Abstract
The current study was conducted to investigate the effect of ExcelMOS® in enhancing the immune system of Sparus aurata broodstock and their impact on offspring health through displaying the maternal transfer of immunity. Broodstock were divided into two groups: one was injected intraperitoneally with ExcelMOS® 1 month before spawning, while the other group was used as a control (without injection). Comprehensive increase in survival rate was observed for larvae hatched from ExcelMOS®-injected broodstock than those of the control (P ≤ 0.05). Hematological analysis showed increases in leukocyte count and hematocrit percentage (P ≤ 0.05) and significant enhancement in immune assays as phagocytic, respiratory burst, lysozyme activities in ExcelMOS®-injected broodstock (P ≤ 0.05). Additionally, total immunoglobulin levels in the serum, eggs, and larvae resulted from ExcelMOS®-injected broodstock were highly significant (P ≤ 0.05) than those in the control ones. Transmission electron microscopy and semi-thin sections in posterior intestine of ExcelMOS®-injected broodstock revealed reinforcement of the epithelial barrier structure, intestinal integrity, and functionality in combination with the stimulation of innate immune system. In conclusion, immunostimulation of Sparus aurata broodstock using ExcelMOS® has improved survival of larvae and enhanced both innate and adaptive immune defense mechanisms. Further investigations are required to show the effect of ExcelMOS® on fish cultured in intensive culture systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Significant mortalities are routinely recorded at early and late larval stages of fishes in marine hatcheries all over the world. The technique to enhance the survival rate of fish larvae is a serious issue of practical importance (Swain and Nayak 2009). This is the case of the gilthead seabream “Sparus aurata” being one of the most important aquaculture candidate species of the intensively reared fish in the Mediterranean Basin. Nowadays, aquaculture becomes the fastest growing food-producing sector in the world with the paramount potential to meet the increasing demand for aquatic food products (FAO 2012). One of the main constraints for marine aquaculture development in Egypt is the weak control and lack of reliable marine hatcheries causing pressures on natural fry (Kara et al. 2018).
Many strategies have been adopted to enhance the health of Sparus aurata larvae in hatcheries. One of these include the use of antibiotics, which have been introduced as a solution to solve these serious aquaculture problems related to expansion, cost, and economic return in fish farms (Swain and Nayak 2009). However, increasing economic and social concerns about antibiotics usage as well as other chemicals in fish farming have promoted more eco-friendly approaches to solve aquaculturist problems (Maqsood et al. 2011). The long-term application of antibiotics has been widely criticized due to the emergence of resistant strains, contamination of aquatic environment, elimination of gut microbial flora, increase in costs, and potential side effects (Karimzadeh et al. 2013). One of the alternative techniques to limit the use of antibiotics in aquaculture is prebiotics. Prebiotics are defined as fibers that are healthy and non-digestible compounds. They are working as substrate for the beneficial bacteria of the host’s gut. Prebiotics generally include nutrients such as non-digestible carbohydrate, resistant starch nutrient fiber, sugars, some peptides, and proteins (Song et al. 2014). Many investigations have reported that prebiotics are known to improve disease resistance, gut morphology, and growth parameters and modulate the intestinal microbiota, which helps the immune system’s proficiency in various aquatic species. Prebiotic application in large farmed fish and juveniles is done either through its incorporation into formulated diets, or through injection (intraperitoneal, pre-anal, or intravenous), or by direct administration to rearing water as immersion baths during early larval development stages (Dimitroglou et al. 2011). The protection gained by oral administration may often be comparatively low compared to injection in addition to the large individual variation in response to immunostimulants (Robertsen et al. 1990). Additionally, Esteban et al. (2000) indicated that entirely immune parameters in Sparus aurata remained unaffected when chitin was intravenously administered, while intraperitoneal administration showed amplified humoral and cellular immune responses.
