Abstract
The present study aimed to investigate whether the Gfra1/Gdnf and/or Kit/Kitlg regulatory pathways could be involved in the regulation of spermatogonial cell proliferation and/or differentiation in fish. Homologs of the mammalian gfra1, gdnf, kitr, and kitlg genes were identified in gnathostomes and reliable orthologous relationships were established using phylogenetic reconstructions and analyses of syntenic chromosomal fragments. Gene duplications and losses occurred specifically in teleost fish and members of the Salmoninae family including rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Some duplicated genes exhibited distinct spatiotemporal expression profiles and were differently regulated by hormones in rainbow trout. Undifferentiated type A spermatogonia expressed higher levels of kitrb and kitra2 making them possible target cells for the gonadal kitlgb and somatic kitlga before the onset of spermatogenesis. Interestingly, gdnfa and gdnfb ohnologous genes were poorly expressed before the onset of spermatogenesis. The expression level of gdnfb was correlated with that of the vasa gene suggesting that the late increased abundance of gdnfb during spermatogenesis onset was due to the increased number of spermatogonial cells. gfra1a2 was expressed in undifferentiated type A spermatogonia whereas gfra1a1 was mainly detected in somatic cells. These observations indicate that the germinal gdnfb ligand could exert autocrine and paracrine functions on spermatogonia and on testicular somatic cells, respectively. Fsh and androgens inhibited gfra1a2 and gdnfb whereas gfra1a1 was stimulated by Fsh, androgens, and 17α, 20β progesterone. Finally, our data provide evidences that the molecular identity of the male germ stem cells changes during ontogenesis prior to spermatogenesis onset.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spermatogenesis is a highly organized process that allows undifferentiated spermatogonia to differentiate into spermatozoa. In mammals, undifferentiated type A spermatogonia are classified in three different main subpopulations. Single A spermatogonia (As) are isolated cells. Recent investigations suggest that only a subset of As spermatogonia expressing high levels of the Id4 protein is capable to colonize the seminiferous tubules, to self-renew, and to initiate new spermatogenetic waves after transplantation assays (Helsel et al. 2017). These cells should be considered as the spermatogonial stem cells (SSC). A paired spermatogonia (Apr) derive from the symmetric division of the As spermatogonia but the daughter cells remain interconnected by a cytoplasmic bridge. Aligned A spermatogonia (Aal) result from symmetrical divisions of paired spermatogonia, and they successively form chains of 4, 8, and 16 interconnected spermatogonia. It has been shown that A aligned spermatogonia could revert to less-differentiated germ cells through spermatogonial cyst fragmentation. Using live imaging in vivo on the ngn3/eGFP mouse model, it has been observed that a chain containing eight Aal spermatogonia dividing synchronously were undergoing a fragmentation process leading to the apparition of one 12-cell cyst and two doublets of interconnected cells corresponding to Apr. The latter gave rise to new Aal 4-cell cysts while the remainder 12-cell cyst underwent cell death (Nakagawa et al. 2010). These observations led to the assumption that the stock of As is also maintained in adult animal through clonal fragmentation with a continuous interconvert between equipotent As and syncytial Apr and Aal cells (Hara et al. 2014). In consequence, Apr and Aal should be considered as progenitor germ cells that maintain their stemness status while undergoing a reversible differentiation process.
Based on morphological studies, the population of undifferentiated spermatogonia is also heterogeneous in teleost fish species (Lacerda et al. 2010; Loir 1999). Although different nomenclatures were used, studies report the presence of multiple As spermatogonial subpopulations, syncytial doublets, and 4- to 64-cell cysts containing interconnected spermatogonia in adult fish testes. The presence of SSC in juvenile or adult fish has been evidenced through transplantation assays in salmonids (Bellaiche et al. 2014b; Okutsu et al. 2006), cichlids (Lacerda et al. 2010), and cyprinids (Nobrega et al. 2010). However, the subpopulation of fish SSC and the cellular dynamics leading to their homeostasis remain poorly understood.
SSC are not autonomous cells. It is well admitted that their survival and decision to self-renew or to differentiate into progenitors are under the control of a specific microenvironment provided by the “germinal niche” formed mainly by the Sertoli and peritubular cells in mammals. SSC and somatic cell interactions could be mediated directly though cell-cell contacts involving adherence molecules or indirectly through the secretion of soluble factors. In mammals, the glial cell line-derived neurotrophic factor (Gdnf) is expressed by somatic cells located in the vicinity of the SSC and its action is mediated by the glial cell line neurotrophic factor receptor alpha 1 (Gfra1) and the multifunctional Ret co-receptor (Jing et al. 1996; Treanor et al. 1996). Invalidation of one gdnf allele in mouse results in spermatogenesis failure in aging animal due to the loss of SSC (Meng et al. 2000). Similarly, a reduction of gfra1 gene expression in undifferentiated spermatogonia decreases SSC proliferation and promotes their differentiation. The transplantation of Gdnf-, Gfra1-, and Ret-deficient neonatal testes under the back skin of immunodeficient mice demonstrated that Gdnf/Gfra1 was a key physiological regulatory pathway regulating self-renewal and differentiation of SSC in adult mouse (Naughton et al. 2006).
Recent studies have addressed the potential involvement of the Gfra1 signaling pathway in the regulation of fish gametogenesis in cartilaginous and bony fishes. In dogfish (Scyliorhinus canicula), one type of gfra1 transcript was detected in undifferentiated spermatogonia and the addition of mammalian Gdnf to cultured undifferentiated spermatogonia supports their long term survival and proliferation (Bosseboeuf et al. 2013; Gautier et al. 2014). However, no gdnf transcript was detected in the germinative zone of the dogfish testis. In bony fishes, gfra1 and gdnf transcripts were identified in adult testes. In trout (Oncorhynchus mykiss), low expression levels of a gfra1 transcript were detected mainly in undifferentiated spermatogonia (Bellaiche et al. 2014a; Nakajima et al. 2014). The expression of a gdnf gene copy named gdnfb was barely detected in the testes of immature animals. Its expression was evidenced not only in somatic cells (Bellaiche et al. 2014a) but also in germ cells (Bellaiche et al. 2014a; Nakajima et al. 2014). In addition, gdnfb transcript expression peaks rather lately in pre-spermiating male trout (Bellaiche et al. 2014a). The Gfra1 protein was detected on single undifferentiated spermatogonia of the Nile tilapia (Oreochromis niloticus) using a heterologous antibody (Lacerda et al. 2013) but not in carp (Labeo rohita) (Panda et al. 2011). In contrast to mammals, Fsh represses gdnfb expression in rainbow trout testes (Bellaiche et al. 2014a) whereas Fsh stimulates Gdnf in mammals (Tadokoro et al. 2002). In consequence, the physiological role of the Gfra1/Gdnf pathway remains unclear in adult fish gonads.
