Abstract
The existence of nutritional and energy reserves is fundamental for fish female fertility, so that the existence of a correlation between metabolic reserves and reproductive capacity is suggested. Leptin regulates body weight and energy homeostasis. Estradiol induces the synthesis of vitellogenin, a phospholipoglycoprotein produced by the liver and taken up by the growing oocytes. The objective of this study was to investigate the possible existence of a crosstalk between 17β-estradiol (E2) and leptin in the modulation of E2-induced vtg in the rainbow trout Oncorhynchus mykiss. Liver slices were incubated with recombinant trout leptin (rt-lep) at three different concentrations (1–10–100 ng/ml). rt-lep brought about the decrease of E2-induced vtg secretion in the medium and the down-regulation of vtg mRNA expression. Moreover, rt-lep stimulated the lipase activity and diminished the liver fatty acid content. The combined employment of signal transduction inhibitors and the analysis of signal transduction phosphorylated factors revealed that rt-lep effect on E2-induced vtg occurred through the activation of phosphodiesterase, protein kinase C, MAP kinases, and protein kinase A. In conclusion, our study suggests that leptin influences E2-induced vtg synthesis in the rainbow trout Oncorhynchus mykiss by modifying both the protein and the lipid components.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish reproduction requires the central nervous system (CNS) integration of hormonal and environmental signals such as temperature, photoperiod, and food availability. Such signals influence the production of gonadotropin-releasing hormone (GnRH) in the brain, which in turn stimulates the pituitary release of gonadotropins (GtH) with downstream effects on the ovaries (Zohar et al. 2010; Degani 2016). In response to gonadotropins, the ovary produces the steroid 17β-estradiol (E2), which induces the liver to produce vitellogenin (Vg), the main precursor of egg yolk (Norris 1996). The hormonal control of reproduction and vitellogenin synthesis are extensively studied phenomena in many fish species due to their relevance in aquaculture (Tanaka et al. 2003; Guzmán et al., 2008; Pérez et al., 2011; Degani et al. 2017).
Leptin is a pleiotropic 16-kDa peptide hormone firstly discovered in mammals (Zhang et al. 1994), but found widely distributed phylogenetically (Prokop et al. 2012). Leptin is considered a mediator of the body metabolic status, responsible for informing the CNS about the level of energy reserves (Blundell et al. 2001). As in the case of mammals, also in teleosts, leptin appears to have an established role at the CNS level as a metabolic indicator of energy reserves and regulator of appetite and feeding behavior (Murashita et al. 2008; Li et al. 2010; Gorissen and Flik 2014; Chisada et al. 2014; Yan et al. 2016; Jorgensen et al. 2016).
Leptin is actively involved in the metabolic regulation of the body weight and food intake also at a peripheral level, and altered levels of leptin are associated to dysmetabolic syndromes such as obesity and diabetes (Michalakis et al. 2013). In mammals, leptin regulates peripheral lipid metabolism by reducing triacylglyceride (TAG) deposits and promoting fatty acid oxidation (FA) in both the adipose tissue and liver, sustaining its role as lipolytic hormone (Muoio and Dohm 2002). Triglicerydes are transported outside the liver as lipoproteins, bound to the Apolipoprotein B (ApoB), a protein involved in lipid metabolism and the main protein constituent of very low density and low density lipoproteins (Olofsson and Boren 2005). In the mouse liver, leptin regulates lipid metabolism by promoting the incorporation of triglycerides in VLDL particles, directly affecting ApoB levels and consequently the release in the bloodstream (Huynh et al. 2013). Interestingly, it has long been proposed that vtg and human ApoB possess a common ancestor (Babin et al. 1999), sustaining the role of the liver as a crossroad of energy reserves and reproductive functions. Since vtg is a phospholipoglycoprotein that can be regarded as a lipoprotein (Nagler and Idler 1990) and the liver appears also as the major site of leptin synthesis in fish (Kurokawa et al. 2005; Londraville et al. 2017), it is possible that leptin may be involved in the regulation of vtg synthesis and somehow interact with estradiol.
In the hypothalamus of mammals, where estrogen and leptin are both crucial for the regulation of reproduction and act in overlapping neurons to regulate fertility and long-term energy balance (Xu et al. 2012), there are indications of a direct interaction between the two signals at the level of STAT3 pathway to promote gene expression (Clegg et al. 2006). The JAK/STAT pathway has been identified as the main transduction mechanism of leptin (Fruhbeck 2006; Robertson et al. 2008), although other signaling pathways, such as MAPK/ERK, can be activated (Park and Ahima 2014). Moreover, estrogen effects may include not only the activation of the canonical pathway mediated by estrogen receptors, but also the activation of non-canonical signal transduction pathways, such as PI3K, MAPK, protein kinase C (PKC), and ERK (Etgen and Acosta-Martinez 2003; Marino et al. 2002).
In this frame, we hypothesized that there may be a crosstalk between 17β-estradiol and leptin in the modulation of the 17β-estradiol-induced vtg and consequently of the reproductive responses in relation to the available energy reserves. In order to test this hypothesis, we analyzed the effect of recombinant trout leptin (rt-lep) on 17β-estradiol-induced vtg on liver slices of rainbow trout Oncorhynchus mykiss, one of the most economically relevant species in aquaculture (FAO report 2018) and a widely used laboratory fish model (Bailey et al. 1996; Grunow et al. 2011; Williams 2012). We measured vtg release in the incubation medium, the liver expression of vtg mRNA transcript and lipase activity, and the signal transduction pathways involved in 17β-estradiol-induced vtg synthesis. Furthermore, we analyzed the lipid composition of rt-lep-treated liver slices by 1H-NMR spectroscopy.
Materials and methods
Fish and sampling procedures
All the procedures involving animals were conducted as indicated in the Italian National Guidelines (D.L. No. 116 G.U., suppl. 40, 18.2.1992, circolare No. 8, G.U. July 1994) and in accordance with the European Council Directive 2010/63/EU following the rules of the D.Lgs 26/14, adhering to the Guide for the Care and Use of Laboratory Animals (United States National Research Council, 1996). The protocol was approved by the Committee on the Ethics of Animal Experiments of the same Consortium (DM n. 12/2016–UT, 29/09/2016).
Rainbow trout (Oncorhynchus mykiss) with an average weight of 500 g were used for this study. Fish were obtained from a local farm (Di Mella, Santa Croce del Sannio, Benevento, Italy) and acclimated for 1 week in a recirculating aquaculture system at 18 °C. Twenty trouts were employed for this study. The sacrifice took place 24 h after the last meal. Fish were euthanized by immersion in tricaine methanesulfonate (MS-222) (MP Biomedicals, LLC, Aurora, OH, USA) 80 mg/l (LC50 > 200 mg/l). Gonads were threadlike as evaluated by visual inspection.
