Abstract
Rainbow trout Oncorhynchus mykiss (~ 180 g, 16 °C and < 5 kg m−3) that were feed deprived and kept in total darkness showed a significant increase in critical swimming speed (U crit) between 1 and 12 days of deprivation (from 3.35 to 4.46 body length (BL) s−1) with no increase in maximum metabolic rate (MMR). They also showed a significant decrease in the estimated metabolic rate at 0 BL s−1 over 12 days which leads to a higher factorial aerobic metabolic scope at day 12 (9.38) compared to day 1 (6.54). Routine metabolic rates were also measured in ~ 90 g rainbow trout that were swimming freely in large circular respirometers at 16 °C. These showed decreasing consumption oxygen rates and reductions in the amount of oxygen consumed above standard metabolic rate (a proxy for spontaneous activity) over 12 days, though this happened significantly faster when they were kept in total darkness when compared to a 12:12-h light–dark (LD) photoperiod. Weight loss during this period was also significantly reduced in total darkness (3.33% compared to 4.98% total body weight over 12 days). Immunological assays did not reveal any consistent up- or downregulation of antipathogenic and antioxidant enzymes in the serum or skin mucus of rainbow trout between 1 and 12 days of feed and light deprivation. Overall, short periods of deprivation do not appear to significantly affect the performance of rainbow trout which appear to employ a behavioural energy-sparing strategy, albeit more so in darkness than under a 12:12-h LD regime.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Short- and long-term food deprivation is a common occurrence for both wild and cultured fishes and is often related to seasonal variations in food supply (Love 1975; Wang et al. 2006), migration (Mommsen 2004) or husbandry/slaughter practices in aquaculture (Einen et al. 1998; Álvarez et al. 2008). In the short term, food deprivation requires the utilisation of lipid and glycogen stores to satisfy the minimum energy requirements, i.e. standard metabolic rate (SMR) (Kiessling et al. 1990; Bradford 1993). Depending on species, feeding mode and habitat, it may also lead to various energy-conserving strategies such as decreases in voluntary or spontaneous activity. This has been observed in the roach Rutilus rutilus (van Dijk et al. 2002) and in the surfacing behaviour of the obligatory air-breathing channel snakehead Ophiocephalus striatus (Pandian and Vivekanandan 1976). Despite reductions in voluntary activity, fish that have been food deprived for 12–48 h have higher maximum aerobic swimming speeds (critical swimming speed, U crit) and improved swimming endurance than fed fish (Laurence 1972; Alsop and Wood 1997; Plaut 2001). This is considered to be the result of the reduction in the physiological costs of digestion and assimilation (specific dynamic action, SDA) which results in an expanded capacity for physiological work (Khan et al. 2014b). After an extended period of deprivation, fish should become limited by metabolic fuels (especially active fish) which would reduce swimming endurance or critical swimming speed.
The presence or absence of light is also known to have various effects on fish. In the literature, there has been a particular focus on the effects of photoperiod on the smolting and reproductive cycles of salmonids (Henderson 1963; Hansen et al. 1992; Solbakken et al. 1994; Fjelldal et al. 2011) and moving light stimuli on schooling behaviour and antagonistic interactions (Oppedal et al. 2001; Valdimarsson and Metcalfe 2001; Herbert et al. 2011; Oppedal et al. 2011). Oppedal et al. (2001) found that the voluntary swimming behaviour of Atlantic salmon Salmo salar decreased with light intensity and that many fish sought out low-flow zones to maintain position in the darkest treatments. This suggests that low-light levels and darkness could play a significant role in energy conservation in food-deprived fishes. However, there could be other consequences of light deprivation that have to be considered. In particular, changes in photoperiod have been shown to impact the immune response in fish, particularly components of the innate immunity (Zapata et al. 1992; Magnadóttir 2006). It has been documented that a number of defence molecules in fish, such as lysozymes, complements and peroxidases, exhibit diel variations (Ángeles Esteban et al. 2006; Binuramesh and Michael 2011; Lazado et al. 2015, 2016), indicating the strong influence of light–dark changes in the immunological status of fish. As an example, Leonardi and Klempau (2003) found that the expansion of T lymphocytes in rainbow trout Oncorhynchus mykiss was significantly suppressed after 60 days on a 24:0-h light–dark (LD) regime, likely due to a chronic stress response. A light-induced stress response was also observed in Atlantic cod Gadus morhua reared under continuous light, where higher levels of transcripts coding for antioxidant enzymes and antimicrobial peptides were identified (Giannetto et al. 2014).