Inulin is one of the prebiotics applied to seabream and showed significant increase in serum complement activity, IgM levels, leukocyte phagocytic activity, and leukocyte respiratory burst activity (Cerezuela et al. 2012). The compound, chosen for this study, is one of the most promising prebiotic products; it is a commercial compound known as ExcelMOS®, which is an all-natural source of beta-glucans and mannan oligosaccharides derived from a selected strain of Saccharomyces cerevisiae cell wall. This prebiotic compound showed promising applications. It has the same mode of action as Immunogen (Karimzadeh et al. 2013), and it has many advantages like preventing pathogenic bacteria from attaching to the cell surface of carbohydrates in the gastrointestinal tract, improving feed conversion, promoting beneficial bacteria in the gastrointestinal tract, enhancing immune system functioning, and improving survival rate and growth. Although some studies are available on the effect of prebiotic on fish growth performance, immunity, and microflora characteristics (Song et al. 2014), none of the available information mentioned the use of ExcelMOS® on Sparus aurata hatching procedures and immune response of broodstock using intraperitoneal administration. Therefore, this study was conducted on Sparus aurata broodstock to assess the transfer of maternal immunity from broodstock to offspring and its effect on their survival rate, as a result of improving the mother health state.
Materials and methods
Experimental design
Healthy seabream Sparus aurata (n = 36), weighing between 600 and 900 g, were selected from fish obtained by angling from the Eastern Harbor of Alexandria, Egypt; the experiment was carried out at the National Institute of Oceanography and Fisheries, Alexandria, Egypt, using the new hatchery and larval rearing unit [LARVANNU] and its associated laboratory. The fish were kept in 3 m3-capacity round tanks, filled with 2.5 m3-treated natural seawater (6 fish/tank), and were maintained under ambient temperature of 18–21 °C and salinity of 33.5–35 ppt. The broodstock was observed and examined regularly to ensure that they are in good health and to examine their maturation status. Fish were divided into two groups each with three replicates (6 fish/tank). For each holding tank, the sex ratio was adjusted to be two females to one male. The fish were fed twice daily with clams, small crabs, and small shrimp for 7 days a week. After 2-week acclimation period, the first group of matured fish were anesthetized in 20-ppm MS-222 (Moretti et al. 1999), and each fish was injected intraperitoneally by 1-ml ExcelMOS® (GLOBAL NUTRITECH) that had been dissolved in sterile phosphate-buffered saline (PBS) (0.009 g ExcelMOS/ml PBS/kg fish weight). The other control group received 1 ml of PBS (Cuesta et al. 2007).
Eggs and larvae collection
After spawning and fertilization, fertilized eggs were collected from both control and ExcelMOS-injected brood stocks. Half the amount of eggs were used for detection of antibodies while the rest were moved to the hatching tanks and reared until the 25th day post hatch following the method of Moretti et al. (1999). Larvae were collected at 25th day post hatch from both control and ExcelMOS-injected groups in separated sterile vials and were washed with PBS with pH 7.2 (Swain et al. 2006). They were then stored at − 20 °C till the time of analysis. The eggs (1000/ml) and larvae (n = 1000) were homogenized in PBS with pH 7.2 and the addition of protease inhibitors. The supernatants were collected after centrifugation (Swain et al. 2006). These procedures were established in chilled conditions to avoid any protease activity.
Survival rate
Samples of larvae were collected daily to calculate the survival rate percentage by the following formula:
Percent survival = (initial number of fry − number of died fry)/(initial number of fry) × 100.
Blood collection
Blood samples were collected from the caudal peduncle, from both control and ExcelMOS-injected broodstock after a week of the last injection for various hematological and immunological analyses. Serum was kept in − 20 °C for further analysis following the method of Swain et al. (2006).
Hematological parameters
All the hematological parameters were analyzed within 2 h after sampling. Hematocrit, total red blood corpuscle counts (RBC), total white blood corpuscle counts (WBC), and mean corpuscular volumes (MCV) were all tested with a Neubauer hemocytometer (Marienfeld-Superior, Lauda Königshofen, Germany) by using Natt Herrick’s method (Maqbool et al. 2014). Heparinized as well as EDTA-treated blood (50 μl) was taken in micro hematocrit capillaries and centrifuged at 12,000 rpm for 5 min to obtain hematocrit value. Blood indices including mean corpuscular volume (MCV) were calculated (Maqbool et al. 2014).
Immune parameters
Total immunoglobulin M
Serum, eggs, and larvae extracts were used to determine the total immunoglobulin M (IgM) concentration using mouse anti-gilthead seabream IgM monoclonal antibody (Aquatic Diagnostics Ltd.), according to Swain et al. (2006). Data were evaluated statistically by t test analysis. When the t test referred to statistically significant differences between groups, P ≤ 0.05.