Reagents and Oncorhynchus mykiss recombinant leptin (rt-lep) synthesis
17β-estradiol, IBMX, chelerythrine, PD098059, H89, AG490, MG132, D-Mannitol, saccharose, EDTA, Trizma-base, protease inhibitors cocktail, anti β-actin, methanol, β-mercaptaethanol, SDS, blue bromophenol, glycerol, Tween 20, nonfat dry milk, PBS, and the lipase activity assay kit were purchased from Sigma-Aldrich Corp. (St Louis, MO, USA). ATP Bioluminescence Assay Kit CLSII was from Roche Applied Science (Penzberg, Germany). Vitellogenin ELISA kit was from Biosense Laboratories AS (Bergen, Norway). SV Total RNA Isolation System was from Promega (Madison, WI, USA). DNAse, SuperScript® III Reverse Transcriptase, Novex Sharp protein standard, Express SYBR®GreenER™qPCRSuperMix with Premixed ROX were purchased from Invitrogen (Carlsband, CA, USA). Bio-Rad dye protein assay was from Bio-Rad laboratories Inc. (Perth, UK). Anti-p-JNK, anti-p-ERK, and anti-p-STAT3 were bought by Santa Cruz Biotechnology (Dallas, TX, USA). Immobilon Western Chemiluminescent HRP substrate Kit was from Millipore (Billerica, MA, USA). Rainbow trout recombinant leptin (rt-lep) was produced as described in Mariano et al. (2013). Briefly, the complete trout leptin cDNA sequence was amplified from total liver cDNA by PCR. Purified leptin cDNA coding sequence was cloned into the plasmid vector and transformed into the BL21 E. coli strain for protein expression. Then, cells were collected and lysed by sonication for the extraction and purification by nickel affinity chromatography and stored at − 20 °C until use. The protein recombinant techniques were according to Gellissen (2004).
Slice preparation, incubation, and viability
Liver slices were prepared according to Coccia et al. (2014). Briefly, livers were perfused in situ by insertion of a cannula into the portal vein followed by perfusion with sterile, ice-cold, clearing buffer (a modified Hanks’ balanced salt solution, HBSS, lacking Ca2+ and Mg2+, pH 7.2) for 10 min to remove red blood cells and prevent clotting (Shilling and Williams 2000). Livers were carefully removed, sectioned by an Oscillating Tissue Slicer EMS 5000 (Electron Microscopy Science, Hatfield, PA). Each slice was weighed and placed into a vial with 1.5 ml/100 mg of tissue of the incubation medium (HBSS). The medium was saturated with 95% O2 to 5% CO2 and vials were sealed. The vials were then placed on a vertically mounted wheel that was rotated at a rate of 2.3 rpm. The unit was housed within a temperature-controlled room held at 18 °C at the incubation times of 0, 24, 48, 72 h. Liver slice viability was determined by histological analysis (Imperatore et al., 2019), adenosine triphosphate (ATP) levels (ATP Colorimetric/Fluorometric Assay Kit Sigma-Aldrich, St.Louis MO USA), and genomic DNA integrity visualized by DNA laddering assay (Wyllie 1980).
Experimental design and inhibitors
In a first set of experiments, the liver slices were incubated with three concentrations of 17β-estradiol (E2) (1–10–100 μM) to identify the most effective concentration to stimulate vitellogenin (vtg). The concentration of E2 10 μM gave the best results and was employed in the following incubations. In a second set of experiments, we used E2 10 μM to stimulate vtg and tested rt-lep at three different concentrations (1–10–100 ng/ml). The concentrations of rt-lep were chosen according to the circulating leptin levels in rainbow trout (Francis et al. 2014). In a third set of experiments, the liver slices were incubated for 48 h (chosen as the best compromise between the effect and the incubation time) with 10 μM E2, 10 μM E2 plus 100 ng/ml rt-lep (leptin concentration was chosen as the one showing the best effect on vtg secretion, at 48 h as shown in Fig. 1) for 1H-NMR assay and in presence or absence of specific cell signaling inhibitors. The inhibitors used were the following: IBMX (phosphodiesterase inhibitor that elevates cAMP), chelerythrine (protein kinase C inhibitor), PD098059 (MAP kinase inhibitor), H89 (protein kinase A inhibitor), AG490 (JAK2 tyrosine kinase inhibitor), and MG132 (NF-kB inhibitor) at the concentration of 1, 10, and 50 μM. In a fourth set of experiments, the liver slices were incubated for 30 min with 10 μM E2, 10 μM E2 plus 100 ng/ml rt-lep, and 100 ng/ml rt-lep, to analyze the following phosphorylated signal transduction factors: p-JNK, p-ERK, and p-STAT3.
ELISA vtg assay
Vtg concentrations in the medium were evaluated by an enzyme-linked immunosorbent assay (ELISA) kit specific for Oncorhynchus mykiss (Biosense Laboratories AS, Bergen, Norway), following the manufacturer’s instructions. Vtg concentration standard curve ranged from 0.39 to 200 ng/ml.
RNA extraction and PCR analyses
Total RNA from the liver slices was isolated and quantified with a Nanodrop 1000 Spectrophotometer (Thermo-Scientific, Waltham, Massachusetts, USA) using a kit for Total RNA Isolation (Promega Madison, WI, USA). Reverse transcription was performed using 1 μg of total RNA (previously treated with DNAse), oligodT primers, and the SuperScript® III Reverse Transcriptase (Invitrogen, Carlsband, CA, USA).
cDNA preparations from liver slices were used in PCR amplifications. Vtg primers (F-5′ TAGAGTGCCGGCAGACAAAT 3′, RV-5′ TAAGGAGCTGAATGGGCAATC 3′) were designed on the Oncorhynchus mykiss vtg sequence reported in GenBank (Accession n. X92804), by using the Primer3 software (Rozen and Skaletsky 2000). EF1-α primers specific for Oncorhynchus mykiss are reported in literature (Morash et al. 2008). All primers were designed at the exon-exon intersection.
PCR amplification of vtg was carried out in a Helix Thermal Cycler (DiaTech-Pharmacogenetics S.R.L., AN, Italy). PCR conditions consisted of denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. A final elongation step was performed at 72 °C for 10 min. The PCR product was separated by 2% agarose gel electrophoresis with ethidium bromide and visualized with Chemidoc UV transilluminator (BioRad, MI, Italy). The PCR fragment was purified using a QIAquick Gel Extraction (QiagenValencia, CA, USA). The PCR fragment (116 bp long) was cloned into a pGEM-T Easy Vector (Promega, WI, USA) to transform Escherichia coli (strain DH-5α) using standard methods. Clones containing the PCR insert were isolated and the plasmid DNA was purified using a QIAprep Spin Miniprep Kit (QiagenValencia, CA, USA). The nucleotide sequence was carried out by PRIMM s.r.l. (NA, Italy).
