Abstract
Commonly used aquatic feed naturally contains low-level or no hydroxyproline (Hyp). This study was conducted to evaluate the effects of dietary Hyp inclusion on growth performance, body composition, amino acid profiles, blood biochemistry, and the expression of target of rapamycin (TOR) pathway-related genes in juvenile Nibea diacanthus. Fish with similar size (initial body weight, 133.00 ± 2.14 g) were fed six isonitrogenous and isolipidic practical diets supplemented with graded levels of Hyp (0, 5, 10, 15, 20, and 25 g kg−1 of dry matter) for 8 weeks. The results indicated that growth performance and feed utilization were improved with increased levels of dietary Hyp (P < 0.05), and the optimum amount of dietary Hyp estimated from SGR as 16.6 g kg−1. The crude protein of whole body and swim bladder and the amino acid composition of muscle and swim bladder were significantly (P < 0.05) affected by the addition of dietary Hyp, which reflects the important role of feed composition in animal body composition. In addition, the expression levels of mammalian target of rapamycin (TOR) and ribosomal protein S6 kinase1 (S6K1) genes in the liver, muscle, and swim bladder increased with increasing Hyp content of diets, while the mRNA expression level of eukaryotic translation initiation factor 4E-binding protein (4E-BP) gene in these tissues decreased. These results indicated that Hyp improved fish growth and the ability to synthesize proteins, most likely through the TOR pathway. It is suggested that dietary Hyp supplementation is particularly necessary for application in aquatic feed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydroxyproline (Hyp), which is mainly found in collagen, is not a natural free amino acid (Li and Wu 2018). All animals generate Hyp from proline (Pro), as it is a post-translational metabolite from proline-containing collagen (Li et al. 2009). Although Hyp can be generated from Pro in collagen by vitamin C-dependent prolyl hydroxylase, this metabolic process requires large energy input (6 mol ATP/mol Hyp, i.e., 4 mol ATP for collagen synthesis and 2 mol ATP for collagen degradation) (Wu 2013; Li and Wu 2018). Thus, it is speculated that direct provision of Hyp in diets could save the huge energy required for promoting the growth of animals. Previous studies have shown that the addition of Hyp to feed has beneficial effects in animals. For instance, a plant-based protein diet supplemented with crystalline Hyp enhanced weight gain of salmon (Salmo salar L.) (Aksnes et al. 2008). Hou and Wu (2017) reported that adding an appropriate amount of Hyp to the diet can affect animal growth, muscle structure, and collagen content. It has also been reported that Hyp can scavenge oxidants and regulate the redox state of cells (Phang et al. 2008). Considering that the Hyp content in the feed is low, and there is energy waste in the biosynthesis of Hyp, it is therefore valuable to investigate the effect of dietary Hyp and to determine the possible impact of Hyp on growth when formulating diets.
Nibea diacanthus is a large predatory fish that grows fast and lives long due to its disease resistance, as well as its strong adaptability, for which reasons that it is easy to breed. Due to these excellent characteristics, N. diacanthus has been well received by farmers in the south of China (Chen et al. 2011). In recent year, we found that N. diacanthus is regarded as an excellent food fish, especially, its large swim bladder, which is considered a tonic with a high market value and sales volume in Southeast Asia, particularly in Hong Kong and Southern China (Rong et al. 2019, 2020). However, there are few studies on N. diacanthus, especially the effects of dietary Hyp on growth performance of juvenile N. diacanthus, have not been well explored. Similarly, little is known about the effect of dietary Hyp on cell signaling, amino acid-sensing pathways, nutritional regulation, and the pathways for Hyp metabolism in fish. It is known that the mammalian target of rapamycin pathway (mTOR) has an important amino acid-sensing mechanism, and affects cell growth and function by integrating signals from nutrients (glucose and amino acid) (Holz et al. 2005; Wullschleger et al. 2006). Many studies have shown that various amino acids, e.g., tryptophan (Tang et al. 2013), arginine (Chen et al. 2012), proline (Liao et al. 2008), leucine (Zhou et al. 2019), lysine (Li et al. 2019), and methionine (He et al. 2019), improve growth and enhance protein synthesis of fish by activating the TOR signaling pathway. However, the effects of dietary Hyp on growth performance of N. diacanthus and whether this is through the activation of TOR signaling have not been studied. Therefore, this study sought to investigate the effects of Hyp on growth, biochemical composition, and the expression of TOR-related genes in juvenile N. diacanthus-fed diets with different levels of Hyp. It is hoped that the findings here would give more insight on the functional properties of Hyp and therefore provide theoretical basis for the development of aquatic feed.
