, seasonal expression and pituitary localization. J Exp Zool Part A: Ecol Genet Physiol 323:567–585. https://doi.org/10.1002/jez.1949
Acharjee A, Chaube R, Joy KP (2017) Effects of altered photoperiod and temperature on expression levels of gonadotrophin subunit mRNAs in the female stinging catfish Heteropneustes fossilis. J Fish Biol 90:2289–2311. https://doi.org/10.1111/jfb.13305
Alvarado MV, Carrillo M, Felip A (2015) Melatonin-induced changes in kiss/gnrh gene expression patterns in the brain of male sea bass during spermatogenesis. Comp Biochem Physiol A Mol Integr Physiol 185:69–79. https://doi.org/10.1016/j.cbpa.2015.03.010
Badruzzaman M, TaroIkegami AKM, Amin R, Shahjahan M (2020) Melatonin inhibits reproductive activity through changes of serotonergic activity in the brain of freshwater catfish (Mystus cavasius). Aquaculture 526:735378. https://doi.org/10.1016/j.aquaculture.2020.735378
Borg B (2009) Photoperiodism in fishes. In: Photoperiodism, Nelson RJ, Denlinger DL, Somers DE (eds) The biological calendar. Oxford University Press, New York, pp 371–398
Chang JP, Pemberton JG (2018) Comparative aspects of GnRH-stimulated signal transduction in the vertebrate pituitary - contributions from teleost model systems. Mol Cell Endocrinol 463:142–167. https://doi.org/10.1016/j.mce.2017.06.002
Chang JP, Mar A, Wlasichuk M, Wong AOL (2012) Kisspeptin-1 directly stimulates LH and GH secretion from goldfish pituitary cells in a Ca2+-dependent manner. Gen Comp Endocrinol 179:38–46
Chaube R, Rawat A, Inbaraj RM, Bobe J, Guiguen Y, Fostier A, Joy KP (2017) Identification and characterization of a catechol-o-methyltransferase cDNA in the catfish Heteropneustes fossilis: tissue, sex and seasonal variations, and effects of gonadotropin and 2-hydroxyestradiol-17β on mRNA expression. Gen Comp Endocrinol 246:129–141. https://doi.org/10.1016/j.ygcen.2016.12.001
Chaube R, Rawat A, Sharma S, Senthilkumaran B, Bhat SG, Joy KP (2019) Molecular cloning and characterization of a gonadotropin-releasing hormone 2 precursor cDNA in the catfish Heteropneustes fossilis: expression profile and regulation by ovarian steroids. Gen Comp Endocrinol 280:134–146. https://doi.org/10.1016/j.ygcen.2019.04.021
Chaube R, Sharma S, Senthilkumaran B, Bhat SG, Joy KP (2020) Identification of kisspeptin2 cDNA in the catfish Heteropneustes fossilis: expression profile, in situ localization and steroid modulation. Gen Comp Endocrinol 294:113472. https://doi.org/10.1016/j.ygcen.2020.113472
Chi L, Li X, Liu Q, Liu Y (2017) Photoperiod regulate gonad development via kisspeptin/kissr in hypothalamus and saccus vasculosus of Atlantic salmon (Salmo salar). PLoS One 12:e0169569. https://doi.org/10.1371/journal.pone.0169569
Cowan M, Davie A, Migaud H (2012) Photoperiod effects on the expression of kisspeptin and gonadotropin genes in Atlantic cod, Gadus morhua, during first maturation. Comp Biochem Physiol A Mol Integr Physiol 163:82–94. https://doi.org/10.1016/j.cbpa.2012.05.191
Escobar S, Servili A, Espigares F, Gueguen MM, Brocal I, Felip A (2013) Expression of kisspeptins and kiss receptors suggests a large range of functions for kisspeptin systems in the brain of the European sea bass. PLoS One 8:e70177. https://doi.org/10.1371/journal.pone.0070177
Falcón J, Migaud H, Muñoz-Cueto JA, Carrillo M (2010) Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol 165:469–482. https://doi.org/10.1016/j.ygcen.2009.04.026
Guzman JM, Rubio M, Ortiz-Delago JB, Klenke U, Kight K, Cross I, Sanchez-Ramos I, Riaza A, Robordinos L, Saraquete C, Zohar Y, Mananos EK (2009) Comparative gene expression of gonadotropins (FSH and LH) and peptide levels of gonadotropin-releasing hormones (GnRHs) in the pituitary of wild and cultured Senegalese sole (Solea senegalensis) broodstocks. Comp Biochem Physiol A Mol Integr Physiol 153:266–267. https://doi.org/10.1016/j.cbpa.2009.02.032