Semi-quantitative PCR amplification was carried out in a Helix Thermal Cycler (DiaTech). PCR conditions consisted of denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. A final elongation step was performed at 72 °C for 10 min. The PCR product was separated by 2% agarose gel electrophoresis with ethidium bromide and visualized with Chemidoc UV transilluminator (BioRad). Quantification of PCR products was performed by densitometric analysis using the Quantity-One software (Bio-Rad). All samples were normalized to the housekeeping gene, EF1-α.
Real-time PCR reactions were carried out on a 7900HT Real-Time PCR System (Applied Biosystem, Foster City, CA) using Express SYBR®GreenERTMqPCRSuperMix with Premixed ROX (Invitrogen, Carlsband, CA, USA) as a reference dye in a total volume of 20 μl per reaction. Each reaction contained 10 μl of SYBR Green mix, 0.4 μl each of forward and reverse primer (10 μM), 4 μl of 10× diluted cDNA. The thermal program included 2 min at 95 °C, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Each reaction was run in duplicate. A no-template control and dissociation curve were performed to confirm the specificity of the assays. A dilution series was prepared to ensure the efficiency of the reactions. All samples were normalized to the housekeeping gene, EF1-α. Data were analyzed using the 2−ΔΔCt method to calculate relative expression levels. All molecular techniques described were according to Farrell Jr (2009).
Lipase assay
Lipase activity in the liver slices was evaluated by a lipase activity assay kit (Sigma-Aldrich, St Louis, MO, USA) following the manufacturer’s instructions. Lipase activity was reported as nmole/min/ml = milliunit/ml.
Western blot analysis
Western blot was performed as described in Mahmood and Yang (2012). Proteins from liver slices were extracted with lysis buffer (220 mM D-mannitol, 70 mM saccharose, 1 mM EDTA, 20 mM Tris, pH 7.4) containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The slices were homogenized and centrifuged at 12,000×g for 30 min at 4 °C. The supernatants were collected and underwent protein determination with the Bio-Rad dye protein assay (Bio-Rad laboratories Inc., Perth, UK). Samples were boiled for 10 min in loading buffer (50 mM trisHCl pH 6.8, 100 mM β-mercaptaethanol, 2% SDS, 0.1% blue bromophenol, 10% glycerol). Then, the proteins were separated on a 12% SDS-PAGE and electrotransferred onto a nitrocellulose membrane at 100 V at 4 °C for 2 h. Membranes were blocked at 4 °C for 1 h in blocking buffer containing 0.1% Tween 20 and 5% no fat dry milk in PBS. Then, membranes were incubated overnight at 4 °C with the primary antibodies raised against Janus Kinase (p-JNK), p-ERK, p-STAT3 (Santa Cruz Biotechnology, Dallas, TX, USA) diluted 1:500 and polyclonal antibodies against β-actin (Sigma-Aldrich Corp., St Louis, MO, USA) as internal marker. The incubation with the secondary anti-mouse and anti-goat IgG (1:3000) was carried out for 1 h at RT. Signals were detected by chemiluminescence with the ImmobilonWestern Chemiluminescent HRP substrate Kit (Millipore, Billerica, MA, USA) with Chemidoc (Bio-Rad). A prestained molecular weight ladder (Novex Sharp protein standard, LC5800, Invitrogen, Hilden, Germany) was used to determine the protein size. Western blotting bands were quantified by Quantity One (Bio-Rad) software version 4.6.1.
Nuclear magnetic resonance (1H-NMR) spectroscopy
1H-NMR spectroscopy was carried out according to Coccia et al. (2019). Briefly, liver samples were suspended in H2O and methanol and sonicated for 30 s. Then, chloroform was added and the samples were centrifuged at 10,000 rpm for 10 min at 4 °C. The lipophilic (apolar) phases were collected, transferred to a glass vial, and dried under nitrogen flow. Thereafter, they were dissolved in deuterated chloroform. A 600-MHz BrukerAvance spectrometer with a TCI cryoprobe was used to acquire 1H-NMR spectra at 300 K as reported in Costantini et al. (2018). A double-pulsed field gradient echo was used, with a soft square pulse of 4 ms at the water resonance frequency and the gradient pulses of 1 ms each in duration adding 128 transients of 64 k complex points, with an acquisition time of 4 s/transient. Time domain data were all zero filled to 256 k complex points and an exponential amplification of 0.6 Hz prior to Fourier transformation was applied. Metabolites were assigned based on the comparison of chemical shifts and spin-spin couplings with reference spectra and tables present in the SBASE-1-1-1 database in AMIX package (Bruker, Biospin, Germany) and the biological magnetic resonance database (BMRB) (Ulrich et al. 2008). The spectral 0.50–8.60 ppm region of 1H-NMR spectra was integrated in buckets of 0.04 ppm by AMIX package (Bruker, Biospin, Germany). Orthogonal Projections to Latent Structures discriminant analysis (OPLS-DA) by Metabo Analyst tool 3.0 (Xia et al. 2015) was used to analyze the different groups.
Statistical analysis
Data were analyzed by one-way analysis of variance (ANOVA) at a significance level of 0.05, following confirmation of normality and homogeneity of variance. Where significant differences were detected by ANOVA, data were subjected to Tukey’s multiple range test. All values were reported as mean ± SEM, and all analyses were carried out with the statistical software Statistica version 7.0 (Statsoft Inc., Tulsa, OK, USA). For statistical analysis theory, we referred to the manual “An Introduction to Statistical Learning: with Applications in R” (James et al. 2013).
Results
Tissue slice viability
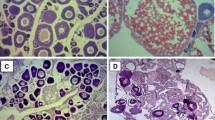
The histological analysis of the liver tissue slices showed normal cells with an intact nucleus and an absence of apoptotic cells with a condensed fragmented nucleus (Fig. 1). No cellular DNA fragmentation was recorded, and no statistically significant differences in the ATP levels in liver tissue slices over the entire experimental period were detected (Figs. 2 and 3). The results confirmed that the liver tissue slices used in the experimentation were viable across the experimental period.
In vitro effects of E2 and rt-lep on vtg secretion
The in vitro effect of E2 and E2 plus rt-lep on vtg secretion is reported in Fig. 4. The increase of vtg concentration in the incubation medium after the treatment with E2 was time dependent, reaching the highest concentration after 72 h of incubation. The co-incubation of E2 plus rt-lep (1, 10, 100 ng/ml) significantly reduced the vtg concentration in the incubation medium. The decrease was both time and dose dependant.
