Abstract
To determine the effects of Roundup, a commercial formulation of glyphosate, gametes, and embryos of common carp (Cyprinus carpio L) was exposed to wide range of herbicide concentrations (0.0, 0.1, 0.5, 2.0, 5.0, 10.0, 20.0, and 50.0 mg/l). The obtained results showed different effects of Roundup on common carp gametes. Herbicide reduced swelling of eggs (but the effect was not concentration-related), while sperm showed low sensitivity to Roundup (time of spermatozoa motility was reduced in a significant way only at 20 mg/l, and at remaining concentrations, only a slight tendency was observed). During the embryonic development, Roundup caused a decrease of common carp embryonic survival (and the effect was concentration-related); however, it had no effect on development rate. During the embryogenesis, three types of embryo body malformation were observed: yolk sac edema, spine curvature, and shortening of body, but their frequencies were not associated with the presence or concentration of herbicide. However, Roundup affected quality of newly hatched larvae of common carp by increasing their mortality. No effect of herbicide on percentage of deformed larvae was observed but larvae hatched in water with Roundup tended to show more complex anomalies compared to those from the control. Obtained data showed that even low concentrations of this herbicide in waters can significantly reduce egg swelling, survival of embryos, and quality of fish larvae.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Roundup is one of the most popular non-selective herbicides containing isopropylamine salt of glyphosate (GIS), as the active ingredient, and water and polyoxyethylene amine (POEA), as the surfactant agent (Jiraungkoorskuletal 2002). It is used for weed control in agricultural, silvicultural, and urban environments (Alonzo and Correa 2008; Baylis 2000; Franz et al. 1997). The herbicidal function of glyphosate is to prevent the plant from producing aromatic amino acids (phenylalanine, tryptophan, tyrosine). Animals are unable to synthesize these amino acids (Gimsing et al. 2004; Monheit 2007), and thus glyphosate was recently considered relatively safe (Busse et al. 2001; Cox and Surgan 2006; Howe et al., 2004; Monheit 2007). However, the results of many recent studies showed toxicity of Roundup to animals from invertebrates (Folmar et al. 1979; Contardo-Jara et al. 2008) to vertebrates including human (Acquavella et al. 2004). Cavalli et al. (2013) stated that fertility in rats can be affected by Roundup and glyphosate due to the induction of death of Sertoli cells which are responsible for the maintenance of spermatogenesis. Moreover, glyphosate was reported to disrupt the steroidogenic acute regulatory (StAR) protein expression in cultured tumoral Leydig cells (Walsh et al. 2000) which resulted in reduction of steroidogenesis, eliciting the endocrine disruption role of this agrichemical. These results are in agreement with the findings that Roundup alters sperm production in rats (Romano et al. 2012) as it has been demonstrated that glyphosate and Roundup negatively affect Leydig cells, causing apoptosis in the first hours of exposure (Clair et al. 2012).
Roundup also easily reaches the aquatic ecosystems by runoff, drainage, leaching, or in advertent aerial overspray, thus it represents a dangerous and widely spread environmental contaminant (Giulherme et al. 2012). Some studies show that toxicity of Roundup to fish is caused more by POEA (Giesy et al. 2000) than by glyphosate itself. Toxic effects of the commercial formulation Roundup on aquatic organisms concern their metabolism (Glusczak et al. 2006; Glusczak et al. 2007; Costa et al. 2008; Jofre et al. 2013), growth (Tsui and Chu 2003), oxidative status and gene expression (Velasques et al. 2016), hematological parameters (Neskovic et al. 1996; Szarek et al. 2000; Cavas and Konen 2007; Modesto and Martinez 2010; Kreuz et al. 2011), histopathological changes (Ayoola 2008; Ramírez-Duarte et al. 2008; Fan et al. 2013), behavior (Nwani et al. 2013), growth, mortality, metamorphosis, and biomass (Relyea 2004, 2005). However, little information is available on sensitivity of early developmental stages of fish to this herbicide (Uren-Webster et al., 2014) or glyphosate, (Sulukan et al., 2017). According to Sancho et al. (2000), teleost fish have proved to be good models to evaluate the toxicity and effects of contaminants on animals, since their biochemical responses are similar to those of mammals and of other vertebrates. The presented data indicate that Roundup has various negative effects on living organisms, including fish.