Joy KP (1999) Role of central monoamines in regulation of gonadotropin-II secretion. In: Prasada Rao PD, Peter RE (eds) Neural regulation in the vertebrate endocrine system. Springer, Boston, MA, pp 111–126. https://doi.org/10.1007/978-1-4615-4805-8_8
Joy KP, Agha AK (1991) Seasonal effects of administration of melatonin and 5- methoxytryptophol on ovarian activity in the catfish, Heteropneustes fossilis (Bloch). J Pineal Res 10:65–70. https://doi.org/10.1111/j.1600-079X.1991.tb00012.x
Joy KP, Senthilkumaran B (1995) A serotonergic control of pituitary- gonadal activity in the female catfish, Heteropneustes fossilis, under normal and long photoperiods. In: Goetz FW, Thomas P (eds) Austin, U.S.AReproductive Physiology of Fish, pp 67–70
Kang KS, Shimizu K, Azuma M, Ui Y, Nakamura K, Uchiyama M, Matsuda K (2011) Gonadotropin-releasing hormone II (GnRH II) mediates the anorexigenic actions of α-melanocyte-stimulating hormone (α-MSH) and corticotropin-releasing hormone (CRH) in goldfish. Peptides 32:31–35. https://doi.org/10.1016/j.peptides.2010.10.013
Kim MH, Oka Y, Amano M, Kobayashi M, Okuzawa K, Hasegawa Y, Kawashima S, Suzuki Y, Aida K (1995) Immunocytochemical localization of sGnRH and cGnRH-II in the brain of goldfish, Carassius auratus. J Comp Neurol 356:72–82. https://doi.org/10.1002/cne.903560105
Kim NN, Shin HS, Choi YJ, Choi CY (2014) Kisspeptin regulates the hypothalamus- pituitary-gonad axis gene expression during sexual maturation in the cinnamon clownfish, Amphiprion melanopus. Comp Biochem Physiol B Biochem Mol Biol 168:19–32. https://doi.org/10.1016/j.cbpb.2013.11.002
Lamba VJ, Goswami SV, Sundararaj BI (1983) Circannual and circadian variations in plasma levels of steroids (cortisol, estradiol-17β estrone, and testosterone) correlated with the annual gonadal cycle in the catfish, Heteropneustes fossilis (Bloch). Gen Comp Endocrinol 50:205–225. https://doi.org/10.1016/0016-6480(83)90221-6
Lee YR, Tsunekawa K, Moon MJ, Um HN, Hwang JI, Osugi T, Otaki N, Sunakawa Y, Kim K, Vaudry H, Kwon HB, Seong JY, Tsutsui K (2009) Molecular evolution of multiple forms of kisspeptins and GPR54 receptors in vertebrates. Endocrinology 150:2837–2846. https://doi.org/10.1210/en.2008-1679
Li S, Zhang Y, Liu Y, Huang X, Huang W, Lu D, Zhu P, Shi Y, Cheng CHK, Liu X, Lin H (2009) Structural and functional multiplicity of the kisspeptin/GPR54 system in goldfish (Carassius auratus). J Endocrinol 201:407–418
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2-DDCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Martinez-Chavez CC, Minghetti M, Migaud H (2008) GPR54 and rGnRH I gene expression during the onset of puberty in Nile tilapia. Gen Comp Endocrinol 156:224–233. https://doi.org/10.1016/j.ygcen.2008.01.019
Martins RS, Gomez A, Zanuy S, Carrillo M, Canário AV (2015) Photoperiodic modulation of circadian clock and reproductive axis gene expression in the pre-pubertal European sea bass brain. PLoS One 10:e0144158. https://doi.org/10.1371/journal.pone.0144158
Migaud H, Davie A, Taylor JF (2010) Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J Fish Biol 76:27–68. https://doi.org/10.1111/j.1095-8649.2009.02500.x
Miranda LA, Strüssman CA, Somoza GM (2009) Effects of light and temperature conditions on the expression of GnRH and GtH genes and levels of plasma steroids in Odontesthes bonariensis females. Fish Physiol Biochem 35:101–108. https://doi.org/10.1007/s10695-008-9232-3
Muñoz-Cueto JA, Zmora N, Paullada-Salmeróna JA, Marvel M, Mañanos E, Zohar Y (2020) The gonadotropin-releasing hormones: lessons from fish. Gen Comp Endocrinol 291:113422
Nakajo M, Kanda S, Karigo T, Takahashi A, Akazome Y, Uenoyama Y, Kobayashi M, Oka Y (2018) Evolutionally conserved function of kisspeptin neuronal system is nonreproductive regulation as revealed by nonmammalian study. Endocrinology 159:163–183. https://doi.org/10.1210/en.2017-00808