Vitellogenin (vtg) concentration in the incubation medium in response to 17β-estradiol (E2) and recombinant trout leptin (rt-Lep) in in vitro incubation of rainbow trout (Oncorhynchus mykiss) liver slices at different incubation times (24, 48, and 72 h). The data represent the mean ± SEM of different trout samples (n = 6). Different letters on the columns indicate statistically significant intergroup differences
In vitro effects of E2 and rt-lep on vtg mRNA expression
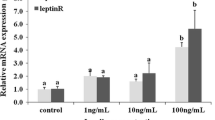
In vitro effect of E2 and rt-lep on vtg mRNA expression is shown in Fig. 5. The treatment of liver tissue slices with E2 resulted in a significant increase in vtg mRNA expression with respect to the control. When liver slices were incubated with both E2 and rt-lep, at any of the concentration tested (1, 10, 100 ng/ml), vtg mRNA expression was abolished. The incubation with rt-lep alone (1, 10, 100 ng/ml) did not cause any significant change in mRNA expression of vtg.
Vitellogenin (vtg) mRNA expression evaluated by semi-quantitative PCR (a) and real-time PCR (b) in response to 17β-estradiol (E2) and recombinant trout leptin (rt-Lep) in in vitro incubations of rainbow trout (Oncorhynchus mykiss) liver slices after 48 h of incubation time. The data represent the mean ± SEM of different fish samples (n = 6) and the different letters on the columns indicate statistically significant differences. The housekeeping gene was EF1α
In vitro effects of E2 and rt-lep on lipase activity
The administration of E2 did not cause significant changes in the lipase activity compared to the control (2.80 ± 0.24 and 3.10 ± 0.32 mU/g of tissue for untreated and E2 treated, respectively). The administration of rt-lep and E2 plus rt-lep resulted in a significant increase in the lipase activity (5.36 ± 0.18 and 5.45 ± 0.16 mU/g of tissue, respectively) with respect to the control (2.80 ± 0.24) (Fig. 6).
Lipase activity in response to 17β-estradiol (E2) and recombinant trout leptin (rt-Lep) in in vitro incubations (48 h) of rainbow trout (Oncorhynchus mykiss) liver slices. The data represent the mean ± SEM of different fish samples (n = 6) and the different letters on the columns indicate statistically significant differences
Signal transduction pathway identification
In order to determine the signal transduction pathway activated by E2, rt-lep, and E2 plus rt-lep in liver, two sets of experiments were carried out. In one set, liver slices were incubated with inhibitors of specific pathways; in a second set, liver slices were incubated with E2, rt-lep, and E2 plus rt-lep for 30 min and antibodies against cell signaling phosphorylated molecules were employed in Western blotting analysis.
In vitro effects of signal transduction inhibitors on E2-induced vtg secretion
As reported in Fig. 7, the co-incubation with both E2 and rt-lep reduced significantly the vtg release in the medium at 48 h of incubation. The use of inhibitors blocking the pathway of vtg secretion influenced by leptin restores the vtg synthesis. The concentration of vtg in the medium significantly increased following the co-incubation of E2 and rt-lep together with IBMX (10 and 50 μM), cheleritine (50 μM), PD98059 (1 and 10 μM), H89 (10 μM), AG490 (50 μM), and MG132 (50 μM).
Vitellogenin (Vtg) concentration in the incubation medium in response to 17β-estradiol (E2), E2 + rt-Lep, E2 + rt-Lep + inhibitor of signal transduction pathways (IBMX, cheleritine, PD98059, H89, AG490, MG132) in in vitro incubations of rainbow trout (Oncorhynchus mykiss) liver slices after 48 h. Concentrations employed were 10 μM for E2, 100 ng/ml for rt-Lep, and 1–10–50 μM for each inhibitor. The data represent the mean ± SEM of different fish samples (n = 6). Different letters on the columns indicate statistically significant differences. The percentage of vtg concentration in the incubation medium was calculated by setting the E2-induced vtg value at 100
In vitro effects of E2 and rt-lep on signal transduction pathways
In order to determine which signal transduction pathways were activated, liver slices were incubated with E2, rt-lep, and E2 plus rt-lep for 30 min. MAP kinase pathway activation was evaluated by analyzing the phosphorylation of JNK and ERK. The increase in JNK phosphorylation was observed in liver slices incubated with E2 compared with the control, while liver slices incubated with rt-lep or E2 plus rt-lep revealed a decrease of JNK phosphorylation with respect to the control (Fig. 8a). The increase in ERK phosphorylation was observed in liver slices incubated with rt-lep compared with the control, while incubation with E2 or E2 plus rt-lep did not have significant effects on ERK phosphorylation (Fig. 8b). The Jak/STAT pathway activation was evaluated analyzing the phosphorylation of STAT3. The increase in STAT3 phosphorylation was observed in liver slices incubated with rt-lep compared to the control, while liver slices incubated with E2 or E2 plus rt-lep did not have significant effects on STAT3 phosphorylation (Fig. 8c).
Western blotting analysis of a pJNK, b pERK, and c pSTAT3 after 30 min of incubation of rainbow trout (Oncorhynchus mykiss) liver slices with 17β-estradiol (E2) (10 μM), recombinant trout leptin (rt-Lep), and E2 plus rt-Lep. The data represent the mean ± SEM of different fish samples (n = 6) and the different letters on the columns indicate statistically significant differences. β-actin was used as an internal marker
1H-NMR spectroscopy analysis
The 1H-NMR spectroscopic analysis is reported in Fig. 9. OPLS-DA plot related to the comparison between the lipid fractions of the control liver and liver slices after incubation with E2, rt-lep, and E2 plus rt-lep showed that the groups clustered into four separate classes (Fig. 9a), suggesting the presence in these groups of lipids with statistically different levels (Fig. 9b). Laden plot showed that untreated liver slices had high level content of cholesterol, phospholipids, n-3 PUFAs such as DHA, and triacylglycerols (TAGs) and the levels decreased in treated liver slices, according to the following intensity: E2 < rt-lep < E2 plus rt-lep.
1H-NMR spectroscopy analysis of liver slices in response to 17β-estradiol (E2) (10 μM) and recombinant trout leptin (rt-Lep) (100 ng/ml) after an incubation time of 48 h. Orthogonal projections to latent structure discriminant analysis (OPLS-DA) plots (a) and loading plot related to the apolar fractions (b). The Y-axis reports the metabolites while the X-axis indicates the significativity values of the top ten significant metabolites
Discussion
In this study, we investigated the leptin role in the modulation of E2-induced vtg in liver slices of rainbow trout Oncorhynchus mykiss.