Interestingly, the testicular morphology of Gdnf-, Gfra1-, and Ret-deficient mice is normal before birth (Meng et al. 2000). This suggests that other regulatory pathways are involved in the production of the initial stock of germ stem cells before birth and/or Gdnf-dependent SSC appear only after birth. In mouse, primordial germ cells (PGC) originate from a population of pluripotent epiblast cells that migrate towards the genital ridge at E10.5. The kit receptor and its cognate ligand named either kit ligand (Kitlg), steel factor, or stem cell factor (SCF) are essential for survival, proliferation, and migration of PGC (Runyan et al. 2006). Between E12.5 and E14.5, germ stem cells cease to proliferate due to the decreased release of soluble kit ligand. The germ stem cells differentiate into prospermatogonia and enter mitotic arrest (De Felici 2000). Similarly to mammals, a duplicated form of kit ligand, named kitlga, stimulates the proliferation of cultured PGC collected from 24-h zebrafish embryos (Fan et al. 2008). However, the in vivo spatial and temporal expression profiles of the genes involved in Kit/Kitlg pathway remain unexplored in fish.
The present study aimed to gain information on the possible involvement of the Kit/Kitlg and Gfra1/Gdnf regulatory pathways in the regulation of germ line stem cell homeostasis in rainbow trout during ontogenesis. We first identified homologous genes in rainbow trout and in other vertebrate species to validate the orthologous relationships and to understand the complex evolutionary history of the different receptors and cognate ligands. The cellular localization and temporal expression pattern of the transcripts encoding different receptors and cognate ligands were further examined in combination of known germ cell-specific transcripts. We provide evidence that the molecular identity of the germ stem cells evolves during ontogenesis and different combinations of receptor/ligand interactants may act sequentially prior and after spermatogenesis onset.
Materials and methods
Phylogenetic analysis
Homologs of the candidate mammalian genes were searched in 25 vertebrate species by querying different types of nucleotide databases (genome, mRNA, and EST collections) hosted at the NCBI, EMBL and INRA institutes. The full amino acid sequences of the proteins deduced from the homologous genes were subsequently aligned using the CLUSTALW algorithm of the BioEdit software and a phylogenetic reconstruction was carried out using the neighbor-joining method of the MEGA7 freeware.
Animals and tissue sampling
Male rainbow trout (O. mykiss) were obtained from the INRA experimental fish farm (PEIMA, Sizun, France) and kept in the laboratory facilities at 12 °C under natural photoperiod. In our rearing conditions, most of the male trout mature at 2 years old (60%) but precocious male puberty can occur at the end of the first year of life (40%). Fish were euthanized in 0.03% 2-phenoxyethanol. In order to validate the gdnfa primer sets, different organs (brain, pituitary, heart, muscle, gill, stomach, liver, intestine, skin, spleen, and kidney) were sampled from five pre-pubertal male rainbow trout (11 months of age).
The genetically male monosex population was generated by fertilizing eggs collected from two females with the sperm of one YY rainbow trout male. Offspring was maintained at 10 °C for 55 days and then reared at 12 °C. Gonads were collected from male alevins at 55, 90,120, and 180 days of age. Five replicates comprising 250 gonads (at 55 and 90 days of age), 100 gonads (at 120 days of age), or 20 gonads (at 180 days of age) were generated. All samples were immediately frozen in liquid nitrogen and subsequently stored at − 80 °C until RNA extraction.
Germ cell separation using centrifugal elutriation
Populations of undifferentiated spermatogonia were obtained from 40 testes of immature male trout (Stage I, 11 months of age) whereas B spermatogonia, spermatocytes, spermatids, and spermatozoa containing fractions were obtained from 10 testes of maturing males (stages III to IV, 18 months of age). Germ cell fractions were prepared as previously described (Loir and Sourdaine 1994). Briefly, testes were minced and submitted to an enzymatic digestion in collagenase followed by mechanical dissociation. Cell suspensions were filtered through nylon membrane (50- and 32-μm pore size) and centrifuged for 10 min at 100g. The cell pellets were suspended in L15 medium supplemented with 1% BSA and mixed with a 45% Percoll solution. Cells were centrifuged for 40 min at 500g and 20 min at 100g to remove erythrocytes. The upper cell layer was recovered and submitted to centrifugal elutriation (JE5 Beckman Instruments). Cell separation was performed at constant rotation speed (2000 rpm) and increasing flow rates in L15 medium supplemented with 0.5% BSA as described previously (Bellaiche et al. 2014b). Different germ cell fractions were collected and part of each fraction was fixed in Bouin’s fixative, embedded first in agarose and then in Paraplast (Sigma). Then, 7-μm sections were stained with Regaud’s hematoxylin, orange G, and aniline blue to determine germ cell identity and enrichment as reported previously (Bellaiche et al. 2014b). The enrichment values were 90% for the type A spermatogonial fractions, 70% for the type B spermatogonial fractions, 70% for the spermatocytes fractions, and 95% for the spermatid/spermatozoa fractions. The remaining part of each fraction was rinsed in L15 medium containing 0.5% BSA, pelleted, and resuspended in TRIzol reagent. Samples were snap frozen and stored at − 80 °C until RNA extraction.
Real-time PCR analysis
Total RNA was isolated using the TRIzol reagent according to manufacturer’s instructions (Invitrogen) and RNA concentrations were quantified using the NanoDrop ND-10 (Thermo Fisher Scientific, Courtaboeuf, France). RNA quality was determined using the Bioanalyser 2100 (Agilent Technologies, Massy, France). Five hundred nanograms total RNA was reverse transcribed using random hexamers and the GoScript™ Reverse Transcriptase (Promega). Quantitative polymerase chain reactions (qPCR) were conducted using the StepOne Plus thermocycler (Applied Biosystems). Primer sets were designed using online tools available at https://eu.idtdna.com/calc/analyzer and https://eu.idtdna.com/scitools/Applications/RealTimePCR/. Forward and reverse primers were chosen in different exons of the candidate genes to prevent amplification from genomic DNA. The primer sets are detailed in Table 1. PCR amplification was performed on 1/20 diluted first-strand cDNA templates, 1X Fast SYBR Green Master Mix (Applied Biosystems) fluorescent dye, and 300 nM of each forward and reverse primers. The cycling conditions were as follows: 95 °C for 20s, followed by 40 cycles at 95 °C for 3 s and 60 °C for 30 s. The efficiency of each primer set was about 100% as determined using twofold serial dilutions of pooled RT products. A melting curve analysis was carried out at the end of each run to control the amplification of a single PCR product. Gene expression levels were measured on five independent biological samples in duplicate and normalized using rs15 as described previously (Rolland et al. 2009). The relative abundance of the transcript was determined in arbitrary units relative to expression of the rs15 gene using the comparative Ct method (Livak and Schmittgen 2001). A t test and a non-parametric Wilcoxon-Mann-Whitney were carried out to determine significant variations of the different transcript abundances.