As the combination of light and feed deprivation has achieved little attention in the literature, the current study aims to determine how these factors affect the physiological performance of an important salmonid, the rainbow trout. The primary hypothesis is that swimming performance (U crit) will be highest in the period shortly after feeding has ceased (~ 2–4 days) and then decrease. The current study also aims to determine if rainbow trout will decrease their metabolism and activity levels to conserve energy during periods of deprivation. This will be conducted under the hypothesis that metabolism and activity will decrease with increasing feed deprivation and that this effect will be greater in fish kept in total darkness compared to those kept under normal light regimes. Humoral and mucosal immune responses in fish exposed to short-term feed and light deprivation will also be assessed to identify the impact on the immunological status of fish under these conditions.
Materials and methods
All handling and experimental procedures were conducted in accordance with national and EU legislation (2010/63/EU) on animal experimentation.
Fish, maintenance, feed and light deprivation
Rainbow trout were obtained from Markmølle Dambrug (Nykøbing, Denmark) as smolts initially weighing ~ 50 g. Fish were quarantined for 2 weeks at 20‰ salinity, after which they were divided into 16 1.5-m3 holding tanks which were connected to a 200-m3 fresh water, recirculating aquaculture system at the DTU Aqua, North Sea Research Centre, Hirtshals, Denmark. They were held in these tanks for several months whilst all experiments were performed. The entire system was maintained at 16 ± 0.1 °C and densities never exceeded 5 kg m−3. During the holding periods, fish were fed a commercial diet (BioMar EFICO Enviro 920 3- and 4.5-mm pellets, 21.9 MJ kg−1 digestible energy) at ~ 2% of tank biomass daily (as per the recommendations of the feed manufacturer) via a 12-h belt feeder. Oxygen levels never fell below 90% saturation for any extended period.
After 2 weeks of acclimation to the holding tanks, feeding was halted in four adjacent tanks over 4 days, i.e. 1 tank day−1. At the same time that feeding was halted, the tanks were covered with black material so that the fish were in total darkness (DD). Oxygen levels and temperature remained constant for the entire deprivation period. Each tank was maintained in this way for a total of 12 days. The only disturbance to the darkness was when fish were removed for swim flume respirometry and immune function sampling which occurred after one whole day of being covered and then on days 4, 8 and 12 for each tank (see Table 1). This took no longer than 40 s, and light exposure was kept to an absolute minimum. As this produced only an n = 4 for each time point for the swim flume respirometry (1, 4, 8 and 12 days of deprivation, max. 1 fish day−1 throughput), the entire process was immediately repeated on another subset of four holding tanks to produce n = 8 for this particular experiment.
Swimming performance with progressive feed and light deprivation
Swimming performance was investigated in rainbow trout (179.61 ± 8.48 g and 226.73 ± 8.18 mm fork length, FL) which had been subjected to light and feed deprivation for either 1, 4, 8 or 12 days (n = 8 for each) to resolve the combined effect on swimming performance. The mass specific rate of oxygen consumption (\( {M}_{{\mathrm{O}}_2} \), mg O2 kg−1 h−1) was determined in a total of 32 fish over 32 days using a Loligo® Systems 30-L Steffensen-type swim flume respirometer thermostatted to 16 ± 0.1 °C with continuous water exchange. Oxygen saturation in the respirometer was measured continuously using a PreSens® Fibox 3 (PreSens, Germany) connected to an oxy-dipping probe in a position anterior to the swimming section. The oxygen meter was calibrated at the beginning of every experiment against daily atmospheric pressure. The swim flume was operated using AutoResp™ (V 2.2.0; Loligo Systems, Denmark) which controlled water speed in the swimming section and the cycling between flush, wait and measure periods (5, 1 and 4 min, respectively, a total of 10 min) and calculated \( {M}_{{\mathrm{O}}_2} \).