Materials and methods
Experimental diets
The basal practical diet (42.34% crude protein, 8.35% crude lipid, and 10.94% crude ash) was formulated according to our previous study (Rong et al. 2019), and Hyp was considered as the limiting factor to support the optimum growth of N. diacanthus. Six isonitrogenous and isolipidic diets with graded levels of extra addition of Hyp (0, 5, 10, 15, 20, and 25 g kg−1 diet) were formulated (Table 1). Crude Hyp powder (a mixture of 4-hydroxylprolyl and 3-hydroxylprolyl, with the main components being 4-hydroxyproline) was purchased from Hebei Runying Biotechnology (Hebei, China). As shown in Table 1, fish meal, mixed amino acids, and crystalline amino acids were used as the main dietary protein sources, while fish oil was the main dietary lipid source. The quantity of Hyp was increased at the expense of glutamic acid to make the diets isonitrogenous, as glutamate levels not affect the parameters being measured. The levels of other nutrients met the requirements for N. diacanthus according to the National Research Council (NRC 2011. The levels of dietary amino acids are shown in Table 2. Feedstuffs were sifted through a 0.3-mm sieve and mixed thoroughly in ratios before being puffed with a 2.5-mm diameter extruder, and then air-dried in a natural ventilated environment. Dried pellets were sealed in plastic bags and stored at − 20 °C until use.
Experimental systems and feeding
Experiments were carried out in floating pens at Nan’Ao Marine Biology Station (NAMBS), Shantou University, Shantou, China. Fish (N. diacanthus) were obtained from a local marine fish hatchery (Raoping, Guangdong, China). Fish were reared in floating net pens (3 × 3 × 2 m, L:W:H) and fed with a commercial diet (40.0% crude protein, 10.0% crude lipid, Tongwei, Jieyang, China) for 2 weeks to allow them acclimatize to the new environment. Before being used for the experiments, fish were fasted for 24 h after which they were weighed and anesthetized with 40 mg/L eugenol solution (Aladdin reagent Co., Ltd., China) to avoid being stressed. Next, 450 fish (133 ± 2.14 g) were randomly distributed into 18 pens (1 × 1 × 1.5 m, L:W:H) with 25 fish per pen. Three pens were randomly assigned to one of the six dietary treatments. Fish were hand-fed to apparent satiation twice (07:00 and 16:30 h) daily for 8 weeks. Satiation of fish was considered when they did not show feeding behavior towards the pellets. The amount of food (pellets) consumed in each pen was recorded. During the experiments, the temperature ranged from 23 to 30 °C, pH 7.8 to 8.1, ammonia nitrogen lower than 0.05 mg L−1, salinity 31 to 33 g L−1, and dissolved oxygen 5.2 to 6 mg L−1. All the animal experiments were conducted according to the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of the People’s Republic of China on October 31, 1988.
Sample collection
Samples were collected following similar procedures previously described (Rong et al. 2019). At the end of the feeding trial, fish were fasted for 24 h before being used for subsequent experiments. All experimental fish were anaesthetized with 1:10,000 eugenol (purity 99%, Shanghai Reagent, China) to avoid stress before being weighed and counted to calculate fish growth and feed utilization. Four randomly collected fish from each pen was stored at − 20 °C for whole body composition analysis. Another six fish from each pen were collected for blood samples, dorsal muscle, and swim bladder tissues. First, blood was withdrawn from the caudal vein using syringes, centrifuged (4000g for 10 min) at 4 °C to obtain serum samples before being snap frozen in liquid nitrogen and stored at − 80 °C until analysis. Next, the fish were slaughtered and the dorsal muscle and swim bladder tissues sampled for amino acids analysis, while individual body weight, liver weight, and visceral weight were recorded for the calculation of hepatosomatic index and viserosomatic index. Four fish from each pen were sampled to obtain liver, muscle, intestinal, and swim bladder tissues. The obtained tissues were snap frozen in liquid nitrogen before being stored at − 80 °C for later RNA extraction and real-time quantitative PCR analysis.
Calculation of growth parameters
Fish body weight (BW), initial body weight (IBW), final body weight (FBW), body length (BL), feed consumed (FC), protein intake (PI), viscera weight (VW), and liver weight (LW) were measured and recorded at the feeding trial. Growth performance was assessed in terms of percentage and efficiency ratio of feed and protein, while the body weight and body length were used to calculate the following parameters:
Proximate composition analysis
Proximate composition of diets and whole body expressed on a wet-based mass was determined in triplicates according to standard methods of the Association of Official Analytical Chemists (AOAC 1995). Analysis of dry matter (105 °C, 24 h), crude protein (Kjeldahl nitrogen N × 6.25), crude lipid (Soxhlet method), and crude ash (550 °C, 6 h) was performed following standard laboratory procedures.
Amino acid content determination
The amino acid composition of samples was analyzed using an HPLC-Ultimate 3000 (Thermo Scientific Dionex, USA) based on a national standard method (GB/T 18246-2000) and method used in our previous work (Rong et al. 2020). Briefly, freeze-dried samples were hydrolyzed at 110 °C for 24 h with 15 mL of 6 mol L−1 HCl. Next, the protein hydrolysate was filtered through a 0.45-μm filter with a sterile syringe, and 10 uL of filtered solution was injected into the HPLC system with a sodium cation-exchange column (3.0 × 250.0 mm; Pickering, CA, USA) for the chromatographic separation at 48 °C. Ninhydrin was used as the post-column derivatization reagent, and pump flow rate was 0.3 mL min−1 at 130 °C. The absorbance was recorded by ultraviolet detection at a wavelength of 440 nm (for proline) and 570 nm (for other amino acids). The identity and quantity of the amino acids were assessed by comparing their retention times and peak areas with that of amino acid standards. The tryptophan content was determined by the colorimetric method after alkaline hydrolysis of each sample, because tryptophan could not be detected after acid hydrolysis.