Organotypic culture of testicular fragments
Testes were collected from immature male rainbow trout at stage III and cultured as previously reported (Sambroni et al. 2013). Note that the gonadal stage was later determined by histological examination of a testicular fragment fixed in Bouin’s fixative. Testes were minced into 1 to 3 mm pieces with a tissue chopper and ten testicular fragments were randomly distributed (60–80 mg per well) onto Nunc polycarbonate membrane inserts into 24-well plates filled with 300 μl per well of culture medium (synthetic L15 medium as modified by Loir and supplemented with 2% Ultroser SF (n = 6 replicates per condition). Tissue fragments were incubated at 12 °C for 96 h. A single dose (300 ng/ml) of three different androgens [11-ketotestosterone (11KT), testosterone (T), and 17α methyltestosterone (MT)] and one progestin [17α, 20β-Dihydroprogesterone (DHP)] was tested. Purified pituitary rainbow trout Fsh was also used at 500 ng/ml. The medium was changed every 2 days. At the end of the incubation, tissues were snap frozen and stored at − 80 °C in TRIzol reagent until RNA extraction.
Results
Phylogenetic reconstructions reveal species-specific gene duplication and losses in teleosts
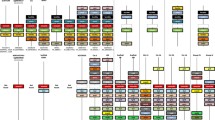
The homologous genes corresponding to mammalian gfra1, gdnf, kit, and kitlg were searched in different vertebrate species and the orthologous relationships were first established using amino acid sequence alignments (Supplemental data 1, 2, 3 and 4) and phylogenetic reconstructions (Figs. 1 and 2), and then confirmed through analyses of syntenic chromosomal regions (Supplemental data 5 and 6).
Evolutionary history of Gfra1 receptors and cognate Gdnf ligands in gnasthostomes. The evolutionary relationships of the genes encoding for distinct Gfra1 receptors and Gdnf ligands was examined using a sequence-based phylogenetic reconstruction. Amino acids sequences homologous to the mammalian Gfra1 receptor and Gdnf were searched using the tblastn algorithm in NCBI and ENSEMBL nucleotide databases including genomes, mRNA, and EST collections. Amino acid sequences deduced from the nucleotide sequences were aligned using the CLUSTALW multiple alignment software (Thompson et al. 1994). The phylogenetic tree was built using the Molecular Evolutionary Genetics Analysis (MEGA) software version 7 (Kumar et al. 2016). The analysis of the amino acids sequences was carried using the Neighbor-Joining (NJ) method. The reliability of the inferred tree was estimated using the bootstrap method with 100 replications. The tree is represented as a rooted tree using the elephant shark sequences. The third (3R) and fourth (4R) whole genome duplications are indicated. The accession number of each protein is indicated after the common name of the species. The scale bar indicates the number of expected amino acid substitutions per site per unit of branch length
Evolutionary history of the Kit receptors and cognate Kit ligands in gnasthostomes. The evolutionary relationships of the genes encoding for Kit receptors and cognate Kitlg ligands was examined using a sequence based phylogenetic reconstruction as described in Figure legend 1. The tree is represented as a rooted tree using the whale shark and elephant shark sequences. The third (3R) and fourth (4R) whole-genome duplications are indicated. The accession number of each protein is indicated after the common name of the species. The scale bar indicates the number of expected amino acid substitutions per site per unit of branch length
The analysis of the phylogenic tree of gfra1 genes reveals that a single gfra1 gene copy has been conserved in jawed animals including cartilaginous fishes, non-teleost bony fishes, birds, and mammals (Fig. 1). However, two orthologous gene copies, named gfra1a and gfra1b, were detected in most teleostean fish species suggesting this gene duplication event occurred during the teleost-specific third-round whole genome duplication (3R). Surprisingly, no gfra1b gene copy was discovered in species belonging to the Salmoninae family (rainbow trout and Atlantic salmon). However, the two salmonids gained an additional gfra1a gene copy. The two distinct gfra1a gene copies were named gfra1a1 and gfra1a2 in these two salmonids. Regarding the phylogenetic analysis of the Gdnf ligand (Fig. 1), a different evolutionary history is observed in gnathostomes although a single-copy gene named gdnf is also conserved in cartilaginous fishes, non-teleost bony fish, birds, and mammals. In most teleost fish, two duplicated gene copies named gdnfa and gdnfb were identified. In contrast to gfra1b that was not identified in rainbow trout and Atlantic salmon genomes, gndfb gene was maintained in Salmoninae as a single-copy gene.
A similar evolutionary history was observed with the genes involved in Kit/Kitlg pathway (Fig. 2). A single copy of the kitr and kitlg genes was identified in cartilaginous fishes elephant shark (Callorhinchus milii) and whale shark (Rhincodon typus), in non-teleost bony fishes, birds, and mammals. In teleost, the ancestral duplication 3R led to the emergence of two gene copies for kitlg (kitlga and kitlgb) and kitr (kitra and kitrb). The additional whole-genome duplication (4R) that occurred in Salmoninae resulted in the duplication of kitlga (kitlga1 and kitlga2) and kitra (kitra1 and kitra2) in rainbow trout and Atlantic salmon. The kitrb and kitlgb genes remained unique copy genes in these two salmonids.
In summary, genes encoding for the mediators of the Gfra1/Gdnf and Kit/Kitlg regulatory pathways are conserved during the evolutionary history of the gnathostomes suggesting they exert important physiological functions. However, two successive duplication events occurred, a first one in the ancestor of teleost fish and a second one in the ancestor of Salmoninae, resulting in the retention of additional gene copies or loss of paralogs (gfra1b). In order to further understand the biological significance of gene duplications and losses, we examined the spatial and temporal expression patterns of the duplicated gene copies in rainbow trout.