The aim of the present study was to determine the effects of Roundup on gametes: sperm and eggs as well as on parameters of embryonic development, such as rate of development, survival, frequency of deformations, and quality of larvae of common carp (Cyprinus carpio L).
Material and methods
Sperm and egg collection and maintenance
The study was done on eggs and sperm and embryos of common carp. The eggs and sperm were obtained from the commercial hatchery of fishing farm “Samoklęski” in Kamionka, Poland. Gametes were stripped manually during artificial spawning from mature females and males. The samples of about 3 ml of eggs and 1 ml of sperm from each female or male, respectively, were placed in Eppendorf test tubes. The material was transported in a cold box (at 5 °C) to the laboratory of the Department of Animal Physiology (Siedlce University of Natural Sciences and Humanities). The eggs from three females and sperm from five (common carp) males were fertilized in about 2 h after stripping using the method described by Lugowska (2009): the eggs from all females and sperm from all males were pooled and mixed with a small amount of water (the temperature 22 °C) for 3 min.
Media preparation
Fertilized eggs were incubated in dechlorinated tap water (dissolved oxygen saturation about 80%; hardness 167 mg/dm3 as CaCO3; pH 7.8, temperature 22 °C) containing different concentrations of Roundup. The Roundup Ultra 170 SL Transorb containing 41% glyphosate acid equivalent, glyphosate concentration 170 g/l (Monsanto Europe SA/NV). Concentration range was selected based on literature data (WHO 1994; Jiraungkoorskul et al. 2002).
Sperm analysis
Motility of spermatozoa was measured using light microscope (magnification 40 × 12.5), after combining 10 μl of sperm with 10 μl Roundup solution (0.0, 0.1, 0.5, 2.0, 5.0, 10.0, 20.0, or 50.0 mg/l, as glyphosate) on a glass slide in order to activate the spermatozoa. The sperm concentration was not measured, using any specific method. Time of sperm activity was measured from the moment of activation until cessation of spermatozoa movements in three randomly chosen slide fields (five replicates for each concentration).
Egg analysis
For evaluation, the effects of Roundup on egg swelling 25 fertilized eggs were placed in Petri dishes for egg diameter measurement containing Roundup (0.0, 0.1, 0.2, 0.5, 2.0, 5.0, or 10.0 mg/l, as glyphosate). Diameters of whole egg and the yolk were measured 20, 40, 60, and 120 min after fertilization using stereoscopic microscope (magnification 1.6 × 12) to establish exact time of maximum swelling (in fisheries swelling is traditionally measured after 120 min). The percent of swelling was calculated as the following: S = (c − d) × 100/d, where S—swelling (as increase in egg diameter), c—egg diameter, and d—yolk diameter (base line). As a base yolk diameter was used, because before swelling, egg diameter is equal to the yolk diameter (there is no perivitelline space which develops and increases during swelling).
Embryo incubation
Development of common carp embryos took place in 2 l aquaria containing different Roundup concentrations (0.0, 0.1, 0.2, 0.5, 2.0, 5.0, or 10.0 mg/l, as glyphosate). Embryos in aquaria were incubated in additional glass dishes of 8 cm diameter (n = 4) in which just after fertilization were place about 45 eggs. All embryos were observed three times a day to evaluate the development. Time of achievement of each development stage was evaluated (when more than 50% of embryos in group reached particular stage), deformed embryos were counted, and cumulative percent of deformations was calculated at the stage of cleavage and organogenesis. Dead embryos (whitish opaque eggs) were counted and removed at five embryonic stages (body formation, metamere formation, development of eye, and brain germs, before hatching and at hatching) to calculate embryonic survival.