Determination of biochemical indices in serum and liver
Serum and liver triglyceride (TG), total cholesterol (T-CH), and serum calcium (SC) levels were all measured using a Diagnostic Reagent Kit (Art. No. A110-2, A111-2, C004-2, respectively; Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer`s instructions. Briefly, liver was homogenized in nine volumes (w/v) of chilled phosphate buffer (0.1 mol/L pH 7.4) or 0.9% saline solution and then centrifuged (2500g) at 4 °C for 10 min. The floating lipid layer was then removed, and the supernatant was divided into aliquots in 1.5-mL tubes. All the tubes were then stored at − 80 °C until analysis. Aliquots of 10-uL sample (serum or liver), 10-uL ultra-pure water, or 10-uL standard sample were mixed with 1-mL working liquid (provided by each kit) and incubated for 10 min at 37 °C, and then the absorbance of TG and T-CH was determined at 510 nm. The absorbance of SC was determined at 610 nm, while the concentration was determined from each standard curve.
Analysis of metabolism-related gene expression
The total RNA of each tissue (liver, muscle, intestines, and swim bladder) was isolated using Trizol (Invitrogen™) according to manufacturer’s instructions, while genomic DNA was eliminated by DNase I digestion (Takara, Dalian, China). The concentration and quality of RNA were determined by measuring the absorbance at 260 nm/280 nm using a NanoDrop 2000 spectrophotometer (Thermo Scientific Nanodrop, USA). The integrity of purified RNA was determined using agarose gel electrophoresis. cDNA was synthesized using TransScript® One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen Biotech, Beijing, China). Specific primers for TOR-related genes were designed by the Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA, USA) based on the sequences of Larimichthys crocea (Genbank accession nos. KC510145, XM_010732552, XM_010748995, XM_010736764, and XM_019275101) (Table 3). The polymerase chain reaction (PCR) was carried out on an Applied Biosystems VeritiTM Thermal Cycler (Thermo Fisher Scientific) to obtain the amplified cDNA fragment of target gene. The PCR conditions were as follows: initial denaturation step at 95 °C for to 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, primer annealing at 58 °C for 30 s, primer extension 72 °C for 1 min, final, extended at 72 °C for 10 min. The PCR fragments were subjected to electrophoresis on a 1.2% agarose gel to confirm the product size, and then sequenced (The Beijing Genomics Institute, Shenzhen city). The cloned sequences were verified by comparison with those on NCBI.
For the qRT-PCR analysis, the total reaction volume was 20 uL including 1 uL (10 mM) primer (housekeeping or target gene), 2 uL cDNA, 7 uL ultra-pure water, and 10 uL SYBR® Premix Ex Taq™ II (Applied Takara). The qRT-PCR program was as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 20 s. After the amplification phase, a melting curve of 0.5 °C increments from 65 to 95 °C was performed to confirm only single PCR product in each reaction. The housekeeping gene was used for normalization. Each sample was run in triplicate, and samples without templates used as negative controls. The qRT-PCR reaction was run on a Roche Light Cycler® 480 System and the relative quantification calculated using the ΔΔCT method (Livak and Schmittgen 2001).
Statistical analysis
The results were expressed as means ± SEM (standard error of the mean). All data were tested for normality by using the Kolmogorov–Smirnov test and homogeneity of variances by using Levene’s test before statistical analyses. One-way ANOVA was used to determine the significance of each parameter among different treatments using the SPSS 20.0 (SPSS Inc., Chicago, IL, USA) followed by Tukey’s test. The optimum dietary Hyp supplementation for juvenile Nibea diacanthus based on SGR was estimated by polynomial regression analysis. Significance levels were set at P < 0.05. Data are presented as means ± standard error.
Results
Growth performance
The results of growth performance, survival rate, and feed utilization including SGR, WG, FCR, PER, SR, HSI, and VSI of juvenile N. diacanthus fed different experimental diets are shown in Table 4. There were no pathological differences observed during the trial. Survival of each group was over 90%, and there were no significant differences among the dietary treatments. The PER of fish fed on diets containing 10–15 g kg−1 dietary Hyp increased significantly compared with those on the basal diet (0 g kg−1 Hyp). WG and SGR increased significantly with Hyp content up to 10 g kg−1 in diets. The polynomial regression analysis of SGR (y = − 1857.1x2 + 61.743x + 0.8671 R2 = 0.9222) against dietary Hyp levels in diets is presented in Fig. 1. The amount of dietary Hyp added, which was estimated from SGR, was 16.6 g kg−1. PER, HSI, and VSI of juvenile N. diacanthus increased initially and decreased subsequently with increasing Hyp content of diets, while FCR had a reverse trend (P < 0.05).