Cellular expression patterns of the candidate genes
Although not discriminating different ohnologous copies, our attempts to detect the cellular expression pattern of the transcripts on gonadal sections using the in situ RNA hybridization technique failed. In order to determine the cellular expression pattern of each gene copies using qPCR, we prepared total RNA from testes of immature animals and from enriched germ cell populations. The molecular identity of the different germ cell fractions was first validated by an expression profiling analysis of genes known to be expressed in germ cells or somatic cells (Supplemental data 7). As expected, the vasa transcript was detected in stage I testes and in all germ cell fractions. The nanos2 and dnd genes were previously detected mainly in zebrafish undifferentiated spermatogonia using the in situ RNA hybridization technique (unpublished data). Both nanos2 and dnd transcripts showed a two- to threefold increased accumulation in immature testis and undifferentiated type A spermatogonia compared to type B spermatogonia, meiotic, and post-meiotic germ cell fractions. The relative abundance of the rshl2 gene was highest in spermatocytes and post-meiotic cell fractions. The expression levels of the Sertoli cell-specific gsdf1 gene were highest in immature whole testes and were barely detected in germ cell fractions.
The expression profiles of the genes involved in the Gfra1/Gndf regulatory pathway are presented in Fig. 3. Transcripts corresponding to gfra1a1 and gfra1a2 gene copies were detected in immature whole testis and in undifferentiated type A spermatogonia. Note that the gfra1a1 transcript was predominantly detected in whole immature testes compared to undifferentiated type A spermatogonia suggesting that this transcript is also expressed in somatic cells. The expression levels of the gndfb and gdnfa1 transcripts were higher in undifferentiated type A spermatogonia compared to whole immature testis. No expression of gdnfa2 transcript was detected in the testis of immature male trout. Taken together, our data indicate that the main source of Gdnf ligands originated from the germinal compartment itself. The Gdnfa1 and Gdnfb ligands could exert an autocrine regulation on undifferentiated type A spermatogonia through their binding to the germinal Gfra1a2 receptor and a paracrine action on somatic cells through the activation of the Gfra1a1 receptor.
Cellular expression patterns of the genes involved in the Gfra1/Gdnf regulatory pathway. Gene expression was determined using qPCR from a panel of samples including whole testes (Testes Im) and enriched A spermatogonia (Spg A) collected from immature male rainbow trout at stage I. Additional samples include B spermatogonia (Spg B), spermatocytes (Scytes), and small post-meiotic germ cells (Spt/Spz: secondary spermatocytes, spermatids, and spermatozoa) collected from maturing males. The gfra1a2, gdnfa1, and gdnfb genes are predominantly expressed in undifferentiated type A spermatogonia. The gfra1a1 transcript is accumulated in undifferentiated type A spermatogonia but higher expression levels are observed in whole testes. This suggests that gfra1a1 is also expressed in testicular somatic cells of immature male trout. Statistical analyses were performed using confidence intervals. The different statistical groups are represented by letters a, b, and c (p < 0.05). Values represent means ± SEM of five replicates (n = 5)
The cellular expression profiles of the genes involved in Kit/Kitlg pathway are detailed in Fig. 4. The kitrb transcript showed a sixfold increase of its abundance in undifferentiated type A spermatogonia compared to immature whole testes. The kitrb transcript was barely detected in the other germ cell fractions. The kitra1 and kitra2 were also predominantly expressed in undifferentiated type A spermatogonia. We designed a primer set capable to amplify both kitlga1 and kitlga2 gene copies. The kitlga transcripts were significantly detected in whole testes but not in germ cell fractions indicating that kitlga1 and/or kitlga2 are expressed in somatic cells. In contrast, kitlgb was mainly detected in undifferentiated type A spermatogonia and immature testes. In summary, there are two distinct sources of Kit ligand in testes: kitlga and kitlgb transcripts arise mainly from somatic testicular cells and undifferentiated type A spermatogonia, respectively. The high expression levels of kitrb, kitra1, and kitra2 in undifferentiated type A spermatogonia indicate that this cell type could be the target cells.
Cellular expression patterns of the genes involved in the Kit/Kitlg regulatory pathway. Gene expression was determined using qPCR from a panel of samples including whole testes (Testes Im) and enriched A spermatogonia (Spg A) collected from immature male rainbow trout at stage I. Additional samples include B spermatogonia (Spg B), spermatocytes (Scytes), and small post-meiotic germ cells (Spt/Spz: secondary spermatocytes, spermatids, and spermatozoa) collected from maturing males. The kitrb, kitra1, and kitlgb genes are predominantly expressed in undifferentiated type A spermatogonia. In contrast, much higher expression levels of the kitlga transcript are observed in whole testes. This suggests that kitlga is expressed mainly in testicular somatic cells. The different statistical groups are represented by letters a, b, and c (p < 0.05). Values represent means ± SEM of five replicates (n = 5)
Histological analysis of the male gonads and temporal gene expression patterns during ontogenesis
To determine the temporal expression patterns of the candidate genes in the testes using qPCR, an all-male rainbow trout offspring was produced and gonads were dissected out at 55, 90, 120, and 180 days post fertilization (dpf). Note that in our rearing conditions, 45% of the male trout sexually matured precociously at 1 year of age. Histological observations of testicular sections indicated that undifferentiated type A spermatogonia were single cells in the male gonads from 55 to 90 dpf (Supplemental data 8). The organization of the seminiferous tubules and additional clusters of two to four undifferentiated type A spermatogonia were observed from 120 dpf onwards only in sexually maturing males. The appearance of type B spermatogonia was observed between 120 and 180 dpf.
A gene expression profiling of the sample panel was first carried out with control genes to examine the evolution of the Sertoli cells and spermatogonial populations before and after spermatogenesis onset (Supplemental data 9). The expression levels of the vasa gene remained unchanged between 55 and 90 dpf. This indicates that the proportion of undifferentiated type A spermatogonia remains constant in the male gonads during this time period. Interestingly, the expression levels of the nanos2 and dnd genes were significantly increased between 55 and 90 dpf indicating that the initial spermatogonia had undertaken a differentiation process. This population of spermatogonia was referred as prospermatogonia. Concomitantly with the apparition of clustered type A spermatogonia (named progenitors), the abundance of the dnd and nanos2 transcripts increased drastically between 90 and 120 dpf. The dnd and nanos2 gene expression levels reached a plateau between 120 and 180 dpf whereas vasa gene expression levels continued to strongly increase. This reveals that the proportion of progenitors reaches a plateau between 120 and 180 dpf while other spermatogonial cell populations differentiate and proliferate (type B spermatogonia). In contrast to germ cell-specific genes, gsdf1 gene expression levels showed moderate variations.