Larvae analysis
Newly hatched larvae were counted and inspected to evaluate their quality. The larvae were divided into three groups: normal (live, motile, without visible abnormalities), deformed (live, moving erroneously, showing body malformations), dead (immobile, opaque, whitish). The percentage of each group among the entire pool of hatched larvae was calculated. The deformed larvae were photographed and classified according to Jezierska et al. (2000). The number of different types of deformations in each group was counted as a percent comparing to the total number of deformed larvae in particular group (without statistical analysis).
Embryos and larvae were observed and photographed using a computer MultiScan image analysis system and stereoscopic microscope Nikon SMZ-2 T. For observation of embryos, each glass dish in which development took place was transferred to the microscope table and all embryos were inspected without any handling. Newly hatched larvae were harvested using plastic pipette with wide opening and placed separately on concave glass slide filled with small amount of adequate solution of Roundup.
Analysis of data
Normality of distribution was tested by the Shapiro-Wilk’s test and homogeneity of variance using Levene’s test. Only one parameter—egg swelling—showed normal distribution, and the results were analyzed by ANOVA, followed by Tukey’s post hoc test. For the data that did not meet the assumptions of ANOVA (motility of sperm, survival of embryos, percentage of deformed embryos, quality of newly hatched larvae), a non-parametric U Mann–Whitney test was performed. The level of significance was set at p < 0.05. Data were presented as means ± SD. Results were analyzed using STATISTICA 10 program.
Results
The effects of Roundup on gametes
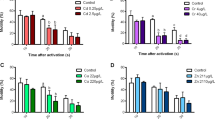
Eggs of common carp eggs in control group swelled already in 20 min from fertilization (Fig. 1a). Swelling eggs in Roundup groups did not depend on concentration. Comparing to the control result (39%) percent of swelling was significantly lower in groups R0.1, R5, and R10. Among Roundup-incubated groups, the highest swelling was observed in R0.5 and R2 (results with no significant difference to control and between groups). After 120 min (Fig. 1b), only in group R0.1 and R10, egg swelling was significantly lower (the lowest R0.1 28.8%) comparing to the control.
a Swelling of common carp eggs (Tukey’s post hoc test; different letter superscripts indicate significant differences among experimental groups): after 20 min from fertilization. b Swelling of common carp eggs (Tukey’s post hoc test; different letter superscripts indicate significant differences among experimental groups): after 120 min from fertilization
Average time of common carp sperm activity at each concentration of herbicide (about 65 s) was lower compared to the control (72.4 s), but no significant differences occurred (except for 20 mg/l—52.6 s) due to high variability (Fig. 2).
The effects of Roundup on embryonic development of common carp
The rate of embryonic development as well as start of hatching (Table 1) was similar in all experimental groups. Roundup caused elongation of hatching and in result a delay of its end (by 1–6 h).
Survival of embryos gradually decreased during the embryonic development (Fig. 3) and was significantly lower at each stage in groups exposed to Roundup compared to the control. The survival of embryos significantly decreased with increase of herbicide concentration. Hatching percent (as final survival of embryos) in control was 78.5% (the hatching success of common carp under optimum conditions is usually above 70%, Jezierska et al. 2009), whereas at 10 mg/l of Roundup was only 23%.
During the embryogenesis, three types of embryo body malformation were observed: yolk sac edema (Acquavella et al., 2004), spine curvature (Alonzo & Correa, 2008), and shortening of body (Ayoola, 2008). These deformations were observed only at 0.1 (1 and 2 in 0.16 ± 0.315% and 3 in 0.16 ± 0.515% of embryos), 0.5 (1 in 0.45 ± 0.614% and 2 in 0.48 ± 0.334% of embryos), and 10.0 mg/l (only 2 in 0.25 ± 0.5% of embryos) of Roundup, but the results did not significantly differ among the groups.