Weight, length, depth and width of fish [to compensate for the solid-blocking effect (Steffensen 1989)] were measured, and specimens were placed in the sealed swimming section of the respirometer (550 × 140 × 145 mm). This data was also used in the calculation of the condition factor (Froese 2006). The entire apparatus was then enclosed in curtains to minimise external disturbance. This occurred at 1600 hours and provided fish an overnight period to acclimate to the conditions of the respirometer at 0.5 body length (BL) s−1 and with the system cycling automatically through flush, wait and measure periods. From 0800 hours the following day, a critical swimming speed (U crit) test commenced where the flow speed inside the swimming section was increased by 0.25 BL s−1 every 30 min (i.e. after three 10-min flush–wait–measure cycles). Fish were considered to have stopped swimming when they rested their caudal aspect against the rear of the swimming section or erratic and non-directional burst activity was observed. Fish swimming behaviour was monitored at all times with a Logitech® C525 HD webcam on an external monitor. Background oxygen consumption levels were measured after the completion of every second trial and confirmed that bacterial respiration was nil. All equipment was cleaned thoroughly between experiments with hot, fresh water.
For each fish, critical swimming speed (U crit) was calculated according to Brett (1964). The 15% quantile method of Chabot and Claireaux (2008) and Franklin et al. (2013) was used to obtain a near-resting value of \( {M}_{{\mathrm{O}}_2} \) from overnight measures at 0.5 BL s−1 in order to remove values from periods of excessive activity. This estimate closely matched the first modal peak in \( {M}_{{\mathrm{O}}_2} \) values for the overnight 0.5 BL s−1 measurements. Thereafter, the \( {M}_{{\mathrm{O}}_2} \) values obtained from each of the 3 cycles at each speed were averaged to resolve the relationship between swimming speed and \( {M}_{{\mathrm{O}}_2} \) (Korsmeyer et al. 2002; Brown et al. 2011; Khan et al. 2014b). In order to yield an estimate of SMR for every individual fish, average \( {M}_{{\mathrm{O}}_2} \) at all speeds was fitted to an exponential function (Pettersson and Hedenström 2000; Yanase et al. 2012) and extrapolated to estimate \( {M}_{{\mathrm{O}}_2} \) at 0.0 BL s−1. Using all \( {M}_{{\mathrm{O}}_2} \) values from the point that fish first entered the respirometer, maximum metabolic rate (MMR) was calculated using the 99% quantile method of Khan et al. (2014a) as this yielded higher values with less inter-individual variation than \( {M}_{{\mathrm{O}}_2} \) values at U crit. Aerobic metabolic scope (AMS) was calculated by subtracting SMR from MMR for each individual. Factorial AMS was calculated as MMR/SMR for each individual.
Immunological function with feed and light deprivation
The effect of combined feed and light deprivation on immunological function was investigated in rainbow trout that had been subjected to light and feed deprivation for either 1, 4, 8 or 12 days (n = 9 at each, only taken from the first three tanks in the sequence, Table 1). Serum and skin mucus samples were collected at each time point. To minimise the effect of daily rhythms in immune response, sample collection was performed between 0800 and 0900 hours. Nine fish were used to represent the initial control group. They were not fed at 24 h prior to the first sample collection. In the succeeding sampling points, three fish were taken from each tank replicate. After euthanising the fish with an overdose of ethylene glycol (Merck, Darmstadt, Germany), skin mucus was obtained by placing the fish in a polyethylene plastic bag and gently rubbing in a downward motion at least ten times (Lazado et al. 2015). This was performed as gently as possible to avoid fecal contamination. Skin mucus extract was prepared by mixing the collected mucus with 1 volume of sterile phosphate-buffered saline (PBS, pH = 7.4), followed by a 2-min vigorous agitation. The suspension was centrifuged at 10,000g for 10 min at 4 °C (Sigma 3-18K, Osterode, Germany). The supernatant was collected and kept at − 80 °C until analysis. Blood was drawn from the caudal vein using a non-heparinised syringe. It was allowed to clot at room temperature (~ 18 °C) for 2 h and then at 4 °C overnight. Serum was collected after centrifugation at 750g for 10 min, divided into several aliquots and stored at − 80 °C until use. Both serum and skin mucus were analysed for all of the factors described below.