Phagocytic activity
Leukocyte phagocytic function was determined by the method of Cai et al. (2004). The number of leukocytes that engulfed bacteria was counted as percentages in relation to total leukocyte number in the smear from the phagocytosis assay.
Leukocyte respiratory burst by nitroblue tetrazolium assay
The nitroblue tetrazolium (NBT) assay followed the protocol of Glasser and Fiederlein (1990), with some modifications. Blood smears stained with Leishman’s stain were prepared to determine percent of cells containing intracellular blue formazan particles using a light microscope (× 1000).
Lysozyme activity
Lysozyme level in blood serum was determined by turbidimetric assay according to the method described by Anderson and Siwicki (1995) using hen egg white lysozyme (Sigma, St. Louis, MO, USA) in PBS as a standard. Results were presented as lysozyme unit ml−1 (Torrecillas et al. 2011).
Complement activity
Alternative complement activity (ACH50) was evaluated following the procedure of Yano (1992) by using rabbit red blood cells (RaRBC). A lysis curve was obtained by plotting the percentage of hemolysis against the volume of serum added. The volume of serum producing 50% hemolysis (ACH50) was determined, and the number of ACH50 units/ml was obtained for each fish (Asadi et al. 2012).
Peroxidase activity
The total peroxidase content was measured according to the method used by Cuesta et al. (2007). The peroxidase activity (unit/ml plasma) was determined defining one unit of peroxidase as that which produces an absorbance change of one OD (optical density) (Asadi et al. 2012).
Histological study (transmission electron microscope)
Posterior portion of control and immunized seabream intestines was dissected out. It was then fixed in 4% glutraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4 °C for 2 to 3 h, post fixed in 1% osmium tetroxide for 1 h after washing in 5% sucrose and in 0.5 M sodium cacodylate buffer (pH 7.5) for 1 h. Tissues were dehydrated in ascending ethanol series and embedded in epoxy resin. Semi-thin sections (1 μm) were cut using a LKB ultramicrotome with a glass knife and stained with toluidine blue (Abou Shabana 2012).
Statistical analysis
Means and standard deviations (SD) were calculated for each parameter measured. Student’s t test was used to determine significant differences between control, and immunized broodstock for the parameters had been estimated by using Statistica 10, StatSoft.
Results
Survival rate
Continuous decrease in larval survival was observed from the 11th day post hatch for the control group, whereas larvae produced from ExcelMOS®-injected group showed higher survival till the end of the experiment at the 25th day (Fig. 1).
Hematological and immunological analysis
Hematological analysis
Highly significant increase in total WBC count (P ≤ 0.05) was observed in the ExcelMOS®-injected group than in control one, and no significant differences in total RBC count, MCV, and hematocrit between groups were observed (Table 1).
A large number of macrophages engulfing toxic bodies were displayed from the photomicrographs of blood films of ExcelMOS®-injected Sparus aurata broodstock, while normal neutrophils for control (Fig. 2).
Immunological analysis
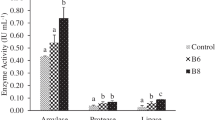
Highly significant differences were perceived in the immune potential of the treated broodstock than in control ones. Phagocytic and respiratory burst activities of blood leukocytes were highly significant (P ≤ 0.05) in ExcelMOS®-injected broodstock than in control. Consequently, the serum peroxidase percentage, serum lysozyme activity percentage, and serum natural complement activity were highly significant (P ≤ 0.05) in ExcelMOS®-injected broodstock than in control (Table 2).
The total mean concentrations of immunoglobulin in the serum of treated broodstock were higher than those in the control. Higher concentrations of IgM were detected in eggs and larvae of ExcelMOS®-treated broodstock than those in the control ones (Table 3).
Histological examination
Semi-thin sections of the posterior portion of seabream broodstock injected with ExcelMOS® showed highly organized microvilli displaying high leukocyte infiltration in mucosa lumen, in comparison to the control group (Fig. 3). The histometric measurements indicated that the ExcelMOS®-injected broodstock enterocytes were significantly greater in terms of length in comparison to the control group with significantly larger intestinal fold (Fig. 3c, d) (Table 4). Remarkable differences in intestinal microbiota were observed. Total heterotrophic aerobic bacterial and lactic acid bacteria (LAB) levels in broodstock injected with ExcelMOS® were significantly higher (P ≤ 0.05) compared to those in the control (Fig. 5). The number of acid mucin-secreting cells by 106 unit area of the posterior gut obtained was high for ExcelMOS®-injected than control broodstocks (Table 4).