In fish, vtg represents the major egg yolk precursor, synthesized in the liver under estrogen control and transported through the bloodstream to the ovary where it is taken up by the growing oocytes (Reading and Sullivan, 2017). It is widely reported in the literature that estrogens, mainly 17β-estradiol, have a powerful effect on the liver synthesis of vtg (Green and Tata 1976; Nagler et al. 1987). In particular, 17β-estradiol activates the transcription of the vtg gene and increases the protein synthesis, improving the stability of the transcript (Baker and Shapiro 1977; Shapiro 1982). Accordingly, we report here the increase in the vtg concentration in the medium of liver slices of rainbow trout Oncorhynchus mykiss, incubated with E2. Incubation with rt-lep at any of the employed concentrations reduced the concentration of vtg induced by E2 in the medium, and the E2-induced vtg mRNA expression was inhibited by rt-lep at any of the doses used. Literature studies reporting the effect of leptin on vtg transcription are lacking. Therefore, we can only hypothesize if leptin is able to physically interfere with the promoter of the vitellogenin gene. Our reasoning is based on the evidence that vtg represents an important carrier of lipids into the growing oocytes; thus, it can be regarded as a lipoprotein (Nagler and Idler 1990). The similarity between vtg and lipoproteins has led us to hypothesize that leptin could influence the synthesis of vtg, either the protein component or the lipid component, or both. Vtg is related to other serum proteins that bind lipids in vertebrates, the so called apolipoproteins (Babin et al. 1999). Indeed, vtg and apolipoprotein B, the protein core of very low density lipoprotein (VLDL) and low density lipoprotein (LDL) (Chan 1992), are members of the same multigene superfamily (Babin et al. 1999). In this study, we report that rt-lep downregulates the E2-induced vtg mRNA. However, leptin does not significantly influence the expressions of apoB in human HepC2 cells, while it shows a downregulation on hepatic apoM transcription and secretion in vitro (Luo et al. 2005). The presence of apoM in the human is associated with the production of HDL, in particular metabolism and transfer of cholesterol in the blood stream (Luo et al. 2004).
It is important to underline here that the similarities between apolipoproteins of mammals and teleosts must be considered with caution, as in teleost apolipoprotein genes have undergone a long phylogenetic differentiation, which may have led to a diversification of functions with respect to mammals. Indeed, the highest sequence similarities between zebrafish and human apolipoproteins hortologs are 54% (Otis et al. 2015).
It is worth pointing that these results are based on in vitro studies and their transposition to the in vivo level has to be carefully considered. It is interesting to note that the rt-lep minimum concentration capable of eliciting the effect of E2-induced vtg is 1 ng/ml. The leptin circulating concentration in rainbow trout ranges from about 1.5 ng/ml before meals to about 3.5 ng/ml after meals (Francis et al. 2014). This opens the possibility that physiologically circulating levels of leptin can affect the expression of vtg and makes possible that leptin can modulate the amount of vtg released by the liver during the reproductive cycle. Unfortunately, no reports are available about the circulating levels of leptin during the reproductive cycle in rainbow trout. However, some studies report a correlation between the levels of 17β-estradiol and leptin, although a clear picture is not deductible. Nagasaka et al. (2006) determined leptin, 17β-estradiol, and prolactin levels in the blood of the salmonid species ayu Plecoglossus altivelis, showing that leptin levels were synchronized with both 17β-estradiol and prolactin, with plasma leptin levels low before spawning and high during and after spawning, suggesting the possibility of a regulatory link between sex hormones and leptin. Similarly, female burbot (Lota lota) showed low leptin levels before and during the spawning period, compared to the after-spawning period when the leptin levels increase significantly (Mustonen et al. 2002). Yu et al. (2012) showed a relationship between overexpression of leptin gene and inhibited 17β-estradiol synthesis in zebrafish. Choi et al. (2014) reported that leptin, 17β-estradiol, and vtg levels increase during sexual maturation and reproduction in chum salmon Oncorhynchus keta. Trombley et al. (2015) indicated the ability of 17β-estradiol to stimulate the leptin gene expression in Atlantic salmon hepatocytes, reporting that the response may depend on the developmental stage of the fish.
In addition to acting on the vtg transcript, leptin may have caused the decrease in vtg release in the medium (present study), by acting on the lipid component of the protein. To investigate this possibility, we evaluated the liver lipase activity and the lipid composition of liver slices by 1H-NMR spectroscopy, before and after the treatments. Through 1H-NMR spectroscopic analysis, we found that untreated liver slices showed the highest content of cholesterol, phospholipids, n-3 PUFAs such as DHA, and triacylglycerols (TAGs) compared to E2, rt-lep, and E2 plus rt-lep-treated liver slices. The liver of teleost species is the main site of lipid metabolism and it has been reported that its lipid content reflects the composition of vtg (Silversand and Haux 1995). In particular, vtg is a phospholipoglycoprotein and its total lipid content has been reported to be 16–18% in rainbow trout Oncorhynchus mykiss. The fatty acid (FA) composition of vtg total lipids in rainbow trout is characterized by a high content of phospholipids and a preponderance of long chain polyunsaturated fatty acids (PUFA), with considerably more n-3 than n-6 PUFAs. Precisely, the predominant n-3 PUFAs were eicosapentaenoic acid (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6) (Silversand and Haux 1995). Furthermore, it is known that vtg binds cholesterol and is directly involved in its transport into the oocytes (Matyash et al. 2001; Akhavan et al. 2016). We also observed a high content of TAGs in the untreated liver slices. However, this class of lipids is more likely associated with VLDL lipoprotein, a lipid transporter with a TAG rich core (Patino and Sullivan 2002; Akhavan et al. 2016).
In our study, the liver slices treated with E2 and rt-lep showed a decrease in the levels of cholesterol, phospholipids, n-3 PUFAs such as DHA, and triacylglycerols with respect to the control. Such decrease was augmented in the liver slices treated with E2 plus rt-lep. We believe that the greatest decrease in the case of the cotreatment with E2 and rt-lep is the sum of two different physiological events. On one hand, the long known 17β-estradiol lipogenic effect (Emmerson et al. 1979; Ng et al. 1984; Cleveland and Manor, 2015) may be masked by the vtg release into the medium, and on the other hand, there is the lipolytic action exerted by rt-lep. In mammals, 17β-estradiol is a potent energy regulator and appears to interact with leptin signaling (Gao and Horvath, 2008). In teleosts, leptin induces an increase in lipolysis and a decrease in lipogenesis (Deck et al. 2017). In the liver of the grass carp Ctenopharyngodon idellus, leptin induced an increase in the mRNA level of hormone-sensitive lipase and a decrease in the expression of the enzyme involved in the FA synthesis (Li et al. 2010). It has been reported that in the catfish Pelteobagrus fulvidraco hepatocytes, leptin caused the increase in the activity and in the mRNA levels of lipolytic enzymes together with a decrease in the overall lipid content (Song et al. 2015). Furthermore, in the hepatocytes of the goby Synechogobius hasta, leptin increased mRNA levels of several lipolytic genes, reduced the levels of lipogenic genes, and decreased the overall content of triglycerides (Wu et al. 2017). These data support the evaluation of enzymatic activity of lipase reported in the present study. Lipase is considered the key enzyme responsible for lipolysis. We reported that its activity was higher in the liver slices treated with rt-lep (both in the presence and absence of E2) compared to the liver slices untreated or treated with 17β-estradiol alone. Leptin and lipid lipase in the liver of fish increase during times of stress (Deck et al. 2017). Stress affects all aspects of vertebrate physiology, including reproduction. Our data support the hypothesis that the simultaneous presence of rt-lep and E2 in the liver of rainbow trout Oncorhynchus mykiss could reflect an inconvenient condition for the establishment of reproductive responses, thus directing the metabolism towards the mobilization and use of energy during periods of stress.