Alkaline phosphatase (ALP) activity was measured following a kinetic assay, employing p-nitrophenyl phosphate as a substrate. One unit of activity was defined as the amount of enzyme required to release 1 μmol of p-nitrophenol product in 1 min (Ross et al. 2000). Antiprotease activity was quantified by a trypsin inhibition assay. Activity values were calculated based on a 100% trypsin control (Caipang et al. 2014). p-Nitrophenyl myristate was used as a substrate in a kinetic assay to quantify esterase activity. One unit of activity was expressed the same as with ALP (Ross et al. 2000). A microplate-based turbidimetric protocol using Micrococcus luteus as the reaction substrate was followed to determine lysozyme activity. One unit of lysozyme activity was defined as the amount of enzyme that resulted in the reduction in absorbance of 0.001 min−1 (Sitjà-Bobadilla et al. 2008). Myeloperoxidase activity was measured by utilising 3,3′,5, 5′-tetramethyl-benzidine as a chromogenic substrate. Activity value was expressed as absorbance at 450 nm (Caipang et al. 2009). Colorimetric determination of antioxidant enzyme activities was performed using commercially available kits (Cayman, MI, USA) that were previously standardised for fish samples (Lazado et al. 2015). Glutathione peroxidase activity was measured indirectly by a coupled reaction with glutathione reductase. Catalase activity was quantified based on the reaction of enzyme with methanol in the presence of an optimal concentration of H2O2. Superoxide dismutase activity used tetrazolium salt for the detection of superoxide radicals generated by xanthine oxidase and hypoxanthine.
Oxygen consumption and activity with feed and light deprivation
The effect of combined feed and light deprivation on oxygen consumption and estimated routine and spontaneous activity was investigated in rainbow trout over 12 days. Fish from the holding tanks, with no previous exposure to extended darkness or feed deprivation (92.28 ± 4.23 g and 201.88 ± 2.51 mm FL), were food deprived for 24 h prior to experimentation. \( {M}_{{\mathrm{O}}_2} \) was measured in 16 fish (eight in DD and eight in a 12:12-h LD cycle) at 16 °C. Measurements were carried out using two automated intermittent flow respirometers similar to the methodologies outlined by Steffensen (1989), Jordan and Steffensen (2007) and Khan et al. (2014a). Two custom-built 13.01-L acrylic, disc-shaped respirometers (with an Eheim® 1046 submersible flush pump on the one side and an outlet tube extending above the water on the opposite side) were placed in a 250-L reservoir filled with fresh water. The reservoir was supplied with vigorously aerated water from a 600-L system previously described by Frisk et al. (2012) with a water replacement rate of 50 L h−1. Water circulation within the respirometers was provided by an external loop of tubing also containing an Eheim® 1046 submersible pump and a cuvette for the fibre optic oxygen probes which were plumbed perpendicular to the flush pump and outlets. The constant action of the recirculation pumps created a water speed of approximately 0.13 m s−1 in the centre of each respirometer. The large size and shape of the respirometers were selected to allow the fish to be spontaneously active rather than confine them.
Oxygen saturation in the respirometer was measured constantly by a PreSens® OXY-4 oxygen meter (PreSens, Germany) connected to a fibre optic dipping probe in the recirculating loop of each chamber. Oxygen saturation was measured constantly across three cycled phases (flushing, wait and measure) that were repeated every 12 min. The dipping probes were calibrated at the beginning of every 12-day experiment.
Fish were weighed in water, and their FL was recorded immediately prior to the start of the experiment. \( {M}_{{\mathrm{O}}_2} \) values were then continually obtained from resting fish over the next 720 h (12 days) either in DD or in an ambient 12:12-h LD cycle (i.e. one fish per respirometer, two respirometers operating under the same light conditions). Fish activity was constantly observed and recorded using infrared cameras. The fish were then reweighed in water at the end of the experiment to determine weight loss and calculate % body weight loss and specific growth rate (SGR) using the following formula: SGR = lnm 2 − lnm 1 / t 2 − t 1 × 100, where m 1 is the initial weight at the start of the growth period (t 1) and m 2 is the final weight at the end of the growth period (t 2). For every day the fish were in the respirometer, SMR was estimated modally as the first peak in a frequency distribution using 5 mg O2 kg−1 h−1 bins for each individual. After each experiment was complete, background oxygen consumption readings were taken to ensure that it remained negligible. All equipment was cleaned thoroughly between specimens with hot, fresh water. Routine and spontaneous activity was defined as any \( {M}_{{\mathrm{O}}_2} \) above that individual’s daily estimate of SMR and was expressed as the amount of oxygen (g) consumed in measurements of \( {M}_{{\mathrm{O}}_2} \) above SMR. As \( {M}_{{\mathrm{O}}_2} \) is a function of both the fish’s mass and the net volume of the respirometer [see Steffensen (1989)], estimates of \( {M}_{{\mathrm{O}}_2} \) are ultimately affected by the change in both of these variables over a 12-day period which could introduce a possible source of error. However, the exact body mass at different time points was not known. Loss in body mass ranged from 2.7 to 8.9%, but because weight loss during food deprivation occurs in a non-linear manner, no specific corrections were made.