Semi-thin sections in posterior intestine showing high leukocyte infiltration in the mucosa lumen of ExcelMOS®-injected Sparus aurata broodstocks (b), in comparison to control group (a). Higher intestinal fold length in ExcelMOS®-injected broodstocks (d) than control ones (c). Magnification, A&B × 100, C&D × 40
Transmission electron microscopy (TEM) sections displayed that ExcelMOS® injection increased the length of microvilli in posterior intestinal region in seabream broodstock (Fig. 4). Posterior gut of ExcelMOS®-injected broodstock (Fig. 4b) showed regular microvillar morphology with distinctly intact enterocytes and low cytoplasmic density in comparison to control group (Fig. 4a).
Lower electron density in the apical zone of the cytoplasm at which the cytoplasmic microtubules allow the identification of the tight junction, adherent junction, and the desmosomes was observed in the posterior intestine of the ExcelMOS®-injected broodstock (Fig. 4d). In the control broodstock, the enterocytes were less packed showing significant disruption in the tight junction and displaying adhering junction with clear debris (Fig. 4c).
Discussion
Recently, the aquaculture industry has faced a serious relapse due to infectious diseases that had affected fish and larvae in different developmental stages, causing huge economic loss. In all stages of life, mortalities have been recorded. However, the highest mortalities were specifically reported at larval stages in most of the cultivated fish species. Immunostimulation by prebiotics is a method conducted for enhancing disease resistance in fish (Song et al. 2014) due to deficiency in immune competence at the early life stages (Torrecillas et al. 2014).
The current study is based on the administration of ExcelMOS® (βG-MOS) through the intraperitoneal injection of Sparus aurata broodstock during the ripening stage. The present results revealed a comprehensive increase in the survival rate of the offspring when compared to the control. Some unspecified compounds of maternal origin may induce innate protection to opportunistic bacteria. The fish eggs are rich in some natural or non-specific defense mechanisms like lysosomes, lectins, and immunoglobulins that differentiate between the macrophages in the early embryonic developmental stages (Torrecillas et al. 2014). These compounds diminish with larval age (Browman et al. 2003). The current results were in accordance with those recorded by Mulero et al. (2007). During the application of βG-MOS, it enhanced and intensified the immunity of the Sparus aurata larvae that is transmitted by the mother at the early developmental stages, mainly during the period in which the larvae have gotten rid of the yolk sac (Mulero et al. 2007). This means that the mother state of health has a distinct effect on the innate defense mechanisms produced by eggs and larvae (Browman et al. 2003). Therefore, the age at which the prebiotic is administrated affects the culture of Sparus aurata (Hanif et al. 2004).
The present study showed that the immunostimulated fish displayed a significant increase in total antibodies’ concentration through indirect ELISA that was successfully transferred from mother to offspring. However, antibodies’ concentration in those offsprings is relatively lower than that in the mother. The transfer of antibodies from mother to offspring was enhanced after immunostimulating the broodstock. The current results agree with investigations on maternal transfer of immunity in many fish species such as tilapia and Atlantic salmon (Magnadottir et al. 2005). In the same context, low antibody concentrations recorded in eggs and larval homogenates were detected, when compared to the serum of the immunostimulated mothers. These results coincide with the findings on several fish species that have shown that maternal immunoglobulins might be partly responsible for the early defense mechanisms against pathogens that attack eggs. These immunoglobulins were transferred from the mother to the offspring (Mulero et al. 2007) due to the relatively late formation of autologous humoral IgM (Magnadottir et al. 2005). Furthermore, the increased antibodies in maternal circulation after immunostimulation were incorporated into vitellogenic oocytes and transferred from the larval yolk sac into the larval circulation. However, they retained the ability to bind antigens and concede relatively higher survival to the larval offspring (Hanif et al. 2005), as proved in the present study. In some species, immunostimulation did not result in protection of offspring, and therefore, the maternal IgM may be responsible of an additional role, as being a nutritional yolk protein (Magnadottir et al. 2005).