Quadratic regression analysis of specific growth rate (SGR, %/d) according to dietary hydroxyproline levels. Each point represents the mean of six groups of Nibea diacanthus with twenty-five fish per group. Addition amount of dietary hydroxyproline estimated from SGR was 16.6 g kg−1 (y = − 1857.1x2 + 61.743x + 0.8671 R2 = 0.9222)
Proximate composition of whole body, muscle, and swim bladder
The proximate compositions of whole body, muscle, and swim bladder of N. diacanthus are presented in Table 5. In the dietary treatment groups, all proximate compositions for swim bladder and muscle had the same trend (Table 5). The moisture content of the different tissues followed the same trend with increasing Hyp content of diets, with no significant differences between any two dietary treatment groups. Similarly, there were no significant differences in the crude lipid and crude ash contents among the dietary treatments. For fish fed with 10 g kg−1 Hyp, the crude protein content in whole body and swim bladder was significantly higher than that of the control group (0 g kg−1 Hyp) (P < 0.05). There were no significant differences in all proximate compositions of muscle among the dietary treatments.
Amino acid content of muscle and swim bladder
The amino acid profile of muscle and swim bladder is presented in Tables 6 and 7, respectively. Apart from leucine, the essential amino acid (EAA) content of muscle initially increased (almost reaching maximum in diet (10 g kg−1 Hyp)) followed by a decrease with increasing Hyp content of diets. Similarly, the non-essential amino acid (NEAA) content of muscle generally increased first and then decreased with increasing dietary Hyp content (Table 6). For the EAA of swim bladder, there were no significant differences in the retention of histidine, valine, phenylalanine, isoleucine, and methionine among the dietary treatments. On the other hand, the retention of EAA (arginine, leucine threonine, and lysine) in swim bladder generally increased initially followed by a decrease with increasing Hyp content of diets (Table 7). Generally, the proline content of muscle and swim bladder varied similarly with increasing Hyp content of diets, reaching the peak value with diet (15 g kg−1 Hyp), and then slowly decreased with increasing Hyp content of diets.
Biochemical indices in serum and liver
As shown in Table 8, there were no significant differences in the SC, TG, and T-CH contents of serum and liver among the dietary treatments with increasing dietary Hyp levels.
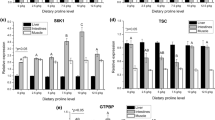
Expression of TOR signaling pathway-related genes
The qRT-PCR analysis of juvenile N. diacanthus tissues revealed that swim bladder and muscle had high mRNA expression levels of TOR, which were significantly higher than liver and intestines (P < 0.05), with no significant difference between liver and intestines. For the basal diet, swim bladder had significantly higher TOR mRNA levels compared with muscle (P < 0.05, Fig. 2). On the other hand, in the same tissues, the TOR mRNA levels in liver and muscle increased gradually with increasing levels of Hyp (P < 0.05), but there was no significant difference in intestines. The mRNA levels of TOR in swim bladder initially increased and decreased subsequently with increasing Hyp content of diets, with the highest observed for fish fed with diets containing 15 g kg−1 Hyp (P < 0.05). In terms of 4E-BP gene expression in fish (juvenile N. diacanthus) fed with diets containing graded levels of Hyp (Fig. 3), it was observed that for different tissues, muscle had the highest level of 4E-BP, which was significantly higher than that of the liver, intestines, and swim bladder (P < 0.05). In muscle, there was no significant difference in 4E-BP gene expression with increasing levels of Hyp. However, there was an initial increase in 4E-BP expression followed by a decrease in the liver, with the highest levels found in fish fed with diets containing 10 g kg−1 supplemented Hyp (P < 0.05). In swim bladder and intestines, the relative expression of 4E-BP gene decreased significantly with increasing Hyp (P < 0.05). The expression of the S6K1 gene in swim bladder of fish fed with diets containing graded levels of Hyp showed a significant (P < 0.05) initial increase followed by a decrease (Fig. 4). The highest S6K1 gene expression in swim bladder was found in fish fed with diets supplemented with 10 g kg−1 Hyp. However, there was no significant difference in S6K1 gene expression in intestines with increasing levels of Hyp. In the muscle and liver, the S6K1 gene expression increased gradually with increasing levels of Hyp, while the S6K1 gene expression of fish on diets containing 15–25 g kg−1 Hyp increased significantly compared with those on the basal diet (0 g kg−1 Hyp) (P < 0.05). For different tissues, the S6K1 gene expression of fish fed basal diets was significantly different, with the transcript level of S6K1 in the liver being the highest among all tested tissues.