Statistical analyses
The relationship between days of feed and light deprivation and each of U crit, \( {M}_{{\mathrm{O}}_2} \) at 0.0/1.1/2.3 BL s−1 and factorial AMS was analysed using a one-way analysis of variance (ANOVA). Where a significant effect was observed, a post hoc Holm–Sidak pairwise comparison was applied. Condition factor at different days of deprivation was analysed with a non-parametric Kruskal–Wallis one-way ANOVA on ranks.
Differences between time points in the immunological studies were determined by one-way ANOVAs. When the data did not follow the Gaussian distribution, values were log10 transformed before performing a parametric one-way ANOVA followed by Tukey’s multiple comparison test. For non-parametric data, a Kruskal–Wallis ANOVA on ranks with Dunn’s multiple comparison test was used. To provide a pictographic representation of fish immune response following light and feed deprivation, a heatmap was constructed using PermutMatrix, version 1.9.3 (Caraux and Pinloche 2005). Activity values for each immune parameter were expressed as the relative response against the initial 1 day of deprivation sample. Thereafter, the values were normalised by expressing them relative to the maximum value of a data set. A 256-color-level spectrum with yellow representing negative values whilst blue corresponding to positive values was adopted.
The \( {M}_{{\mathrm{O}}_2} \) data from each fish was pooled (within light and days of deprivation groups) to produce frequency distributions with 5 mg O2 kg−1 h−1 bins and representative peaks in distribution. The normality of these \( {M}_{{\mathrm{O}}_2} \) distributions was tested individually using the Kolmogorov–Smirnov test. SMR was analysed with a non-parametric Kruskal–Wallis one-way ANOVA on ranks as the data was not normally distributed. The grams of O2 consumed above SMR was analysed with one-way ANOVAs within deprivation treatments (1, 4, 8 and 12 days) and within light treatments (DD and LD). Where significant effects were found between days of deprivation in light treatments, a post hoc Holm–Sidak pairwise comparison was applied. A two-way ANOVA was also applied to both variables with light treatment and days of deprivation as factors. SGR and percentage body weight (%BW) lost were analysed using a one-way ANOVA. Where parametric analyses were applied, the data was first checked for equal variance and normality. Statistical analyses were performed on Sigmastat, V 13.0 (Systat Software, Inc., London, UK). Significance was accepted at P ≤ 0.05.
Results
Swimming performance with progressive feed and light deprivation
U crit increased significantly from 3.35 to 4.46 BL s−1 (F = 3522) between 1 and 12 days of deprivation (Fig. 1a). The estimate of \( {M}_{{\mathrm{O}}_2} \) at 0.0 BL s−1 decreased significantly from 123.71 to 85.06 mg O2 kg−1 h−1 (F = 6586,) between 1 and 12 days of deprivation (Fig. 1b). Condition did not change significantly over 12 days of deprivation (H = 4.48).
The swimming performance of rainbow trout that have been deprived of feed and light for either 1, 4, 8 or 12 days. a Critical swimming speed (U crit) in body lengths per second (BL s−1), y = 0.004x 2 + 0.052x + 3.28, R 2 = 0.99. b Estimated mass-specific rate of oxygen consumption (\( {M}_{{\mathrm{O}}_2} \), mg O2 kg−1 h−1) at 0.0 BL s−1 from extrapolation, y = 0.046x 2 − 4.10x + 127.73, R 2 = 0.99. c Factorial aerobic metabolic scope (the 99th percentile of all \( {M}_{{\mathrm{O}}_2} \) values for each individual, i.e. MMR divided by the 0.0 BL s−1 estimate, i.e. SMR), y = 0.289x + 5.908, R 2 = 0.91. Letters denote a significant difference between deprivation treatments. All values are shown ± standard error
Factorial metabolic scope increased significantly between 4 and 12 days of deprivation (F = 4.52, Fig. 1c).