In order to determine how leptin affects the E2-induced vtg synthesis, we analyzed the effects of signal transduction inhibitors on vtg secretion and the phosphorylation of signal transduction pathway factors. We expected that blocking the pathway of vtg secretion influenced by leptin, its synthesis would be restored. Vtg secretion was re-established following the use of inhibitors of phosphodiesterase, PKC, MAPK, and PKA, suggesting that these could be the pathways through which leptin inhibits the effect of E2 on the secretion of vtg, as also sustained by the inhibition of JNK (a MAPK), phosphorylation by rt-lep. Interestingly, the estrogen activation of MAPK and PKC pathways, among other, has been reported (Etgen and Acosta-Martinez, 2003; Marino et al. 2002), leading to the hypothesis that E2 and leptin effects may converge on such pathways.
Our results show vtg inhibition and an increase in lipase activity after incubation with rt-lep and E2 plus rt-lep in presence of IBMx, an inhibitor of the phosphodiesterase. Phosphodiesterase increases intracellular cAMP, which is the physiological activator of protein kinase A (PKA) which in turn stimulates lipase activity and lipolysis (Duncan et al. 2007). Thus, this pathway could be involved in the regulation of the lipid component of vtg. Moreover, Solinas and Becattini (2017) sustained that JNK acts as a negative regulator of peroxisome proliferator-activated receptor alpha (PPARα) a gene involved in the fatty acid oxidation. Further, ERK and Jak/STAT3 are canonical pathways of leptin acting on lipolysis and fatty acid oxidation (Procaccini et al. 2009). As we can see, rt-lep activated the phosphorylation of these proteins. However, when rt-lep was administered together with E2, these pathways were inhibited, suggesting that a crosstalk between 17β-estradiol and leptin may occur in the liver of the rainbow trout at the level of these pathways, as it occurs in the hypothalamus (Gao and Horvath, 2008). Furthermore, phosphorylation sites recognized by MAPK and PKC have been recently identified on the estradiol receptor (ER) in the liver of a teleost, indicating that ER might activate the MAPK and PKC pathways to trigger the transcription of the vtg gene (Ding et al. 2016). Interestingly, the cAMP/PKA pathway has been recently reported as an intracellular pathway involved in vtg mRNA expression in the hepatopancreas of Litopenaeus vannamei (Chen et al. 2018). Although the results shown in this paper are not definitive, they represent crucial indications that the actions of E2 and leptin can converge on the same cellular pathways. Further studies are needed to precisely define these routes.
Conclusions
In conclusion, our study shows that leptin could influence vtg synthesis by modifying both the protein and the lipid component. In particular, we propose that leptin negatively influences the E2-induced Vtg mRNA transcript, while decreasing the vtg lipid component through its lipolitic activity. The proposed model is summarized in Fig. 10. Collectively, these data reveal an interesting role for hepatic leptin signaling in the modulation of E2-induced vtg in teleosts.
References
Akhavan SR, Salati AP, Falahatkar B, Jalali SAH (2016) Changes of vitellogenin and Lipase in captive Sterlet sturgeon Acipenser ruthenus females during previtellogenesis to early atresia. Fish Physiol Biochem 42(3):967–978
Babin PJ, Bogerd J, Kooiman FP, Van Marrewijk WJA, Van der Horst DJ (1999) Apolipophorin II/I, Apolipoprotein B, Vitellogenin, and Microsomal Triglyceride Transfer Protein Genes Are Derived from a Common Ancestor. J Mol Evol 49:150–160
Bailey GS, Williams DE, Hendricks JD (1996) Fish Models for Environmental Carcinogenesis: The Rainbow Trout. Environ Health Perspect 104:5–21
Baker HJ, Shapiro DJ (1977) Kinetics of estrogen induction of Xenopus laevis vitellogenin messenger RNA as measured by hybridization to complementary DNA. J Biol Chem 252(23):8428–8434
Blundell JE, Goodson S, Halford JCG (2001) Regulation of appetite: role of leptin in signalling systems for drive and satiety. Int J Obes 25:S29–S34
Chan L (1992) Apolipoprotein B, the major protein component of triglyceride-rich and low density lipoproteins. J Biol Chem 267:25621–25624
Chen T, Chunhua RC, Jiang X, Zhang L, Li H, Huang W, Hu C (2018) Mechanisms for type-II vitellogenesis inhibiting hormone suppression of vitellogenin transcription in shrimp hepatopancreas: Crosstalk of GC/cGMP pathway with different MAPK-dependent cascades. PLoS One 13(3):e0194459. https://doi.org/10.1371/journal.pone.0194459
Chisada S, Kurokawa T, Murashita K, Rønnestad I, Taniguchi Y, Toyoda A, Sakaki Y, Takeda S, Yoshiura Y (2014) Leptin receptor-deficient (knockout) medaka, Oryzias latipes, show chronical up-regulated levels of orexigenic neuropeptides, elevated food intake and stage specific effects on growth and fat allocation. Gen Comp Endocrinol 195:9–20
Choi YJ, Kim NN, Shin HS, Choi CY (2014) The Expression of Leptin, Estrogen Receptors, and Vitellogenin mRNAs in Migrating Female Chum Salmon, Oncorhynchus keta: The Effects of Hypo-osmotic Environmental Changes. Asian Australas J Anim Sci 27:479–487
Clegg DJ, Brown LM, Woods SC, Benoit SC (2006) Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes 55:978–987
Cleveland BM, Manor ML (2015) Effects of phytoestrogens on growth-related and lipogenic genes in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C 170:28–37
Coccia E, Varricchio E, Vito P, Turchini GM, Francis DS, Paolucci M (2014) Fatty acid-specific alterations in leptin, PPARα, and CPT-1 gene expression in the rainbow trout. Lipids 49:1033–1046
Coccia E, Imperatore R, Orso G, Melck D, Varricchio E, Volpe MG, Paolucci M (2019) Explants of Oncorhynchus mykiss intestine to detect bioactive molecules uptake and metabolic effects: Applications in aquaculture. Aquaculture 506:193–204
Costantini S, Parrillo L, Guerriero E, Melck D, Colonna G, Volpe MG, Paolucci M (2018) 1H-NMR metabolomic profiling of the crayfish Astacus leptodactylus subjected to polyphenol-enriched diets. Aquac Nutr 24:524–538