Relative expression of TOR gene of juvenile Nibea diacanthus fed with diets containing graded levels of supplemented hydroxyproline. Values are means ± SEM (n = 3). Mean values with different capital letters are significantly different among the dietary treatments in the same tissues (P < 0.05). Mean values with different lowercase letters are significantly different among the different tissues in the basal diet group (diets I) (P < 0.05)
Relative expression of 4E-BP gene of juvenile Nibea diacanthus fed with diets containing graded levels of supplemented hydroxyproline. Values are means ± SEM (n = 3). Mean values with different capital letters are significantly different among the dietary treatments in the same tissues (P < 0.05). Mean values with different lowercase letters are significantly different among the different tissues in the basal diet group (diets I) (P < 0.05)
Relative expression of S6K1 gene of juvenile Nibea diacanthus fed with diets containing graded levels of supplemented hydroxyproline. Values are means ± SEM (n = 3). Mean values with different capital letters are significantly different among the dietary treatments in the same tissues (P < 0.05). Mean values with different lowercase letters are significantly different among the different tissues in the basal diet group (diets I) (P < 0.05)
Discussion
Dietary hydroxyproline supplementation promote growth of juvenile Niber diacanthus
There has been growing interest in research on Hyp metabolism and nutrition as well as dietary supplementation of Hyp in fish diets (Liu et al. 2014; Zhang et al. 2013, 2015; Wei et al. 2016). Some studies have revealed that the appropriate level dietary Hyp supplementation has an effect on growth, muscle texture, and collagen content in fish (Aksnes et al. 2008; Zhang et al. 2013). In the current study, significant growth promotion was observed in juvenile N. diacanthus when supplemented with dietary Hyp. This observation is similar to a previous report (Wei et al. 2016), where the growth of large yellow croaker was significantly improved by optimum supplemented levels of dietary Hyp. However, fish fed with excess or insufficient dietary Hyp experienced nutritional stress, which resulted in decreased fish growth and protein deposition. On the other hand, Albrektsen et al. (2010) reported that there was no significant effect of dietary Hyp supplementation on growth performance of Atlantic salmon (Salmo salar L.). Similarly, Zhang et al. (2015, 2013) found that growth performance of juvenile turbot (Scophthalmus maximus L.) was not significantly affected by dietary Hyp level. These reports on dietary Hyp supplementation on fish growth indicate that moderate levels of Hyp are important for growth promotion. Nonetheless, large differences existed in fish sizes (among species), experimental diets, and aquaculture conditions in these previous studies, which suggest that the effect of dietary Hyp on fish growth could partly depend on size, dietary amino acid composition, and duration of supplementation.
Dietary Hyp supplementation affects protein-related body composition and amino acid contents
Proteins are the main components of tissue structure. In this study, dietary Hyp levels significantly affected the crude protein contents of the whole body and swim bladder of N. diacanthus; however, muscle protein was not affected (Table 5). The crude protein contents of whole body and swim bladder showed a positive correlation with dietary protein, while the moisture, ash, and lipid contents were not affected. In similar previous studies, Wei et al. (2016) revealed that muscle crude protein of large yellow croaker slowly increased with increasing dietary Hyp levels, reaching the highest level with 6.9 g kg−1 Hyp supplementation. On the other hand, Liu et al. (2014) found no significant differences in the proximate composition (moisture, crude protein, crude lipid, ash) of the whole body of turbot (Scophthalmus maximus L.) among all dietary treatments with increasing Hyp levels. The differences observed in these studies could be due to specific function of Hyp, i.e., major amino acid in collagen proteins, and major extracellular components of connective tissues such as skin, tendon, cartilage, and swim bladder. Thus, the demand for Hyp would vary in different tissues or body parts. Protein deposition is actually the deposition of amino acids. Gunasekera et al. (1997) reported that muscle amino acid composition could be affected by the nutritional quality of diets. The amounts and levels of EAA in diets must follow the amino acid balance. Whole-body EAA retentions are considered the most sensitive indicators of an inadequate supply of amino acids in diets (Bulbul et al. 2014), as excess EAA for amino acid balance in diets is channeled towards catabolism and energy production, thereby reducing the retention efficiency (Alam et al. 2008; Unnikrishnan and Paulraj 2010). In the present study, except leucine, significant differences were observed in EAA contents in the muscle of fish-fed diets with increasing Hyp content (Table 6). The EAA of swim bladder showed no significant differences in the retention of histidine, valine, phenylalanine, isoleucine, and methionine among the dietary treatments (Table 7). The maximum EAA retention in muscle and swim bladder were observed in fish fed with diet Hyp 10 g kg−1. It has been reported that growth and efficient protein synthesis depend on the availability of sufficient levels of all EAA in diets (Wu 2014). In this study, it was observed that the appropriate Hyp level in the experimental diets improved the amino acid balance of diets, which therefore promoted the growth and protein synthesis of fish. The optimum supplemented level of Hyp in the experimental diets was 10 g kg−1. For growth and protein synthesis, it is necessary to consider both dietary protein content and amino acid content (EAA intake), in order to fulfill amino acid balance. We observed variations in the EAA of muscle and swim bladder with increasing Hyp levels among all dietary treatments. It has been suggested that the retention of EAA is greater when one of them is limited in the diet (Silvia et al. 2012). A lower retention could suggest an excess of EAA in diets and a higher retention by a deficiency (Sanchez-Lozano et al. 2011; Deng et al. 2017). Thus, the difference in retention of EAA in the different tissues (muscle and swim bladder) could be explained by the fact that the amino acid requirement of muscle and swim bladder is different. Fish bladders, for example, are rich in collagen, which is more Hyp-dependent than muscle because of the relationship between hydroxyproline and collagen. On the basis of this, it is plausible to state that the restrictive amino acids of various tissues are different. On the other hand, NEAA seem to be affected differently, as among the dietary treatments in this study, the variation of EAA contents in muscle and swim bladder correlated with body PER and WG. This observation seems to suggest that the addition of 10–15 g kg−1 dietary Hyp might be linked with EAA deposition in tissues and the changes in PER and WG.