The highest percentage of newly hatched normal larvae was obtained in the control group (97%) with only small amount of deformed and dead ones (Fig. 4). In all groups exposed to Roundup percentage of normal larvae decreased, while the frequency of dead larvae significantly increased, significant effect of herbicide on percentage of deformed larvae was observed 0.1 and 5 mg/l.
In the control, deformed larvae showed only two types of malformations: spine curvatures (about 77%) and deformations of yolk sac. Similar results were observed at 0.1 mg/l of Roundup, while at higher concentration frequency and complexity of deformations increased. At 0.5 mg/l, heart edema occurred, and 2.0, 5.0, and 10.0 mg/l, heart and yolk sac edema were observed together with spine curvature or head deformations (Table 2).
Discussion
The obtained results showed that Roundup reduced swelling of common carp eggs, but the effect of Roundup was not concentration-related. At our best knowledge, there are no other data concerning the effects of Roundup on swelling of fish eggs. Therefore, the comparisons can be done only with the effects of other toxic agents (Jezierska and Bartnicka 1995; Witeska et al. 1995; Slominska 1998) which are probably connected with changes in egg shell permeability. Jezierska et al. (2009) reported similar reduction of common carp egg swelling induced by various heavy metals, even below 25%, compared to about 40% under control conditions. Alterations in egg swelling caused by Roundup might have resulted from disturbances in water uptake and ion exchange between the perivitelline fluid and external environment or changes in physical properties of the egg surface.
Motility of spermatozoa is one of the most important characteristics to be examined to evaluate sperm quality because it is a pre-requisite for fertilization (Rurangwa et al. 2004). The results of present study showed low sensitivity of common carp sperm to Roundup that reduced time of spermatozoa motility in significant way only at concentration 20 mg/l and at remaining concentrations only a slight tendency was observed. On the contrary, some authors observed more significant effects of this herbicide for spermatozoa of other fish species. Lopes et al. (2014) suggested that reduction of zebrafish spermatozoa motility can be caused by reduction of mitochondrial functionality at glyphosate or Roundup exposure. The mitochondrion is essential for energy generation during sperm movement; therefore, reduction of mitochondrial functionality may lead to a decrease in sperm motility. Peixoto (2005) explained reduction of spermatozoa mitochondrial functionality caused by Roundup as a result of disturbance of mitochondrial bioenergetics by inducing non-selective membrane permeability at the concentrations 84.535 to 845.35 mg/l.
The results of present study showed that Roundup caused a decrease of common carp embryo survival, and the effect of herbicide increased with increase of its concentration, but development rate in all groups was very similar. Similar effects were described by Uren-Webster et al. (2014) in zebrafish. For embryos exposed to ≥ 100 mg/l of Roundup or glyphosate, they found evidence of progressive delay in development and hatching with increasing concentration. These authors also observed a significant increase in mortality of zebrafish embryos exposed to concentrations ≥ 100 mg/l of glyphosate and ≥ 500 mg/l of Roundup. Yusof et al. (2014) reported that glyphosate significantly affected the survival rate of java medaka in a concentration-related way. The embryos exposed to 300, 400, or 500 mg/l of glyphosate were all dead by the end of the exposure period. As the survival of the embryos decreased, the hatching rate also decreased with the increase of herbicide concentration.
During embryonic development, three types of body malformations occurred: yolk sac edema, spine curvature, and shortening of body. Abnormalities of zebrafish embryos at ≥ 50 mg/l of glyphosate and ≥ 250 mg/l of Roundup at 24 hpf were also observed by Uren-Webster et al. (2014). Yousof et al., 2014 reported several developmental abnormalities (shrunken egg yolk, abnormal body curvature, and disproportionate head and body size) in pre-hatch Oryzias javanicus embryos exposed to different concentrations of glyphosate.