Deck CA, Honeycutt JL, Cheung E, Reynolds HM, Borski RJ (2017) Assessing the Functional Role of Leptin in energy Homeostasis and the Stress Response in vertebrates. Front Endocrinol 8:63. https://doi.org/10.3389/fendo.2017.00063
Degani D (2016) Oogenesis Control in Multi-Spawning Blue Gourami (Trichogaster Trichopterus) as a Model For the Anabantidae Family. Int J Sci Res 5:179–184
Degani G, Din SY, Hurvitz A (2017) Transcription of Insulin-like Growth Factor Receptor in Russian Sturgeon (Acipenser gueldenstaedtii) Ovary during Oogenesis. Univers J Agric Res 5:119–124
Ding W, Cao L, Cao Z, Bing X, Zhao F (2016) Molecular characterization and expression profile of the estrogen receptor α gene during different reproductive phases in Monopterus albus. Sci Rep 6:27924. https://doi.org/10.1038/srep27924
Duncan RE, Maryam AM, Jaworski K, Sarkadi-Nagy E, Sul HS (2007) Regulation of Lipolysis in Adipocytes. Annu Rev Nutr 27:79–101
Emmerson J, Korsgaard B, Petersen I (1979) Dose response kinetics of serum vitellogenin, liver DNA, RNA, protein and lipid after induction by estradiol-17 beta in male flounders (Platichthys flesus L.). Comp Biochem Physiol B 63:1–6
Etgen AM, Acosta-Martinez M (2003) Participation of Growth Factor Signal Transduction Pathways in Estradiol Facilitation of Female Reproductive Behavior. Endocrinology 144:3828–3835
FAO (2018) The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Rome. http://www.fao.org/3/i9540en/i9540en.pdf
Farrell RE Jr (2009) RNA Methodologies: Laboratory Guide for Isolation and Characterization. Elsevier Science, Amsterdam
Francis DS, Thanuthong T, Senadheera SPSD, Paolucci M, Coccia E, De Silva SS, Turchini GM (2014) n-3 LC-PUFA deposition efficiency and appetite regulating hormones are modulated by the dietary lipid source during rainbow trout growout and finishing periods. Fish Physiol Biochem 40:577–593
Fruhbeck G (2006) Intracellular signalling pathways activated by leptin. Biochem J 393:7–20
Gao Q, Horvath TL (2008) Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 294:E817–E826
Gellissen G (2004) Production of Recombinant Proteins: Novel Microbial and Eukaryotic Expression Systems. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Gorissen M, Flik G (2014) Leptin in teleostean fish, towards the origins of leptin physiology. J Chem Neuroanat 6:200–206
Green CD, Tata JR (1976) Direct induction by estradiol of vitellogenin synthesis in organ cultures of male Xenopus laevis liver. Cell 7:131–139
Grunow B, Wenzel J, Terlau H, Langner S, Gebert M, Kruse C (2011) In vitro Developed Spontaneously Contracting Cardiomyocytes from Rainbow Trout as a Model System for Human Heart Research. Cell Physiol Biochem 27:1–12
Guzmán M, Norberg B, Ramos J, Mylonas CC, Mañanós EL (2008) Vitellogenin, steroid plasma levels and spawning performance of cultured female Senegalese sole (Solea senegalensis). Gen Comp Endocrinol 156:285–297
Huynh FK, Neumann UH, Wang Y, Rodrigues B, Kieffer TJ, Covey SD (2013) A Role for Hepatic Leptin Signaling in Lipid Metabolism via Altered Very Low Density Lipoprotein Composition and Liver Lipase Activity in Mice. Hepatology 57:543–554
Imperatore R, D'Angelo L, De Girolamo P, Cristino L, Paolucci M (2019) Identification of Orexin and Endocannabinoid Receptors in Adult Zebrafish Using Immunoperoxidase and Immunofluorescence Methods. Jove 148. https://doi.org/10.3791/59308
James G, Witten D, Hastie T, Tibshirani R(2013) An Introduction to Statistical Learning: with Applications in R, Springer Texts in Statistics 103, https://doi.org/10.1007/978-1-4614-7138-71, © Springer Science+Business Media New York 2013
Jorgensen EH, Bernier NJ, Maule AG, Vijayan MM (2016) Effect of long-term fasting and a subsequent meal on mRNA abundances of hypothalamic appetite regulators, central and peripheral leptin expression and plasma leptin levels in rainbow trout. Peptides 86:162–170
Kurokawa T, Uji S, Suzuki T (2005) Identification of cDNA coding for a homologue to mammalian leptin from puffer fish, Takifugu rubripes. Peptides 26:45–750
Li GG, Liang XF, Guangzhao QX, Yu Y, Lai K (2010) Gene structure, recombinant expression and functional characterization of grass carp leptin. Gen Comp Endocrinol 166:117–127
Londraville RL, Prokop JW, Duff RJ, Liu Q, Tuttle M (2017) On the Molecular evolution of Leptin, Leptin receptor, and endospanin Leptin Receptor and Endospanin. Hypothesis and theory. https://doi.org/10.3389/fendo.2017.00058
Luo G, Zhang X, Nilsson-Ehle P, Xu N (2004) Apolipoprotein M. Lipids Health Dis 3:21
Luo G, Hurtig M, Zhang X, Nilsson-Ehle P, Xu N (2005) Regular paperLeptin inhibits apolipoprotein M transcription and secretion inhuman hepatoma cell line, HepG2 cells. Biochim Biophys Acta 1734:198–202
Mahmood T, Yang P-C (2012) Western Blot: Technique, Theory, and Trouble Shooting. N Am J Med Sci 4:429–434
Mariano G, Terrazzano G, Coccia E, Vito P, Varricchio E, Paolucci M (2013) Effects of recombinant trout leptin in superoxide production and NF-kappaB/ MAPK phosphorylation in blood leukocytes. Peptides 48:59–69
Marino M, Acconcia F, Bresciani F, Weisz A, Trentalance A (2002) Distinct Nongenomic Signal Transduction Pathways Controlled by 17β-Estradiol Regulate DNA Synthesis and Cyclin D1 Gene Transcription in HepG2 Cells. Mol Biol Cell 13:3720–3729
Matyash V, Geier C, Henske A, Mukherjee S, Hirsh D, Thiele C, Grant B, Maxfield FR, Kurzchalia TV (2001) Distribution and Transport of Cholesterol in Caenorhabditis elegans. Mol Biol Cell 12:1725–1736
Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG (2013) The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism 62:457–478
Morash AJ, Kajimura M, McClelland GB (2008) Intertissue regulation of carnitine palmitoyl transferase I (CPTI): Mitochondrial membrane properties and gene expression in rainbow trout (Oncorhynchus mykiss). Biochim Biophys Acta 1778:1382–1389