Dietary Hyp supplementation affects transcript levels of TOR but not 4E-BP and S6K1
Although hydroxyproline (Hyp) has been reported to be a signaling molecule that regulates cellular redox state and apoptosis (Phang et al. 2008), limited information is currently available on the effect of dietary Hyp level on biochemistry indices in serum and liver. In this study, increasing dietary levels of Hyp had no significant effect on SC, TG, and T-CH contents of serum and liver. While Hyp has limited effect on lipid metabolism, it seems to promote protein metabolism, but the pathways involved require further studies. Dietary amino acids are reported to play an important role in controlling gene expression (Zhou et al. 2019; He et al. 2019; Liang et al. 2016, 2019). In addition to their promotion of protein synthesis, recent studies have shown that amino acids regulate metabolism through the TOR pathway in different fish species (Liao et al. 2008; Marine et al. 2011; Chen et al. 2012; Tang et al. 2013; Liang et al. 2016; Li et al. 2019; Zhou et al. 2019; He et al. 2019). However, there is currently no information on the effect of Hyp on the major kinases involved in the TOR pathway in fish. In the present study, we revealed that in N. diacanthus transcript levels of TOR in the liver, muscle, and swim bladder increased with increasing levels of dietary Hyp up to an optimum level (diets containing 25 g kg−1, 20 g kg−1, and 15 g kg−1 Hyp supplements, respectively). Moreover, increased dietary Hyp levels increased the SGR, PER, WG, and the crude protein of whole body, which suggest that dietary Hyp improves these parameters in fish via increased activation of TOR. The expression and activation of TOR are however tissue-specific, as transcript levels of TOR in intestines were not affected by the increase in dietary Hyp. These findings are consistent with Manor et al. (2015), who revealed that the TOR pathway might be involved in nutrient redistribution during maturation, and accounts for the differences in the expression of related genes. In terms of the TOR pathway, our results are in agreement with studies on leucine (Zhou et al. 2019), arginine (Liang et al. 2016), and methionine (He et al. 2019), where it has been demonstrated that these amino acids promote protein synthesis of fish by altering the expression of TOR gene.
Eukaryotic translation initiation factor 4E-binding protein (4E-BP) and ribosomal protein S6 kinase1 (S6K1) are found downstream of TOR and have been implicated in the regulation of protein synthesis (Seiliez et al. 2008). In the Jian carp, the activity of 4E-BP is reported to be stimulated by amino acids (Chen et al. 2012; Tang et al. 2013). Similarly, leucine has been shown to regulate the phosphorylation of S6K1 and 4E-BP through the TOR signaling pathway ( Liang et al. 2019), while dietary arginine is reported to regulate the phosphorylation of 4E-BP in the intestinal epithelia of rats through the TOR signaling pathway (Ban et al. 2004). In this study, the relative expression of 4E-BP decreased with higher dietary Hyp levels in the tissues examined, except muscle. Notably, 4E-BP and TOR had different expression patterns, with transcript levels of TOR downregulated by increased dietary Hyp, while that of 4E-BP were upregulated. On the other hand, S6K1 expression was synonymous with TOR, increased initially followed by a decrease with increasing dietary Hyp level. This phenomenon was only observed in swim bladder. Increased transcript levels of S6K1 were observed in the liver and muscle, but not in intestine with increasing dietary levels of Hyp. These differences in the expression patterns of 4E-BP, S6K1, and TOR in the liver, muscle, and swim bladder suggest that Hyp improved protein synthesis by increasing TOR activity. This observation is however independent of the expression regulation of 4E-BP and S6K1 genes, as the mTOR pathway is regulated by phosphorylation of mTORC1 targets. Thus, the phosphorylation status of these genes in response to dietary Hyp supplementation would have to be further explored. In any case, the current study highlights the beneficial effects of Hyp on promoting N. diacanthus growth and the synthesis of proteins. Hyp seems to regulate the TOR signaling pathway through the phosphorylation of 4E-BP and S6K1, but the underlying mechanism requires further investigation.