As a result of Roundup exposure in the present study, we also observed a decrease in quality of newly hatched larvae compared to the control. All groups exposed to Roundup mortality during hatching resulting in emergence of dead larvae significantly increased. Although the herbicide did not significantly affect frequency of all deformed larvae, its effect on frequency of various types of malformations was visible. Yusof et al. (2014) also reported several developmental impairments in the larvae including absence of pectoral fins and cornea, permanently bent tail, abdominal enlargement, and cell disruption in the fin, head and abdomen. According to these authors, teratogenic impairments might have developed during pre-hatch period but became noticeable post-hatch. More adverse effects were reported after exposure to higher glyphosate concentrations. Kelly et al. (2014) noticed increasing frequency of deformations in Galaxias anomalus, which developed spine malformations after exposure to glyphosate. The results of our study and reported by other authors show also that larval deformations caused by herbicide exposure were very similar to those induced by any other toxicants and/or adverse environmental factors (Jezierska et al. 2009).
Our study pointed possible effect of Roundup on common carp early development, an important species in central Europe fish farming. Obtained data showed that even low concentrations of this herbicide in waters can significantly reduce egg swelling, survival of embryos, and quality of larvae.
References
Acquavella JF, Bruce H, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, Bleeke M (2004) Glyphosate biomonitoring for farmers and their families: results from the farm family exposure study. Environ Health Perspect 112:321–326
Alonzo HGA, Correa CL (2008) In: Oga, S., Camargo, M.M.A., Batistuzzo, J.A.O. (Eds.), Praguicidas. 3aed. Atheneu Editora, São Paulo, pp. 621–642
Ayoola SO (2008) Toxicity of glyphosate herbicide on Nile tilapia (Oreochromis niloticus) juvenile. Afr J Agric Res 3(12):825–834
Baylis AD (2000) Why glyphosate is a global herbicide: strengths, weaknesses and prospects. Pest Manag Sci 56:299–308
Busse MD, Ratcliff AW, Shestak CJ, Powers RF (2001) Glyphosate toxicity and effects of long-term vegetation control on soil microbial communities. Soil Biol Biochem 33:1777–1789
Cavalli VLLO, Cattani D, Heinz Rieg CE, Pierozan P, Zanatta L, Parissoto EB, Filho DW, Mena Barreto Silva FR, Pessoa-Pureur R, Zamoner A (2013) Roundup disrupts male reproductive functions by triggering calcium-mediatedcell death in rats testis and Sertoli cells. Free Radic Biol Med 65:335–346
Cavas T, Konen S (2007) Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 22(4):263–268
Clair A, Mesnage R, Travert C, Seralini GEA (2012) Glyphosatebased herbicide induces necrosis and apoptosis in mature rat testicularncells in vitro, and estosterone decrease at lower levels. Toxicol Vitro 26(2):269–279
Contardo-Jara V, Klingelmann E, Wiegant C (2008) Bioaccumulation glyphosate and its formulation Roundup in lumbriculus variegates and its effects on biotransformation and antioxidants enzymes. Environ Pollut 157:57–63
Costa MJ, Monteiro DA, Oliveira-Neto AL, Rantin FT, Kalinin AL (2008) Oxidative stress biomarkers and heart function in bullfrog tadpoles exposed to Roundup Original®. Ecotoxicology 17(3):153–163
Cox C, Surgan M (2006) Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ Health Perspect 114:1803–1806
Fan J, Geng J, Ren H, Wang X (2013) Time-effect relationship of toxicity induced by Roundup® and its main constituents in liver of Carassius auratus. Comput Water Energy Environ Eng 2:20–25