The current study showed the distinct immunostimulatory activity of ExcelMOS®, displaying elevated leukocyte count, enhancement of the complement activity, phagocytic activity, peroxidase activity, and increase in lysozyme activity. These results were in agreement to those obtained by Dawood et al. (2015) on β-glucan supplementation to Pagrus major juvenile diet as it improved various immunological parameters such as lysozyme activity, peroxidase content, and bactericidal activity.
In contrast, Siwicki and Anderson (1993) proved that levamisole has no significant effect on the hematocrit levels in the levamisole-injected fish or control, estimating that levamisole did not affect the hematocrit levels of fish.
Macrophages, granulocytes, or mononuclear phagocytes have an essential role in innate defense mechanisms in fish (Ispir and Dorucu 2005). In the current study, neutrophil killing activity, phagocytized blood leukocytes increased in ExcelMOS-injected broodstock. Similar results have also been described by Anderson and Jeney (1992). The respiratory burst activity was calculated by (NBT) assay that measures the quantity of intracellular superoxide radicals produced by leukocytes (Ardo et al. 2008). ExcelMOS®-injected broodstock displayed an increase in the respiratory burst activity of broodstock phagocytes, showing similar effect of herbal-based immunostimulants (Abasali and Mohamad 2010). Blood leukocyte production for superoxide anion was improved in Labeo rohita after feeding with Achyranthes aspera seed (Rao et al. 2006). Ardo et al. (2008) described that feeding Nile tilapia with two herbal extracts separately or in mixture significantly had improved the phagocytic and respiratory burst activities of blood phagocytic cells. Likewise, ExcelMOS® significantly increased the respiratory burst activity in injected seabream broodstock compared to controls. White blood corpuscle (WBC) count in ExcelMOS®-injected broodstock was significantly higher, compared to the control group. Similar findings were detected by Abasali and Mohamad (2010) and Sahu et al. (2007), who described that WBC count increased in fingerlings of Labia rohita fed with Mangifera indica kernel in comparison to control. Additionally, Gopalakannan and Arul (2006) stated an increase in the WBC count after feeding the carp with immunostimulant-like chitin. When a fish encounters a pathogen or any foreign body, leukocytes are activated to produce toxic radicals such as O2 and NO in order to kill that pathogen which is known as respiratory burst activity. Furthermore, at the end of this cascade, eosinophil peroxidase and myeloperoxidase (EPO and MPO, respectively) use halide ions and hydrogen peroxide to form chlorides and chloramines to aid in the fight. Both enzymes are found in phagocytic cell granules that are released by degranulation during proper activation (Cuesta et al. 2007). Consequently, peroxidases were known as good indicators for leukocyte activation as recorded in our results, showing the significant increase in the peroxidase.
Lysozymes contain an essential hydrolytic enzyme with a protein character in the innate defense system, which is present in mucus, serum, and phagocytic cells in several fish species (Abasali and Mohamad 2010). There was little evidence on the lysozyme activity of fish (Ispir and Dorucu 2005). In this study, the increase in serum lysozyme activity was detected, showing the immunostimulatory effects of ExcelMOS®. Similar to our results, synbiotics dietary supplementation improved the non-specific immune responses of the Caspian roach. Fish fed 2 g kg−1 synbiotics had significantly increased plasma lysozyme compared to control ones (Chitaz et al. 2016). The lysozyme activity in serum was found to be elevated in rainbow trout fed with natural carotenoids supplemented in diet (Amara et al. 2004). Moreover, significant increase in complement activity for ExcelMOS-injected broodstock, recorded in our study, was in agreement to what was recorded by Chitaz et al. (2016).
Many studies have shown that prebiotic applications affect and enhance gastrointestinal morphology (Torrecillas et al. 2014; Song et al. 2014). In this study, the semi-thin sections and ultra-thin sections elucidated that ExcelMOS® caused significant improvement in the posterior intestinal regions. Its application increased the absorptive surface area by promoting longer mucosal folding. Moreover, the transmission electron micrographs displayed that ExcelMOS® was able to increase microvilli length. Few data were recorded concerning the effect of β-MOS (β-glucan mannan oligosaccharide) on Sparus aurata intestinal histology. However, recent studies in other fish species have provided worthy information. In the same context, MOS (mannan oligosaccharide) supplementation showed significant increase in microvilli length in cobia larvae (Rachycentron canadum) (Salze et al. 2008). Similarly, improvements of gut morphology had been proved in swine and poultry fed dietary MOS (Castillo et al. 2008). On the other hand, Torrecillas et al. (2007) showed how the dietary administration of MOS to the European sea bass for 67 days did not affect villi length.