Conclusion
The findings here revealed that appropriate Hyp supplementation in diet could improve fish growth, feed conversion ratio, and protein efficiency ratio. Regression analysis of the SGR data and dietary Hyp levels revealed that the optimum amount of Hyp to add to the basal diet was 16.6 g kg−1. Proximate composition and amino acid composition of tissues were affected by addition of dietary Hyp, but physiology indices of serum and liver were not affected by dietary Hyp supplementation. Dietary Hyp was shown to affect transcript levels of TOR, 4E-BP, and S6K1 in different tissues, which suggest that Hyp might enhance fish growth by regulating fish growth through the TOR pathway.
References
Aksnes A, Mundheim H, Toppe J, Albrektsen S (2008) The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets. Aquaculture 275:242–249
Alam S, Watanabe WO, Carroll PM (2008) Dietary protein requirements of juvenile black sea bass (Centroperistis striata). J World Aquacult Soc 39:656–663
Albrektsen S, Sirnes E, Aksnes A, Hagen Ø (2010) Impacts of dietary hydroxyproline on growth, muscle firmness, collagen and PYD cross-links formation in Atlantic salmon (Salmo salar). Program and Abstracts of the 14th international symposium on fish nutrition and feeding. P. 79
Association of Official Analytical Chemists (AOAC) (1995) Official methods of analysis of Official Analytical Chemists International, 16th edn. Association of Official Analytical Chemists, Arlington
Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Haisa M, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y (2004) Arginine and leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med 13:537–543
Bulbul M, Kader MA, Koshio S, Ishikawa M, Yokoyama S (2014) Effect of replacing fishmeal with canola meal on growth and nutrient utilization in kuruma shrimp Marsupenaeus japonicus (Bate). Aquac Res 45:848–858
Chen HD, Zheng JH, Shen ZK (2011) Technique for artificial breeding of spotted drum N. diacanthus. Fish Sci 30:501–504 (in Chinese with English abstract)
Chen GF, Feng L, Kuang SY, Liu Y, Jiang J, Hu K, Jiang WD, Li SH, Tang L, Zhou XQ (2012) Effect of dietary arginine on growth, intestinal enzyme activities and gene expression in muscle, hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Br J Nutr 108:195–207
Deng JM, Chen LQ, Mai KS, Mi HF, Zhang L (2017) Effects of replacing soybean meal with rubber seed meal on digestive enzyme activity, nutrient digestibility and retention in tilapia (Oreochromis niloticus×Oreochromis aureus). Aquac Res 48:1767–1777
Gunasekera RM, Shim KF, Lam TJ (1997) Influence of dietary protein content on the distribution of amino acids in oocytes, serum and muscle of Nile tilapia, Oreochromis niloticus (L.). Aquaculture 152:205–221
He Y, Chi S, Tan B, Dong X, Yang Q, Liu H, Zhang S, Han F, Liu D (2019) dl-Methionine supplementation in a low-fishmeal diet affects the TOR/S6K pathway by stimulating ASCT2 amino acid transporter and insulin-like growth factor-I in the dorsal muscle of juvenile cobia (Rachycentron canadum). Br J Nutr 122(7):734–744
Holz MK, Ballif BA, Gygi SP, Blenis J (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123:569–580
Hou YQ, Wu G (2017) Nutritionally nonessential amino acids: a misnomer in nutritional sciences. Adv Nutr 8:137–139
Li P, Wu GY (2018) Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50:29–38
Li P, Mai KS, Trushenski J, Wu GY (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37:43–53
Li X, Wu X, Dong Y, Gao Y, Yao W, Zhou Z (2019) Effects of dietary lysine levels on growth, feed utilization and related gene expression of juvenile hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂). Aquaculture 502:153–161
Liang H, Ren M, Habte-Tsion HM, Ge X, Xie J, Mi H, Xi B, Miao LH, Liu B, Zhou QL, Fang W (2016) Dietary arginine affects growth performance, plasma amino acid contents and gene expressions of the TOR signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 461:1–8
Liang H, Mokrani A, Chisomo-Kasiya H, Ji K, Ge X, Ren M, Liu B, Xi B, Sun A (2019) Dietary leucine affects glucose metabolism and lipogenesis involved in TOR/PI3K/Akt signaling pathway for juvenile blunt snout bream Megalobrama amblycephala. Fish Physiol Biochem 45(2):719–732
Liao XH, Majithia A, Huang XL, Kimmel AR (2008) Growth control via TOR kinase signaling, an intracellular sensor of amino acids and energy availability, with crosstalk potential to proline metabolism. Amino Acids 35:761–770
Liu YZ, He G, Wang QC, Mai KS, Xu W, Zhou HH (2014) Hydroxyproline supplementation on the performances of high plant protein source based diets in turbot (Scophthalmus maximus L.). Aquaculture 433:476–480
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2(-ΔΔC(T)) method. Methods 25:402–408