Muoio DM, Dohm GL (2002) Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab 16:653–666
Murashita K, Uji S, Yamamoto T, Rønnestad I, Kurokawa T (2008) Production of recombinant leptin and its effects on food intake in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol B Biochem Mol Biol 150:377–384
Mustonen AM, Nieminen P, Hyvärinen H (2002) Leptin, ghrelin, and energy metabolism of the spawning burbot (Lota lota, L.). J Exp Zool 293:119–126
Nagasaka R, Okamoto N, Ushio H (2006) Increased leptin may be involved in the short life span of ayu (Plecoglossus altivelis). J Exp Zool Part A 305:507–512
Nagler JJ, Idler DR (1990) Ovarian uptake of vitellogenin and another very high density lipoprotein in winter flounder (Pseudopleuronectes americanus) and their relationship with yolk proteins. Biochem Cell Biol 68:330–335
Nagler JJ, Ruby SM, Idler DI, So YP (1987) Serum phosphoprotein phosphorus and calcium levels as reproductive indicators of Vg in highly vitellogenic mature female and estradiol-injected immature rainbow trout. Can J Zool 65:2421–2425
Ng TB, WooNYS TPPL, Au CYW (1984) Changes in metabolism and hepatic ultrastructure induced by estradiol and testosterone in immature female Epinephelus akaara (Teleostei, Serranidae). Cell Tissue Res 236:651–659
Norris DO (1996) In: Norris DO (ed) Vertebrate endocrinology. Academic Press, London
Olofsson SO, Boren J (2005) Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. JIM 258:395–410
Otis JP, Zeituni EM, Thierer JH, Anderson JL, Brown AC, Boehm ED, Cerchione DM, Ceasrine AM, Avraham-Davidi I, Tempelhof H, Yaniv K, Farber SA (2015) Zebrafish as a model for apolipoprotein biology: comprehensive expression analysis and a role for ApoA-IV in regulating food intake. Dis Model Mech 8:295–309
Park H-K, Ahima RS (2014) Leptin signaling. F1000Prime Reports 6:73
Patino R, Sullivan CV (2002) Ovarian follicle growth, maturation, and ovulation in teleost fish. Fish Physiol Biochem 26:57–70
Pérez L, Peñaranda DS, Dufour S, Baloche S, Palstra AP, Van Den Thillart GEEJM, Asturiano JF (2011) Influence of temperature regime on endocrine parameters and vitellogenesis during experimental maturation of European eel (Anguilla anguilla) females. Gen Comp Endocrinol 174:51–59
Procaccini C, Lourenco EV, Matarese G, La Cava A (2009) Leptin signaling: A key pathway in immune responses. Curr Signal Transduct Ther 4:22–30
Prokop JW, Duff RJ, Ball HC, Copeland DL, Londraville RL (2012) Leptin and leptin receptor: analysis of a structure to function relationship in interaction and evolution from humans to fish. Peptides 38:326–336
Reading BJ, Sullivan CV (2017) Vitellogenesis in Fishes.https://doi.org/10.1016/B978-0-12-809633-8.03076-4
Robertson SA, Leinninger GM, Myers MG Jr (2008) Molecular and Neural Mediators of Leptin Action. Physiol Behav 94:637–642
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press, Totowa, pp 365–386
Shapiro DJ (1982) Steroid hormone regulation of vitellogenin gene expression. CRC Crit Rev Biochem 12:187–203
Shilling AD, Williams DE (2000) The non-aromatizable androgen, dihydrotestosterone, induces antiestrogenic responses in the rainbow trout. J Steroid Biochem Mol Biol 74:187–194
Silversand C, Haux C (1995) Fatty acid composition of vitellogenin from four teleost species. Comp Physiol B 164:593–599
Solinas G, Becattini B (2017) JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol Metab 6:174–184
Song YF, Wu K, Tan XY, Zhang LH, Zhuo MQ, Pan YX, Chen QL (2015) Effects of recombinant human leptin administration on hepatic lipid metabolism in yellow catfish Pelteobagrus fulvidraco: in vivo and in vitro studies. Gen Comp Endocrinol 212:92–99
Tanaka H, Kagawa H, Ohta H, Unuma T, Nomura K (2003) The first production of glass eel in captivity: fish reproductive physiology facilitates great progress in aquaculture. Fish Physiol Biochem 28:493–497
Trombley S, Rocha A, Schmitz M (2015) Sex steroids stimulate leptin gene expression in Atlantic salmon parr hepatocytes in vitro. Gen Comp Endocrinol 221:156–164
Ulrich EL, Akutsu H, Doreleijers JF, Harano Y, Ioannidis YE, Lin J, Livny M, Mading S, Maziuk D, Miller Z, Nakatani E, Schulte CF, Tolmie DE, Wenger RK, Yao H, Markley JL (2008) BioMagResBank. Nucleic Acids Res 36:D402–D408
Williams DE (2012) The rainbow trout liver cancer model: Response to environmental chemicals and studies on promotion and chemoprevention. Comp Biochem Physiol C 155:121–127
Wu K, Tan XY, Xu YH, Shi X, Fan YF, Li DD (2017) JAK family members: molecular cloning, expression profiles and their roles in leptin influencing lipid metabolism in Synechogobius hasta. Comp Biochem Physiol B 203:122–131
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555–556
Xia J, Sinelnikov I, Han B, Wishart DS (2015) MetaboAnalyst 3.0 - making metabolomics more meaningful. Nucleic Acids Res 43:W251–W257
Xu Y, Faulkner LD, Hill JW (2012) Cross-talk between metabolism and reproduction: the role of POMC and SF1 neurons. Front Endocrinol 2:98. https://doi.org/10.3389/fendo.2011.00098
Yan AF, Chen T, Chen S, Tang DS, Liu F, Jiang X, Huang W, Ren C, Hu C (2016) Signal transduction mechanism for glucagoninduced leptin gene expression in goldfish liver. Int J Biol Sci 12:1544–1554
Yu RMK, Chu DLH, Tan T, Tsun LVW, Chan AKC, Giesy JP, Cheng SH, Wu RSS, Kong RYC (2012) Leptin-Mediated Modulation of Steroidogenic Gene Expression in Hypoxic Zebrafish Embryos: Implications for the Disruption of Sex Steroids. Environ Sci Technol 46:9112–9119
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Zohar Y, Muñoz-Cueto JA, Elizur A, Kah O (2010) Neuroendocrinology of reproduction in teleost fish. Gen Comp Endocrinol 165:438–455
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paolucci, M., Coccia, E., Imperatore, R. et al. A cross-talk between leptin and 17β-estradiol in vitellogenin synthesis in rainbow trout Oncorhynchus mykiss liver. Fish Physiol Biochem 46, 331–344 (2020). https://doi.org/10.1007/s10695-019-00720-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00720-8