Additionally, other factors, such as the enhanced intestinal mucus production of fish that were fed MOS (Torrecillas et al. 2012), could partially contribute to a better gut integrity protecting enterocytes from damage and promoting microvilli regularity, as well as length (Anguiano et al. 2013). Dietary Bio-MOS supplementation at 0.4% for 8 weeks to the adult European sea bass increased gut mucosal fold surface by increasing fold length and width in anterior and posterior gut, respectively (Torrecillas et al. 2011). Up to 0.4% of Bio-MOS was supplemented to diet for gilthead seabream for 9 weeks, which resulted in an increased microvilli density and length. Gut bacterial translocation increases when the enterocytes are damaged or when loosening of their cell junctions occur (Ringo et al. 2007). The current examinations showed that junction structures between enterocytes in control broodstocks displayed higher disruption of the tight junctions as well as increased diameter of adhering junction containing unidentified structure between two neighboring intestinal epithelial cells than in immunized broodstocks. In accordance with our results, MOS supplementation to the European sea bass resulted in less disruption of tight junctions and a better-conserved intestinal barrier cyto-architecture surface (Torreciallas et al. 2012).
Dietary 0.4% Bio-MOS supplementation for 8 weeks to European sea bass increased intestinal goblet cell density (Torrecillas et al. 2011), and supplementation for 12 weeks to rainbow trout increased skin mucus weight. Thus, the reduced infection rates found in fish fed MOS could be partially related to an increased mucus production, reducing the adhesion rate of the potential pathogen to the epithelium and preventing its translocation. Indeed, 0.4% Bio-MOS supplementation for the same period of time increases gut mucus immune potential in terms of lysozyme activity in European sea bass; however, no effect was detected on skin mucus. Those findings were in agreement to our results; increased mucus production in intestine was displayed. Accordingly, the enhancement in the secretion of mucus in fish fed with MOS could be directly associated with the lower number of infection in sea bass after direct administration of bacteria in the gut, by means of the anti-adhesive characteristics of mucus (Torrecillas et al. 2007). In conclusion, immunostimulation of Sparus aurata broodstock using ExcelMOS® has improved survival of larvae and enhanced both innate and adaptive immune defense mechanisms. Further investigations are required to show the effect of ExcelMOS® on fish intensive culture systems.
References
Abasali H, Mohamad S (2010) Immune response of common carp (Cyprinus carpio) fed with herbal immunostimulants diets. J Anim Vet Adv 9:18391847
AbouShabana NM (2012) Ultrastructural study of spermatogenic stages in the protandrous sparid fish Diplodus cervinus cervinus (Lowe, 1838) from the South Eastern Mediterranean coast. Afr J Biotechnol 11:7270–7285
Amara EC, Kirona V, Satoh S, Watanabe T (2004) Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish Shellfish Immunology 16:527–537
Anderson DP, Jeney G (1992) Immunostimulants added to injected Aeromonas salmonicida bacterin enhance the defense mechanisms and protection in rainbow trout (Oncorhynchus mykiss). Vet Immunol Immunopathol 34:379–389
Anderson DP, Siwicki AK (1995) Basic haematology and serology for fish health programs. In: Shariff M, Authur JR, Subasinghe RP (eds) Diseases in Asian aquaculture II. Fish Health Section, Asian Fisheries Society, Manila, Philippines, pp 185–202
Anguiano M, Pohlenz C, Buentello A, Gatlin DM (2013). The effects of prebiotics on the digestive enzymes and gut histomorphology of red drum (Sciaenops ocellatus) and hybrid striped bass (Morone chrysops× M. saxatilis). Br. J. Nutr. 109(4):623–629