The expression patterns of the genes involved in the Gfra1/Gdnf regulatory pathway are presented in Fig. 5. The gfra1a1 and gfra1a2 transcripts showed distinct patterns of expression. gfra1a2 expression was highest at 55 dpf and then decreased sharply between 55 and 90 dpf. In contrast, gfra1a1 gene expression increased between 90 and 120 dpf. The temporal expression pattern of gdnfb was similar to vasa suggesting that this gene could be expressed concomitantly with active spermatogonial proliferation and/or differentiation. The gdnfa1 gene expression appeared similar to gfra1a2 but the variations were rather low. In summary, we did not observe obvious correlation between the expression patterns of the genes encoding for the Gfra1 receptors and their cognate ligands. However, a clear shift of expression between gfra1a1 and gfra1a2 occurred between 55 and 120 dpf suggesting that the two receptors could be expressed in different germ cells or are differently regulated. Our data indicate that gdnfa1 and gdnfb are poorly expressed before spermatogenesis onset. The progressive increase of gdnfb gene expression from 90 to 180 dpf favors a late role of the Gfra1/Gdnfb regulatory pathway during spermatogenesis onset.
Temporal expression profiles of the genes involved in the Gfra1/Gdnf regulatory pathway. Gene expression was monitored in the male gonads during rainbow trout ontogenesis using qPCR. Gonadal samples were collected at 55, 90, 120, and 180 days post fertilization (dpf) from an all-male trout population. Note that gfra1a2 expressions levels drop significantly after 55 dpf and before spermatogenesis onset. The expression levels of gfra1a1 and gdnfb increase significantly at 120 dpf concomitantly with the increased number of spermatogonia. The gdnfa1 gene expression pattern shows almost no variation during the time period examined. The different statistical groups are represented by letters a, b, and c (p < 0.05). Values represent means ± SEM of five replicates (n = 5)
In contrast to the Gfra1/Gdnf regulatory pathway, we observed similar expression patterns between the kitra2, kitrb, and kitlgb genes (Fig. 6). These three genes were expressed at higher levels at 55 dpf and a sharp decreased of gene expression was observed from 90 to 180 dpf. The kitra1 gene expression was hardly detectable and showed no significant variation during this time period. The expression profile of kitlga was similar to that of the gsdf1 gene. Our data indicate that the Kit/Kitlg regulatory pathway could be mainly active before spermatogenesis onset.
Temporal expression profiles of the genes involved in the Kit/Kitlg regulatory pathway. Gene expression was monitored in the male gonads during rainbow trout ontogenesis using qPCR. Gonadal samples were collected at 55, 90, 120, and 180 days post fertilization (dpf) from an all-male trout population. Note that kitrb, kitra2, and kitlgb show similar expression profiles with a sharp decrease of their relative abundances at 90 dpf before spermatogenesis onset. The kitra1 and killga transcripts show almost no variation of their relative abundances during the time period examined. The different statistical groups are represented by letters a, b, and c (p < 0.05). Values represent means ± SEM of five replicates (n = 5)
Hormonal regulation of the candidate genes
The hormonal regulation of the genes involved in the two candidate regulatory pathways was examined (Fig. 7). The addition of Fsh to cultured testicular explants slightly decreased gdnfb, gdnfa1, and gfra1a2 but increased gfra1a1 gene expression. gfra1a1 gene expression was mainly increased by 17α, 20β dihydroprogesterone and by testosterone (T) and 17α methyl testosterone although to a lesser extent. This indicates that Fsh action on gfra1a1 is mediated by sexual steroids. Similarly, Fsh action on gdnfb and gdnfa1 is mediated by sexual steroids. The gdnfb transcript expression was slightly decreased by the different sexual steroids used whereas gdnfa1 gene expression was decreased only by T and 11KT. Only gfra1a2 was not regulated by any sexual steroids. No effect of the different hormones was observed on the expression levels of the kitra1, kitra2, kitlga and kitlgb genes (data not shown).
Hormonal regulation of the genes involved in the Gfra1/Gdnf regulatory pathway. Testicular explants collected from maturing rainbow trout males (stage III) were incubated for 4 days in the presence or absence of purified Fsh (300 ng/ml), 11-ketotestosterone (11KT 300 ng/ml), testosterone (T 300 ng/ml), 17α methyl testosterone (MT 300 ng/ml) and 17α-20β dihydroprogesterone (DHP 300 ng/ml). Gene expression levels were measured using qPCR. Note that gfra1a1 gene expression is stimulated by Fsh and sexual steroids including androgens and progestogens. In contrast, gdnfb gene expression is negatively regulated by Fsh and sexual steroids. Note that gdnfa1 is also negatively regulated by Fsh and by physiological androgens only. Different letters indicate significantly different levels of relative abundance at p < 0.05. Values represent means ± SEM of six replicates (n = 6)
Discussion
Genes involved in the Gfra1/Gdnf and Kit/Kitlg regulatory pathways show a complex evolutionary history in teleost fish and in salmonids
Using phylogenetic reconstructions and analyses of syntenic chromosomal fragments, we demonstrate that genes involved in Gfra1/Gdnf and Kit/Kitlg regulatory pathways are conserved in gnathostomes as single-copy genes with the exception of teleost fish in which gene duplications and losses have occurred. It is well admitted that the ancestor of the teleost fish has been submitted to a third round of whole genome duplication (Amores et al. 1998; Meyer and Van de Peer 2005). This event is likely responsible for the duplication of the ancestral gfra1, gdnf, kitr, and kitlg genes. In agreement with an additional fourth round of whole-genome duplication in rainbow trout and in Atlantic salmon (Berthelot et al. 2014; Lien et al. 2016), we observed an additional duplication of gfra1a, gdnfa, kitra, and kitlga in these two salmonids. Whole-genome duplications are followed by gene retentions but also by gene losses to restore a diploid status (Brunet et al. 2006). This implies that duplication of gfra1b, gdnfb, kitrb, and kitlgb occurred in the common ancestor of the two salmonid species but one copy of the gdnfb, kitrb, and kitlgb genes was lost. More, our data indicate that the two gfra1b gene copies were lost. Note that ngr3b and trub1 that were surrounding gfra1b in the ancient fish species localized to different chromosomes (Supplemental data 5) in the two salmonid genomes suggesting that gfra1b could have been lost because of its location close from the breaking spot of the chromosomal fragment. The present study highlights that gene content is different in salmonids compared to other bony fish but the biological significance remains to be further investigated.