Immunological function with feed and light deprivation
ALP activity was significantly downregulated at days 8 and 12 of deprivation compared to day 1 in the serum, but there was no significant effect observed in the skin mucus (Fig. 2). Esterase and lysozyme activity was significantly upregulated at days 8 and 12 in the serum compared to day 1. No significant change was observed in skin mucus.
Serum humoral and skin mucosal immune responses of rainbow trout exposed to short-term light and feed deprivation. The heatmap represents the relative response of several defence molecules in serum and skin mucus. Relative response values are expressed as ratios between the activity value at a specific sampling point and the initial control. Horizontal panel of the array displays the normalised relative response values of the defence molecules at a specific time point (i.e. 4, 8 and 12 days post initiation). Spectral panels enclosed in red box signify that the response was significantly different (P < 0.05) from the initial control group. The color scale below indicates the mode of response relative to initial control: yellow represents negative whilst blue reveals positive response
Of the antioxidant enzymes investigated (i.e. myeloperoxidase, catalase, superoxide dismutase and glutathione peroxidase), only glutathione peroxidase was significantly upregulated in both the serum and skin mucus, both at day 12 (Fig. 2). Myeloperoxidase was only significantly upregulated at day 8 in skin mucus, and superoxide dismutase was only significantly downregulated at day 12 in serum.
Oxygen consumption and activity with feed and light deprivation
For both the DD (Fig. 3a–d) and the LD treatments (Fig. 3e–h), oxygen consumption becomes increasingly unimodal as the days of deprivation increase. The modal peaks in the \( {M}_{{\mathrm{O}}_2} \) distributions also decrease with the number of days of deprivation with the exception of days 8 and 12 in the DD treatment (Fig. 3c, d, respectively) where the peak occurs in the same 75–80 mg O2 kg−1 h−1 bin.
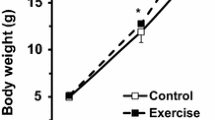
Weight loss over 12 days of deprivation, as measured by SGR and %BW loss, was significantly higher in the LD treatment than in the DD treatment. SGR was − 0.43% BW day−1 in the LD treatment and − 0.35% BW day−1 in the DD treatment (F = 12.66). %BW lost was 4.99% in the LD treatment and 3.33% in the DD treatment (F = 13.06).
SMR decreased significantly between 1 and 12 days of deprivation for both the DD and the LD treatments (Fig. 4a, H = 19.57 and H = 27.35, respectively). There was no significant difference in the SMR between the DD and LD treatments at any of 1, 4, 8 or 12 days of deprivation. A two-way ANOVA revealed that the light treatment had no significant effect on the relationship between SMR and days of deprivation (F = 0.09) and that there was no significant interaction between days of deprivation and the light regime (F = 3.35).
a SMR and b total oxygen consumed above SMR (in grams, a proxy for energy expenditure above maintenance costs) in rainbow trout deprived of feed and kept in either total darkness (DD, filled circles) or 12:12 h LD (open circles) for a total of 12 days (values show are at days 1, 4, 8 and 12). An asterisk denotes a significant difference between light regimes at a particular time point. Letters denote a significant difference between time points within the light treatment. All values are shown ± standard error
The grams of O2 consumed above SMR also decreased significantly between 1 and 12 days of deprivation for both the DD and LD treatments (Fig. 4b, F = 11.04 and F = 9.24). At day 1 of deprivation, the fish in DD consumed significantly less oxygen above SMR on average than those in the LD treatment (F = 4.70). At days 4, 8 and 12, there was no significant difference between the DD and LD treatments. A two-way ANOVA revealed that the light treatment had no significant effect on the relationship between grams of O2 consumed above SMR and days of deprivation (F = 0.17). There was, however, a significant interaction effect between days of deprivation and the light regime (F = 3.84).