Ardo L, Yin G, Xu P, Varadi L, Szigeti G, Jeney Z, Jeney G (2008) Chinese herbs Astragalus membranaceus and Lonicera japonica and boron enhance the non-specific immune response of Nile tilapia Oreochromis niloticus and resistance against Aeromonas hydrophila. Aquaculture 275:26–33
Asadi MS, Mirvaghefei AR, Nematollahi MA, Banaee M, Ahmadi K (2012) Effects of watercress (Nasturtium nasturtium) extract on selected immunological parameters of rainbow trout (Oncorhynchus mykiss). Open Veterinary J 2:32–39
Browman H, St-Pierre JF, Skiftesvik AB, Racca RG (2003). Behaviour of Atlantic cod (Godusmorhua) larvae: an attempt to link maternal condition with larval quality. In: J. Browman& A. Skiftesvik (Ed.). The big fish bang proceedings of the 26th annual larval fish conference. Bergen, Norway, 72–94
Cai WQ, Li SF, Ma JY (2004) Diseases resistance of Nile tilapia (Oreochromis niloticus), blue tilapia (Oreochromis aureus) and their hybrid (female Nile tilapia x male blue tilapia) to Aeromonas sobria. Aquaculture 229:79–87
Castillo M, Martin-Orue SM, Taylor-Pickard JA, Perez JF, Gasa J (2008) Use of mannanoligosaccharides and zinc chelate as growth promoters and diarrhea preventative in weaning pigs: effects on microbiota and gut function. J Anim Sci 86:94–101
Cerezuela R, Guardiola FA, Meseguer J, Esteban MÁ (2012) Increases in immune parameters by inulin and Bacillus subtilis dietary administration to gilthead seabream (Sparus aurata L.) did not correlate with disease resistance to Photobacterium damselae. Fish Shellfish Immunology 32:1032–1040
Chitsaz H, Akrami R, Arab Arkadeh M (2016) Effect of dietary synbiotics on growth, immune response and body composition of Caspian roach (Rutilus rutilus). Iranian Journal Fisheries. Sciences 15:170–182
Cuesta A, Rodrı’guez A, Salinas I (2007) Early local and systemic innate immune responses in the teleost gilthead seabream after intraperitoneal injection of whole yeast cells Fish and Shellfish. Immunology 22:242–251
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S (2015) Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac Nutr 22:148–159
Dimitroglou A, Merrifield D, Carnevali O (2011) Microbial manipulations to improve fish health and production—a Mediterranean perspective. Fish and shellfish. Immunology 30:1–16
Esteban MA, Mulero V, Cuesta A, Ortuno J, Meseguer J (2000) Effects of injecting chitin particles on the innate immune response of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunology 10:543–554
FAO (2012). The state of world fisheries and aquaculture 2012. Rome 209
Glasser L, Fiederlein RL (1990) The effect of various cell separation procedures on assays of neutrophil function. Am J Clin Pathol 93:662–669
Gopalakannan A, Arul V (2006) Immunomodulatory effects of dietary intake of chitin, chitosan and levamisole on the immune system of Cyprinus carpio and control of Aeromonas hydrophila infection in ponds. Aquaculture 255:179–187
Hanif A, Bakopoulos V, Dimitriadis GJ (2004) Maternal transfer of humoral specific and non-specific immune parameters to seabream (Sparus aurata) larvae. Fish Shellfish Immunology 17:411–435
Hanif A, Bakopoulos V, Leonardos I, Dimitriadis GJ (2005) The effect of seabream (Sparus aurata) brood stock and larval vaccination on the susceptibility by Photobacterium damsel subsp. Piscicida and on the humoral immune parameters. Fish Shellfish Immunol 19:345–361
Ispir U, Dorucu M (2005) A study on the effects of levamisole on the immune system of rainbow trout (Oncorhynchus mykiss, Walbaum). Turk J Vet Anim Sci 29:1169–1176
Kara MH, Lacroix D, Rey-Valette H, Mathé S, Blancheton JP (2018). Dynamics of research in aquaculture in North Africa and support for sustainable development and innovation, Reviews in Fisheries Science & Aquaculture. ISSN: 2330–8249 (Print) 2330–8257
Karimzadeh S, Amirkolaie A, Molla A (2013) Effects of different levels of immunogen on growth performance, intestinal bacteria colonization and survival rate in Rutilus kutum larvae. World Journal of Fish and Marine. Sciences 5:664–669