Duplicated gene copies resulting from the recent 4R duplication of the salmonid genome exhibit similar or different spatiotemporal expression profiles and are differently regulated by reproductive hormones
Numerous studies have reported that the ancient third round of whole genome duplication in teleost led either to similar or different cell-specific expression patterns of the duplicated gene copies (Rolland et al. 2009). Therefore, it is not surprising to observe that kitlga and kitlgb were expressed in somatic and germinal cells, respectively. However, information on changes of gene expression profiles resulting from the recent fourth round whole genome duplication in salmonids remains scarce. The present study shows that gfra1a1 was mainly expressed in somatic testicular cells as evidenced by higher expression levels observed in whole immature testes compared to that of undifferentiated type A spermatogonia. In contrast, gfra1a2 was mainly expressed in undifferentiated type A spermatogonia. More, the gfra1a1 and gfra1a2 ohnologs are expressed differently during ontogenesis and are differently regulated by Fsh and sexual steroids. These observations indicate that the fourth round of whole genome duplication in salmonids was followed by a different cell-specific distribution and regulation of the duplicated gfra1a gene copies. Such changes together with discrete amino acids differences in gene product open the way for subfunctionalization or neofunctionalization (Ohno 1970). One could hypothesize that the duplicated genes in fish fulfill all the functions and regulation of the ancestral gene (subfunctionalization) or acquire new functions (neofunctionalization). In salmonids, the gain of an additional gfra1a gene copy with a different regulation may have been sufficient to compensate the loss of gfra1b.
In contrast to gdnfa1, no expression of gdnfa2 was detected in any rainbow trout tissues examined including brain and ovary, two major tissues expressing Gdnf in mammals (data not shown). Further investigations will be required to determine whether the fourth whole-genome duplication resulted in the pseudogenization of the gdnfa2 gene copy in salmonids.
The Kit/Kitlg regulatory pathway could act on germ stem cells prior to spermatogenesis onset
Gene expression profiling during ontogenesis revealed that the kitrb, kitra, and kitlgb transcripts were co-expressed with the highest relative abundance measured at 55 dpf. These genes are likely expressed in germ stem cells at this age since the corresponding transcripts were predominantly detected in undifferentiated type A spermatogonia collected from immature male trout. In contrast to mammalian species in which Kitlg is expressed by somatic cells, our data indicate that kitlgb exerts an autocrine regulation on the male germ stem cells before spermatogenesis onset that occurs between 90 and 120 dpf in precocious males. In mouse, kitr and kitlg genes are also expressed before spermatogenesis onset in the embryonic gonads where they have pleiotropic functions. The kitr and kitlg genes support PGC proliferation and cell survival through a decreased germ cell apoptosis (Dolci et al. 1991; Runyan et al. 2006). In zebrafish, it has been demonstrated that a feeder layer expressing Kitlga stimulated the proliferation of PGC collected from 1 dpf embryos but a Kitlgb conditioned medium had no effect. Interestingly, the sharp decreased expression of kitlgb in trout was not followed by a decreased number of spermatogonia as revealed by monitoring the expression levels of the vasa gene between 55 and 90 dpf. In parallel, note that the expression levels of the somatic kitlga were rather unchanged during the time period examined. Altogether, the observations gained in zebrafish and rainbow trout suggest that Kitlgb may have another function than to stimulate germ cell proliferation. In mouse, both kitr and kitlg genes encode for cell-surface proteins and they play a major role in the adhesion of the PGC to the somatic cells (Pesce et al. 1997). Further investigations will be required to determine whether kitrb, kitra1, and kitlgb have a similar role in fish gonads before spermatogenesis onset.
The Gfra1/Gdnf regulatory pathway could act on germ cells and somatic cells
The present study reveals that both gfra1a1 and gfra1a2 are expressed in rainbow trout undifferentiated type A spermatogonia collected from 11-month immature males. Therefore, undifferentiated type A spermatogonia are likely target cells of the Gdnf ligands. In mammals, Gdnf is required for the maintenance of SSC in vivo and in vitro in long-term cultures of spermatogonia. Gdnf that originates from Sertoli and/or peritubular cells promotes SSC proliferation and inhibits their differentiation through a complex cascade of molecular events (Braydich-Stolle et al. 2005; He et al. 2008). In contrast to mammals, our data provide evidences that Gdnf ligands could exert an autocrine action because gdnfa1 and gdnfb are also expressed in these germ cells. Interestingly, gdnfb expression levels appeared similar to that of the vasa gene between 55 and 180 dpf in rainbow trout male gonads. This similarity suggests that gndfb is co-regulated with vasa in the different spermatogonia or both proteins are involved in the same biological process.
We observed that gfra1a1 was expressed at higher levels in whole immature testes compared to that of undifferentiated type A spermatogonia, suggesting that this gene is also expressed in somatic testicular cells. In mammals, the Gfra1/Gdnf regulatory pathway is known to influence germ stem cell homing (Kanatsu-Shinohara et al. 2012). Gdnf stimulates the proliferation of immature Sertoli cells through its binding to Gfra1 and interaction with the neural cell adhesion molecule (NCAM) co-receptor that mediates a complex intracellular signaling (Yang and Han 2010). NCAM mediates adhesion between Sertoli cells and gonocytes in cocultures from testes of neonatal rats (Orth and Jester Jr. 1995). In contrast to mammals, Sertoli cells continue to proliferate in the testes of adult fish during spermatogenesis (Schulz et al. 2005). In consequence, one could speculate that the increased gndfb expression observed after spermatogenesis onset could be a germ cell derived signal that promotes the proliferation of surrounding Sertoli cells to provide an appropriate homing with nutritional and structural support. Note that gfra1a1 was stimulated by Fsh and other sexual steroids. Fsh and sexual steroids are major mitotic factors for Sertoli cells in mammals (Pitetti et al. 2013). However, further investigations will be required to identify the testicular cells that express gfra1a1 in rainbow trout and the effect of Gdnf ligands on these target cells.