Ohga H, Selvaraj S, Matsuyama M (2018) The roles of kisspeptin system in the reproductive physiology of fish with special reference to chub mackerel studies as main axis. Front Endocrinol 9:147. https://doi.org/10.3389/fendo.2018.00147
Okubo K, Nagahama Y (2008) Structural and functional evolution of gonadotropin releasing hormone in vertebrates. Acta Physiol 193:3–15. https://doi.org/10.1111/j.1748-1716.2008.01832.x
Pasquier J, Lafont AG, Tostivint H, Vaudry H, Rousseau K, Dufour S (2012) Comparative evolutionary histories of kisspeptins and kisspeptin receptors in vertebrates reveal both parallel and divergent features. Front Endocrinol 3:173. https://doi.org/10.3389/fendo.2012.00173
Revel FG, Ansel L, Klosen P, Saboureau M, Pévet P, Mikkelsen JD, Simonneaux V (2007) Kisspeptin: a key link to seasonal breeding. Rev Endocr Metab Disord 8:57–65. https://doi.org/10.1007/s11154-007-9031-7
Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M (2008) New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol 29:48–69. https://doi.org/10.1016/j.yfrne.2007.07.002
Schulz RW, Bosma PT, Zandbergen MA, Van der Sanden MC, Van Dijk W, Peute J, Bogerd J, Goos HJ (1993) Two gonadotropin-releasing hormones in the African catfish, Clarias gariepinus: localization, pituitary receptor binding, and gonadotropin release activity. Endocrinology 133:1569–1577. https://doi.org/10.1210/en.133.4.1569
Scott CJ, Rose JL, Gunn AJ, McGrath BM (2019) Kisspeptin and the regulation of the reproductive axis in domestic animals. J Endocrinol 240:R1–R16. https://doi.org/10.1530/JOE-18-0485
Senthilkumaran B, Joy KP (1995a) Effects of melatonin, p-chlorophenylalanine and α-methylparatyrosine on plasma gonadotropin level and ovarian activity in the catfish Heteropneustes fossilis: a study correlating changes in hypothalamic monoamines. Fish Physiol Biochem 14:471–480. https://doi.org/10.1007/BF00004347
Senthilkumaran B, Joy KP (1995b) Changes in hypothalamic catecholamines, dopamine-β-hydroxylase and phenylanolamine-N-methyltranserase in the catfish Heteropneustes fossilis in relation to season, raised photoperiod and temperature, ovariectomy and estradiol-17β replacement. Gen Comp Endocrinol 97:121–134. https://doi.org/10.1006/gcen.1995.1012
Servili A, Page YL, Leprince J, Caraty A, Escobar S, Parhar IS, Seong JY, Vaudry H, Kah O (2011) Organization of two independent kisspeptin systems delivered from evolutionary-ancient kiss genes in the brain of zebrafish. Endocrinology 152:1527–1540. https://doi.org/10.1210/en.2010-0948
Servili A, Herrera-Perez P, Rendón MC, Munoz-Cueto JA (2013) Melatonin inhibits GnRH-1, GnRH-3 and GnRH receptor expression in the brain of the European sea bass, Dicentrarchus labrax. Int J Mol Sci 14:7603–7616. https://doi.org/10.3390/ijms14047603
Shao YT, Roufidou C, Chung PC, Borg B (2019) Changes in kisspeptin, GnRH, and gonadotropin mRNA levels in male three spine stickleback (Gasterosteus aculeatus) during photoperiod-induced sexual maturation. Evol Ecol Res 20:317–329
Shin HS, Song JA, Choi JY, Kim NN, Choi YJ, Sung SN, Park MS, Min BH, Choi CY (2014) Effects of various photoperiods on kisspeptin and reproductive hormones in the goldfish, Carassius auratus. Animal Cells and Systems 18:109–118. https://doi.org/10.1080/19768354.2014.902863
Somoza GM, Mechaly AS, Trudeau VL (2020) Kisspeptin and GnRH interactions in the reproductive brain of teleosts. Gen Comp Endocrinol. https://doi.org/10.1016/j.ygcen.2020.113568
Song Y, Chen J, Tao B, Luo D, Zhu Z, Hu W (2020) Kisspeptin2 regulates hormone expression in female zebrafish (Danio rerio) pituitary. Mol Cell Endocrinol 513:110858