Magnadottir B, Lange S, Gudmundsdottir S, Bogwald J, Dalmo RA (2005) Ontogeny of humoral immune parameters in fish. Fish Shellfish Immunology 19:429–439
Maqbool A, Ahmed I, Sheikh ZA (2014) Effects of two commonly used anticoagulants on haematology and erythrocyte morphology of rainbow trout (Oncorhynchus mykiss). Int J Fisheries Aquatic Studies 2:239–243
Maqsood S, Singh P, Samoon M, Munir K (2011) Emerging role of immunostimulants in combating the disease outbreak in aquaculture. Int Aquatic Res 3:147–163
Moretti A, Fernandez-Criado M, Cittolin G (1999) Manual on hatchery production of seabass and gilthead seabream. FAO of the United Nations 1:194
Mulero I, Garcı’a-Ayala A, Meseguer J, Mulero V (2007) Maternal transfer of immunity and ontogeny of autologous immunocompetence of fish: a mini- review. Aquaculture 268:244–250
Rao YV, Das BK, Jyotyrmayee P, Chakrabarti R (2006) Effect of Achyranthes aspera on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. Fish Shellfish Immunol 20:263–273
Ringø E, Myklebust R, Mayhew TM, Olsen RE (2007) Bacterial translocation and pathogenesis in the digestive tract of larvae and fry. Aquaculture 268:251–264
Robertsen B, Engstad RE, Jorgensen JB (1990) Enhancement of non-specific disease resistance in Atlantic salmon, Salmo salar L., by a glucan from Saccharomyces cerevisiae cell walls. J Fish Dis 13:391–400
Sahu S, Das BK, Pradhan J, Mohapatra BC, Mishra BK, Sarangi N (2007) Effect of Magnifera indica kernel as a feed additive on immunity and resistance to Aeromonas hydrophila in Labeo rohita fingerlings. Fish Shellfish Immunol 23:109–118
Salze G, McLean E, Schwarz MH, Craig SR (2008) Dietary mannan oligosaccharide enhances salinity tolerance and gut development of larval cobia. Aquaculture 274:148–152
Siwicki AK, Anderson DP (1993) Immunostimulation in fish: measuring the effects of stimulants by serological and immunological methods. US Fish Wildlife Service IFI Poland 1:24
Song S, Beck B, Kim D, Park J, Kim J, Kim H, Ringø E (2014) Prebiotics as immunostimulants in aquaculture: a review. Fish Shellfish Immunology 40:40–48
Swain P, Nayak SK (2009) Role of maternally derived immunity in fish. Fish Shellfish Immunology 27:89–99
Swain P, Dash S, Bal J, Routray P, Sahoo PK, Sahoo SK, Saurabh S, Gupta SD, Meher PK (2006) Passive transfer of maternal antibodies and their existence in eggs, larvae and fry of Indian major carp, Labeo rohita (Ham.). Fish Shellfish Immunology 20:519–527
Torrecillas S, Makol A, Caballero MJ, Montero D, Robaina L, Real F, Sweetman J, Tort L, Izquierdo MS (2007) Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunology 23:969–981
Torrecillas S, Makol A, Caballero MJ, Montero D, Robaina L, Real F, Sweetman J, Tort L, Izquierdo MS (2011) Reduced gut bacterial translocation in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS). Fish Shellfish Immunology 30:674–681
Torrecillas S, Makol A, Caballero MJ, Montero D, Robaina L, Real F, Sweetman J, Tort L, Izquierdo MS (2012) Effects on mortality and stress response in European sea bass, Dicentrarchus labrax (L.), fed mannan oligosaccharides (MOS) after Vibrio anguillarum exposure. J Fish Disease 35:591–602
Torrecillas S, Montero D, Izquierdo M (2014) Improved health and growth of fish fed mannan oligosaccharides: potential mode of action. Fish Shellfish Immunology 36:525–544
Yano T (1992). Assay of hemolytic complement activity. In, Techniques in Fish Immunology (ed.by J.S. Stolen, T.C. Fletcher, D.P. Anderson, S.C. Hattari & A.F. Rowley), 131–141. SOS Publications, Fair Haven
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
AbouShabana, N.M., AbdelKader, R., Abdel-Rahman, S. et al. Enhancement of broodstock health and maternal immunity in gilthead seabream (Sparus aurata L.) using ExcelMOS®. Fish Physiol Biochem 44, 1241–1251 (2018). https://doi.org/10.1007/s10695-018-0517-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0517-x