The molecular identity of the male germ cells changes during rainbow trout ontogenesis and spermatogenesis onset
In the present study, gene expression profiling shows that the molecular identity of germ stem cells changes during ontogenesis. The kitrb transcript was expressed at much higher levels at 55 dpf and was restricted to undifferentiated type A spermatogonia collected at 11 months of age. These observations suggest that kitrb is highly expressed in the early germ stem cell population. Other genes that include kitra2, kitlgb, and gfra1a2 showed a similar expression pattern. In contrast, nanos2 and dnd gene expression levels were low in this germ cell population. It is unlikely that the decreased expression of kitrb, kitra2, kitlgb, and gfra1a2 genes at 90 dpf could result from a decreased number of germ cells because vasa gene expression remained stable and nanos2 and dnd gene expression was increased between 55 and 90 dpf. Altogether, our data suggest that the initial male germ cell population observed at 55 dpf likely differentiates to form a new population of germ stem cells at 90 dpf. Interestingly, in mouse, PGC that reach the gonads between E10.5 and E12.5, stop to proliferate by E14.5 because they do not perceive the Kitlg signal. These cells become prospermatogonia and are arrested in G0/G1 of the cell cycle from E14.5 until 1–2 days post-partum (Busada et al. 2014; Western et al. 2008). Our observations in rainbow trout show that germ cells decrease kitr transcripts expression, but further investigations will be needed to demonstrate whether the initial germ stem cells observed at 55 dpf in the male gonads could differentiate to become “prospermatogonia” between 55 and 90 dpf. We previously observed that nanos2 and dnd were mainly expressed in zebrafish paired A spermatogonia using the in situ hybridization technique (unpublished data). In consequence, we hypothesize that the sharp increased expression levels of nanos2, dnd, and vasa genes between 90 and 120 dpf reflects the differentiation and proliferation of this population of progenitors. This population of progenitors likely reaches a plateau between 120 and 180 dpf because nanos2 and dnd gene expression levels remain constant during this time window. Between 120 and 180 dpf, vasa expression levels continue to increase indicating that other types of spermatogonia proliferate within the gonads.
In conclusion, spatiotemporal gene expression profiling carried out in the present study provides evidences that both Kit/Kitlg and Gfra1/Gdnf regulatory pathways could be active in the male gonads of rainbow trout during ontogenesis. An autocrine Kit/Kitlg regulatory pathway is likely active on germ stem cells before 55 dpf and involved the kitrb, kitra2, and kitlgb genes. A constant paracrine action of Kitlga should not be excluded. An autocrine Gfra1/Gdnf regulatory pathway is also likely activated at 55 dpf though the involvement of gfra1a2, gdnfa1, and gdnfb genes. Again, an additional paracrine action of the regulatory pathway involving gfra1a1 is likely active in somatic testicular cells and is regulated by Fsh and sexual steroids. Further investigations will be required to precise the functional effects of these regulatory pathways on germ cell survival, proliferation and or differentiation.
References
Amores A, Force A, Yan YL, Joly L, Amemiya C, Fritz A, Ho RK, Langeland J, Prince V, Wang YL, Westerfield M, Ekker M, Postlethwait JH (1998) Zebrafish hox clusters and vertebrate genome evolution. Science 282:1711–1714
Bellaiche J, Goupil AS, Sambroni E, Lareyre JJ, Le Gac F (2014a) Gdnf-gfra1 pathway is expressed in a spermatogenetic-dependent manner and is regulated by fsh in a fish testis. Biol Reprod 91:94
Bellaiche J, Lareyre JJ, Cauty C, Yano A, Allemand I, Le Gac F (2014b) Spermatogonial stem cell quest: nanos2, marker of a subpopulation of undifferentiated a spermatogonia in trout testis. Biol Reprod 90:79
Berthelot C, Brunet F, Chalopin D, Juanchich A, Bernard M, Noel B, Bento P, Da Silva C, Labadie K, Alberti A, Aury JM, Louis A, Dehais P, Bardou P, Montfort J, Klopp C, Cabau C, Gaspin C, Thorgaard GH, Boussaha M, Quillet E, Guyomard R, Galiana D, Bobe J, Volff JN, Genet C, Wincker P, Jaillon O, Roest Crollius H, Guiguen Y (2014) The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat Commun 5:3657
Bosseboeuf A, Gautier A, Auvray P, Mazan S, Sourdaine P (2013) Characterization of spermatogonial markers in the mature testis of the dogfish (Scyliorhinus canicula L.). Reproduction 147:125–139
Braydich-Stolle L, Nolan C, Dym M, Hofmann MC (2005) Role of glial cell line-derived neurotrophic factor in germ-line stem cell fate. Ann N Y Acad Sci 1061:94–99
Brunet FG, Roest Crollius H, Paris M, Aury JM, Gibert P, Jaillon O, Laudet V, Robinson-Rechavi M (2006) Gene loss and evolutionary rates following whole-genome duplication in teleost fishes. Mol Biol Evol 23:1808–1816
Busada JT, Kaye EP, Renegar RH, Geyer CB (2014) Retinoic acid induces multiple hallmarks of the prospermatogonia-to-spermatogonia transition in the neonatal mouse. Biol Reprod 90:64
De Felici M (2000) Regulation of primordial germ cell development in the mouse. Int J Dev Biol 44:575–580
Dolci S, Williams DE, Ernst MK, Resnick JL, Brannan CI, Lock LF, Lyman SD, Boswell HS, Donovan PJ (1991) Requirement for mast cell growth factor for primordial germ cell survival in culture. Nature 352:809–811
Fan L, Moon J, Wong TT, Crodian J, Collodi P (2008) Zebrafish primordial germ cell cultures derived from vasa::RFP transgenic embryos. Stem Cells Dev 17:585–597
Gautier A, Bosseboeuf A, Auvray P, Sourdaine P (2014) Maintenance of potential spermatogonial stem cells in vitro by GDNF treatment in a chondrichthyan model (Scyliorhinus canicula L.). Biol Reprod 91:91
Hara K, Nakagawa T, Enomoto H, Suzuki M, Yamamoto M, Simons BD, Yoshida S (2014) Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell 14:658–672
He Z, Jiang J, Kokkinaki M, Golestaneh N, Hofmann MC, Dym M (2008) Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells 26:266–278
Helsel AR, Yang QE, Oatley MJ, Lord T, Sablitzky F, Oatley JM (2017) ID4 levels dictate the stem cell state in mouse spermatogonia. Development 144:624–634
Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM (1996) GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell 85:1113–1124