Sundararaj BI (1981) Aquaculture development and coordination programme. Reproductive physiology of teleost fishes: a review of present knowledge and needs for future research. United Nations Development Programme, Food and Agriculture Organization of the United Nations, Rome
Tang H, Liu Y, Luo D, Ogawa S, Yin Y, Li S, Zhang Y, Hu W, Parhar IS, Lin H, Liu X, Cheng CHK (2015) The kiss/kissr systems are dispensable for zebrafish reproduction: evidence from gene knockout studies. Endocrinology 156:589–599. https://doi.org/10.1210/en.2014-1204
Tharakan B, Joy KP (1996) Effects of mammalian gonadotropin-releasing hormone analogue, pimozide, and the combination on plasma gonadotropin levels in different seasons and induction of ovulation in female catfish. J Fish Biol 48:623–632
Tena-Sempere M, Felip A, Gómez A, Zanuy S, Carrillo M (2012) Comparative insights of the kisspeptin/kisspeptin receptor system: lessons from non-mammalian vertebrates. Gen Comp Endocrinol 175:234–243. https://doi.org/10.1016/j.ygcen.2011.11.015
Tian F, Liu S, Shi J, Qi H, Zhao K, Xie B (2019) Transcriptomic profiling reveals molecular regulation of seasonal reproduction in Tibetan highland fish, Gymnocypris przewalskii. BMC Genomics 20:2. https://doi.org/10.1186/s12864-018-5358-6
Xia W, Smith O, Zmora N, Xu S, Zohar Y (2014) Comprehensive analysis of GnRH2 neuronal projections in zebrafish. Sci Rep 4:3676. https://doi.org/10.1038/srep03676
Yang B, Jiang Q, Chan T, Wendy KWK, Wong AOL (2010) Goldfish kisspeptin: molecular cloning, tissue distribution of transcript expression, and stimulatory effects on prolactin, growth hormone and luteinizing hormone secretion and gene expression via direct actions at the pituitary level. Gen Comp Endocrinol 165:60–71
Zhang H, Zhang B, Qin G, Li S, Lin Q (2018) The roles of the kisspeptin system in the reproductive physiology of the lined seahorse (Hippocampus erectus), an ovoviviparous fish with male pregnancy. Front Neurosci 12:940. https://doi.org/10.3389/fnins.2018.00940
Zmora N, Stubblefield J, Zulperi Z, Klenke U, Zohar Y (2011) Kisspeptin - photoperiod/gonadal steroid relationships in the brain of two perciforms, the striped and hybrid basses. Indian J Sci Technol 4:10–11. https://doi.org/10.17485/ijst/2011/v4iS8/30854
Zohar Y, Muñoz-Cueto JA, Elizur A, Kah O (2010) Neuroendocrinology of reproduction in teleost fish. Gen Comp Endocrinol 165:438–455. https://doi.org/10.1016/j.ygcen.2009.04.017
Acknowledgments
The authors would like to thank the personnel of the research teams for their assistance. The qPCR facility of DBT-ISLS, Banaras Hindu University, is acknowledged.
Funding
The research work was supported by a grant from DST-SERB, New Delhi (EMR/2016/002751 dated 27-03-2017). Dr. K.P. Joy is INSA Senior Scientist (Award No. INSA/SP/SS/2019/629 dated 31-12-2019).
Author information
Authors and Affiliations
Contributions
R. C designed the experiments, analyzed the data, and framed the written part of the manuscript. S. S conducted the study and collected the data. B. S and S. G read the manuscript and gave suggestions. K. P. J supervised the research project, interpreted the data, and finalized the written part of the manuscript. All authors read the final version and approved the final manuscript.
Corresponding author
Ethics declarations
The experiments were conducted according to the general guidelines of the Animal Ethics Committee of Banaras Hindu University, Varanasi.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaube, R., Sharma, S., Senthilkumaran, B. et al. Expression profile of kisspeptin2 and gonadotropin-releasing hormone2 mRNA during photo-thermal and melatonin treatments in the female air-breathing catfish Heteropneustes fossilis. Fish Physiol Biochem 46, 2403–2419 (2020). https://doi.org/10.1007/s10695-020-00888-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00888-4