Folmar LC, Sanders HO, Julin AM (1979) Toxicity of the herbicide glyphosate and several of its formulations to fish and aquatic invertebrates. Arch Environ Contain Toxicol 8:269–278
Franz JE, Mao MK, Sikorski JA (1997) Glyphosate: a unique global herbicide. ACS monograph 189. Am Chem Soc, Washington, DC, pp. 653
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for Roundups herbicide. Rev Environ Contam Toxicol 167:35–120
Gimsing AL, Borggaard OK, Bang M (2004) Influence of soil composition on adsorption of glyphosate and phosphate by contrasting Danish surface soils. Eur J Soil Sci 55:183–191
Glusczak L, Miron DDS, Crestani M, da Fonseca MB, Pedron FDA, Duarte MF, Vieira VUP (2006) Effect of glyphosate herbicide on acetylcholinesterase activity and metabolic and hematological parameters in piava (Leporinusobtusidens). Ecotoxicol Environ Saf 65:237–241
Glusczak L, Miron DDS, Moraes BS, Simoes RR, Schetinger MRC, Morsch VM, Loro VL (2007) Acute effects of glyphosate herbicide on metabolic and enzymatic parameters of silver catfish (Rhamdia quelen). Comp Biochem Physiol 146C:519–524
Guilherme S, Santos MA, Barroso C, Gaivao I, Pacheco M (2012) Differential genotoxicity of Roundup formulation and its constituents in blood cells of fish (Anguilla Anguilla): considerations on chemical interactions and DNA damaging mechanisms. Ecotoxicology 21(5):1381–1390
Howe CM, Berrill M, Pauli BD, Helbing CC, Werry K, Veldhoen N (2004) Toxicity of glyphosate-based pesticides to four North American frog species. Environ Toxicol Chem 23:1928–1938
Jezierska B, Bartnicka B (1995) The effect of pH on embryonic development of carp (Cyprinus carpio L.). Aquaculture 129(1–4):133–134
Jezierska B, Lugowska K, Witeska M, Sarnowski P (2000) Malformations of newly hatched common carp larvae. Electronic Journal of Polish Aquacultural Universities. Fisheries 3 (2)
Jezierska B, Lugowska K, Witeska M (2009) The effects of heavy metals on embryonic development of fish (a review). Fish Physiol Biochem 35:625–640
Jiraungkoorskul W, Upatham ES, Kruatrachue M, Sahaphong S, Vichasri-Grams S, Pokethitiyook P (2002) Histopathological effects of Roundup, a glyphosate herbicide, on Nile tilapia (Oreochromisniloticus). Sci Asia 28:121–127
Jofre DM, Germano García MJ, Salcedo R, Morales M, Alvarez M, Enriz D, Giannini F (2013) Fish toxicity of commercial herbicides formulated with glyphosate. J Environ Anal Toxicol:4–1
Kelly DW, Poulin R, Tompkins DM, Townsend CR (2014) Synergistic effects of glyphosate formulation and parasite infection on fish malformations and survival. J Appl Ecol 47:498–504
Kreutz LC, Gil Barcellos LJ, de Faria Valle S, de Oliveira Silva T, Anziliero D, Davi dos Santos E, Pivato M, Zanatta R (2011) Altered hematological and immunological parameters in silver catfish Rhamdia quelen following short term exposure to sublethal concentration of glyphosate. Fish Shellfish Immunol 30(1):51–57
Lopes FM, Varela Junior AS, Corcini CD, da Silva AC, Guazzelli VG, Tavares G, da Rosa CE (2014) Effect of glyphosate on the sperm quality of zebrafish Danio rerio. Aquat Toxicol 155:322–326
Lugowska K (2009) Embryonic development of barbel (Barbus barbus). Isr J Aquacult Bamidgeh 61:68–72
Modesto KA, Martinez CBR (2010) Effects of Roundup Transorb on fish: hemato-logy, antioxidant defenses and acetylcholinesterase activity. Chemosphere 81:781–787
Monheit S (2007) Glyphosate-based aquatic herbicides. An overview of risk. [Online]. Available: http://teamarundo.org/control manage/docs/glyphosate aqua risk. pdf
Neskovic NK, Poleksic V, Elezovic I, Karan V, Budimir M (1996) Biochemical and histopathological effects of glyphosate on carp, Cyprinus carpio L. Bull Environ Contam Toxicol 56:295–302