Kanatsu-Shinohara M, Inoue K, Takashima S, Takehashi M, Ogonuki N, Morimoto H, Nagasawa T, Ogura A, Shinohara T (2012) Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell 11:567–578
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Lacerda SM, Batlouni SR, Costa GM, Segatelli TM, Quirino BR, Queiroz BM, Kalapothakis E, Franca LR (2010) A new and fast technique to generate offspring after germ cells transplantation in adult fish: the Nile tilapia (Oreochromis niloticus) model. PLoS One 5:e10740
Lacerda SM, Costa GM, da Silva MA, Campos-Junior PH, Segatelli TM, Peixoto MT, Resende RR, de Franca LR (2013) Phenotypic characterization and in vitro propagation and transplantation of the Nile tilapia (Oreochromis niloticus) spermatogonial stem cells. Gen Comp Endocrinol 192:95–106
Lien S, Koop BF, Sandve SR, Miller JR, Kent MP, Nome T, Hvidsten TR, Leong JS, Minkley DR, Zimin A, Grammes F, Grove H, Gjuvsland A, Walenz B, Hermansen RA, von Schalburg K, Rondeau EB, Di Genova A, Samy JK, Olav Vik J, Vigeland MD, Caler L, Grimholt U, Jentoft S, Vage DI, de Jong P, Moen T, Baranski M, Palti Y, Smith DR, Yorke JA, Nederbragt AJ, Tooming-Klunderud A, Jakobsen KS, Jiang X, Fan D, Hu Y, Liberles DA, Vidal R, Iturra P, Jones SJ, Jonassen I, Maass A, Omholt SW, Davidson WS (2016) The Atlantic salmon genome provides insights into rediploidization. Nature 533:200–205
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) method. Methods 25:402–408
Loir M (1999) Spermatogonia of rainbow trout: I. Morphological characterization, mitotic activity, and survival in primary cultures of testicular cells. Mol Reprod Dev 53:422–433
Loir M, Sourdaine P (1994) Testes cells: isolation and culture. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes: analytical techniques. Elsevier, Amsterdam, pp 249–272
Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H (2000) Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287:1489–1493
Meyer A, Van de Peer Y (2005) From 2R to 3R: evidence for a fish-specific genome duplication (FSGD). Bioessays 27:937–945
Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S (2010) Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328:62–67
Nakajima S, Hayashi M, Kouguchi T, Yamaguchi K, Miwa M, Yoshizaki G (2014) Expression patterns of gdnf and gfralpha1 in rainbow trout testis. Gene Expr Patterns 14:111–120
Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J (2006) Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod 74:314–321
Nobrega RH, Greebe CD, van de Kant H, Bogerd J, de Franca LR, Schulz RW (2010) Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS One 5:e12808
Ohno S (1970) Evolution by gene duplication. Springer-Verlag, Berlin, p 160
Okutsu T, Suzuki K, Takeuchi Y, Takeuchi T, Yoshizaki G (2006) Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish. Proc Natl Acad Sci U S A 103:2725–2729
Orth JM, Jester WF Jr (1995) NCAM mediates adhesion between gonocytes and Sertoli cells in cocultures from testes of neonatal rats. J Androl 16:389–399
Panda RP, Barman HK, Mohapatra C (2011) Isolation of enriched carp spermatogonial stem cells from Labeo rohita testis for in vitro propagation. Theriogenology 76:241–251
Pesce M, Di Carlo A, De Felici M (1997) The c-kit receptor is involved in the adhesion of mouse primordial germ cells to somatic cells in culture. Mech Dev 68:37–44
Pitetti JL, Calvel P, Zimmermann C, Conne B, Papaioannou MD, Aubry F, Cederroth CR, Urner F, Fumel B, Crausaz M, Docquier M, Herrera PL, Pralong F, Germond M, Guillou F, Jegou B, Nef S (2013) An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol Endocrinol 27:814–827
Rolland AD, Lareyre JJ, Goupil AS, Montfort J, Ricordel MJ, Esquerre D, Hugot K, Houlgatte R, Chalmel F, Le Gac F (2009) Expression profiling of rainbow trout testis development identifies evolutionary conserved genes involved in spermatogenesis. BMC Genomics 10:546
Runyan C, Schaible K, Molyneaux K, Wang Z, Levin L, Wylie C (2006) Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development 133:4861–4869
Sambroni E, Rolland AD, Lareyre JJ, Le GF (2013) FSH and LH have common and distinct effects on gene expression in rainbow trout testis. J Mol Endocrinol 50:1–18
Schulz RW, Menting S, Bogerd J, Franca LR, Vilela DA, Godinho HP (2005) Sertoli cell proliferation in the adult testis--evidence from two fish species belonging to different orders. Biol Reprod 73:891–898
Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y (2002) Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev 113:29–39
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, Phillips HS, Goddard A, Moore MW, Buj-Bello A, Davies AM, Asai N, Takahashi M, Vandlen R, Henderson CE, Rosenthal A (1996) Characterization of a multicomponent receptor for GDNF. Nature 382:80–83
Western PS, Miles DC, van den Bergen JA, Burton M, Sinclair AH (2008) Dynamic regulation of mitotic arrest in fetal male germ cells. Stem Cells 26:339–347
Yang Y, Han C (2010) GDNF stimulates the proliferation of cultured mouse immature Sertoli cells via its receptor subunit NCAM and ERK1/2 signaling pathway. BMC Cell Biol 11:78
Acknowledgements
The authors thank the animal care facility of the LPGP research department, especially Frédéric Borel, Amélie Patinote, and Cécile Melin. The authors thank Laurent Labbé and Lionel Goardon from the INRA PEIMA experimental fish farm for providing the monosex males and Drs. Alexis Fostier and Yann Guiguen for the gift of the rainbow trout YY sperm.
Funding
The research leading to these results has received funding from the European Community’s Horizon 2020 research infrastructure project (INFRAIA-1-2014/2015) under grant agreement no. 652831 (project AQUAEXCEL2020) and from the French National Research Agency under grand agreement no. 11-INBS-0003 (CRB anim project). Ahmed Maouche gratefully acknowledges the Britany province (Région Bretagne) and the INRA PHASE department for the funding received towards his PhD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maouche, A., Curran, E., Goupil, AS. et al. New insights into the evolution, hormonal regulation, and spatiotemporal expression profiles of genes involved in the Gfra1/Gdnf and Kit/Kitlg regulatory pathways in rainbow trout testis. Fish Physiol Biochem 44, 1599–1616 (2018). https://doi.org/10.1007/s10695-018-0547-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0547-4