Manor ML, Cleveland BM, Weber GM, Brett-Kenney P (2015) Effects of sexual maturation and feeding level on fatty acid metabolism gene expression in muscle, liver, and visceral adipose tissue of diploid and triploid rainbow trout, Oncorhynchus mykiss. Comparative Biochemistry and Physiology Part B 179:17–26
Marine L, Stephane P, Elisabeth PJ, Karine D, Iban S, Sandrine SC (2011) L-leucine, L-methionine, and L-lysine are involved in the regulation of intermediary metabolism-related gene expression in rainbow trout hepatocytes. J Nutr 141:75–80
National Research Council (NRC) (2011) Nutrient requirement of fish and shrimp. National Academy Press, Washington, DC
Phang JM, Donald SP, Pandhare J, Liu YM (2008) The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 35:681–690
Rong H, Zhang YL, Hao ML, Zou WG, Yu J, Yu CQ, Shi QC, Wen XB (2019) Effects of dietary hydroxyproline on collagen metabolism, proline 4-hydroxylase activity, and expression of related gene in swim bladder of juvenile Nibea diacanthus. Fish Physiol Biochem 45(6):1779–1790
Rong H, Zhang YL, Hao ML, Lin F, Zou WG, Zhang HR, Yu CQ, Yu J, Shi QC, Aweya JJ, Wen XB (2020) Effect of hydroxyproline supplementation on growth performance, body composition, amino acid profiles, blood-biochemistry and collagen synthesis of juvenile chu’s croaker (Nibea coibor). Aquac Res 51(3):1264–1275
Sanchez-Lozano NB, Martinez-Llorens S, Tomas-Vidal A, Cerda MJ (2011) Amino acid retention of gilthead sea bream (Sparus aurata, L.) fed with pea protein concentrate. Aquac Nutr 17:604–614
Seiliez I, Gabillard JC, Skiba-Cassy S, Garcia-Serrana D, Gutiérrez J, Kaushik S, Panserat S, Tesseraud S (2008) An in vivo and in vitro assessment of TOR signaling cascade in rainbow trout (Oncorhynchus mykiss). Am J Phys Regul Integr Comp Phys 295:329–335
Silvia ML, Rosa BA, Silvia NM, Miguel JC, Ana TV (2012) Carob seed meal as a partial substitute in gilthead sea bream (Sparus aurata) diets: amino acid retention, digestibility, gut and liver histology. Aquaculture 338–341:124–133
Tang L, Feng L, Sun CY, Chen GF, Jiang WD, Hu K, Liu Y, Jiang J, Li SH, Kuang SY, Zhou XQ (2013) Effect of tryptophan on growth, intestinal enzyme activities and TOR gene expression in juvenile Jian carp (Cyprinus carpio var. Jian): Studies in vivo and in vitro. Aquaculture 412:23–33
Unnikrishnan U, Paulraj R (2010) Dietary protein requirement of giant mud crab (Scylla serrata) juveniles fed iso-energetic formulated diets having graded protein levels. Aquac Res 41:278–294
Wei ZH, Ma J, Pan XY, Mu H, Li J, Shentu J, Zhang WB, Mai KS (2016) Dietary hydroxyproline improves the growth and muscle quality of large yellow croaker Larimichthys crocea. Aquaculture 464:497–504
Wu GY (2013) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu GY (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. Journal of Animal Science and Biotechnology 5(1):34
Wullschleger SS, Loewith RR, Hall MM (2006) TOR signaling in growth and metabolism. Cell 124:471–484
Zhang KK, Ai QH, Mai KS, Xu W, Liufu ZG, Zhang YJ, Peng M (2013) Effects of dietary hydroxyproline on growth performance, body composition, hydroxyproline and collagen concentrations in tissues in relation to prolyl 4-hydroxylase α(I) gene expression of juvenile turbot, Scophthalmus maximus L. fed high plant protein diets. Aquaculture 404:77–84
Zhang KK, Mai KS, Xu W, Zhou HH, Liufu ZG, Zhang YJ, Peng M, Ai QH (2015) Proline with or without hydroxyproline influences collagen concentration and regulates prolyl 4-hydroxylase α (I) Gene expression in juvenile turbot (Scophthalmus maximus L.). J Ocean Univ China 14:541–548
Zhou Z, Wang X, Wu X, Gao Y, Li X, Dong Y, Yao W (2019) Effects of dietary leucine levels on growth, feed utilization, neuro-endocrine growth axis and TOR-related signaling genes expression of juvenile hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂). Aquaculture 504:172–181
Acknowledgments
We are grateful to Zhuangping Expert Workstation and all our laboratory members for technical advice and valuable help during the feeding trial and sample analysis.
Funding
This research was supported by Grant No. A201005D06-1 from China Guangdong Oceanic and Fishery Science and Technology Foundation, Grant No. 2016ZR14 from Natural Science Youth Research Foundation of Yunnan Agricultural University, and Grant No. 2018FG001-044 and 2017FG001-021 from Yunnan Province Agricultural Basic Research Joint Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rong, H., Lin, F., Zhang, Y. et al. The TOR pathway participates in the regulation of growth development in juvenile spotted drum (Nibea diacanthus) under different dietary hydroxyproline supplementation. Fish Physiol Biochem 46, 2085–2099 (2020). https://doi.org/10.1007/s10695-020-00863-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00863-z