Nwani CD, Ibiam UA, Ibiam OU, Nworie O, Onyishi G, Atama C (2013) Investigation on acute toxicity and behavioral changes in Tilapia zilli due to glyphosate-based herbicide, forceup J. Anim. Plant Sci 23 (3)
Peixoto F (2005) Comparative effects of the Roundup and glyphosate on mitochondrial oxidative phosphorylation. Chemosphere 61:1115–1122
Ramírez-Duarte WF, Rondon-Barragan LS, Eslava-Mocha PR (2008) Acute toxicity and histopathological alterations of Roundup® herbicide on “cachama blanca” (Piaractus brachypomus). Pesq Vet Bras 28(11):547–554
Relyea RA (2004) Growth and survival of five amphibian species exposed to combinations of pesticides. Environ Toxicol Chem 23:1737–1742
Relyea RA (2005) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol Appl 15:618–627
Romano MA, Romano RM, Santos LD, Wisniewski P, Campos DA, Souza PB, Vlau P, Bernardi MM, Nunes MT, Oliveira CA (2012) Glyphosate impairs male offspring reproductive development by disrupting gonadotropin expression. Reprod Toxicol 86:663–673
Rurangwa E, Kime DE, Ollevier F, Nash JP (2004) The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture 234:1–28
Sancho E, Cerón JJ, Ferrando MD (2000) Cholinesterase activity and hematological parameters as biomarkers of sublethal molinate exposure in Anguilla anguilla. Ecotoxicol Environ Saf 46:81–86
Slominska I (1998) Sensitivity of early developmental stages of common carp (Cyprinus carpio L.) to lead and copper toxicity. Ph. D thesis, Institute of inland fisheries, Olsztyn, pp. 104 (In Polish)
Sulukan E, Kokturk M, Ceylan H, Beydemir S, Isik M, Atamanalp M, Ceyhun SB (2017) An approach to clarify the effect mechanism of glyphosate on body malformations during embryonic development of zebrafish (Danio rerio). Chemosphere 180:77–85
Szarek J, Siwicki A, Andrzejewska A, Terech-Majewska E, Banaszkiewicz T (2000) Effects of the herbicide RoundupTM on the ultrastructural pattern of hepatocytes in carp (Cyprinus carpio). Mar Environ Res 50:263–266
Tsui MTK, Chu LM (2003) Aquatic toxicity of glyphosate-based formulations: comparison between different organisms and the effects of environmental factors. Chemosphere 52:1189–1197
Uren-Webster TM, Laing LV, Florence H, Santos EM (2014) Effects of glyphosate and its formulation, Roundup, on reproduction in zebrafish (Danio rerio). Environ Sci Technol 48:1271–1279
Velasques RR, Zomer Sandrini J, da Rosa CE (2016) Roundup in zebrafish: effects on oxidative status and gene expression. Zebrafish 00:1–10
Walsh LP, McCormick C, Marin C, Stocco DM (2000) Roundup inhibits steroidogenesis by disrupting steroidogenic acute regulatory (StAR) protein expression. Environ Health Perspect 108:769–776
WHO (1994) Glyphosate. In: Environmental Health Criteria, no. 159, World Health Organization, Geneva, Switzerland, ISBN 92-4-157159-4:177
Witeska M, Jezierska B, Chaber J (1995) The influence of cadmium on common carp embryos and larvae. Aquaculture 129:129–132
Yousof S, Ahmad I, Mohamad SA (2014) Effect of glyphosate-based herbicide on early life stages of java medaka (Oryzias javanicus): a potential tropical test fish. Marine Pollution Bull 85:494–498
Funding
The present study was financed from funds provided by the Siedlce University of Natural Science and Humanities, project No 387/14/S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lugowska, K. The effects of Roundup on gametes and early development of common carp (Cyprinus carpio L). Fish Physiol Biochem 44, 1109–1117 (2018). https://doi.org/10.1007/s10695-018-0498-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0498-9