Discussion
The hypothesis of the current study was based on the assumption that swimming performance would improve as the physiological costs of SDA are reduced and eventually ablated but then decrease as metabolic fuel stores are depleted. This was not the case as swimming performance continued to increase over the 12-day period (U crit, Fig. 1a). The few examples of swimming performance in food-deprived fish have shown a considerable decrease in performance with food deprivation, though after longer periods. For example, Martínez et al. (2003) demonstrated that Atlantic cod Gadus morhua which had been food deprived for 16 weeks had 30% of the swimming endurance (i.e. time/distance swum at 1.8 BL s−1) of fish that had been fed twice weekly for the same period. The same authors also reported a significant decrease in the glycogen content of both red and white muscles. In a later study, Martínez et al. (2004) also determined that short-distance sprint speeds were also significantly decreased in 12-week starved Atlantic cod compared to fed conspecifics. In both studies, there was an obvious preference to retain red muscle oxidative capacity over white muscle glycolytic capacity which has also been seen previously in rainbow trout (Kiessling et al. 1990) and the crucian carp Carassius carassius (Patterson and Goldspink 1973). This suggests that swimming performance at high speeds, where white muscle fibres are recruited, would be reduced before performance at low speeds, though this is not seen between 1 and 12 days of deprivation in the current study. Lapointe et al. (2006) observed reduced swimming performance in food-deprived Atlantic cod, but with the addition of a significantly higher estimate of MMR, AMS and post-exhaustive \( {M}_{{\mathrm{O}}_2} \). Lapointe et al. (2006) observed an increase in AMS but did not observe the decrease in SMR observed in the current study (Fig. 1b). Rainbow trout appear to be able to improve performance at high speed without any reductions in performance across their entire swimming speed range.
Information on the direct effects of photoperiod on swimming performance is sparse. Juvenile largemouth bass Micropterus salmoides have a significantly higher U crit when reared at shorter photoperiods (9:15 h LD), but only at lower temperatures (Kolok 1991). In white crappie Pomoxis annularis, swimming performance was highest at a shorter photoperiod (8:16 h LD) than at all longer photoperiods, as well as DD, independent of temperature (Smiley and Parsons 1997). Although neither of these studies elucidated the exact mechanism, it appears that shorter photoperiods may increase swimming performance.
It is likely that the major mechanism driving the increase in swimming performance is that the short feed deprivation period reduces some of the physiological load that would otherwise reduce the aerobic scope for performance (Khan et al. 2014b). Following a few days of fasting, the energetic costs associated with specific dynamic action [i.e. digestion and assimilation of nutrients, SDA (Jobling 1983; Frisk et al. 2013; Li et al. 2013) and de novo protein synthesis for growth (Coulson et al. 1978; Houlihan et al. 1995; Smith and Houlihan 1995)] are nearly non-existent and that a larger proportion of AMS is available for swimming (Beauchamp et al. 1989; Jobling 1993; Khan et al. 2015; Auer et al. 2016). It is known that rainbow trout fed to satiation have significantly reduced U crit values compared to those that have been deprived of their morning ration (Alsop and Wood 1997). It has also been shown that the duration of the SDA response in rainbow trout can range from 40 to 73 h depending on the individual, ration size and diet composition (LeGrow and Beamish 1986; Secor 2009). It is reasonable to assume that estimations of U crit and swimming performance would increase over this period as less metabolic capacity is being allocated to protein synthesis and growth. A reduction in energetic costs associated with SDA might also explain the significant decrease in the estimate of \( {M}_{{\mathrm{O}}_2} \) at 0.0 BL s−1 between 1 and 12 days of deprivation (Fig. 1b) as rates of protein turnover would decrease to a minimum. Fractional rates of protein synthesis in rainbow trout have been shown to decrease significantly after 14 days of feed deprivation [~ 70% in white muscle and ~ 49% in red muscle (Loughna and Goldspink 1984)] which may account for some of the ~ 30% decrease in \( {M}_{{\mathrm{O}}_2} \) at 0.0 BL s−1 between 1 and 12 days of deprivation (Fig. 1b). Considering that growth and rates of protein accretion are also positively related to photoperiod in fish (Boeuf and Le Bail 1999; Kissil et al. 2001), DD may also reduce the physiological load of protein synthesis.
Several defence molecules responded to feed and light deprivation, though the collective effect was minimal (Fig. 2). The humoral components were more affected than their mucosal counterparts as marked changes were observed more discernibly in serum than in the skin mucus. A decrease in ALP activity in the first 8 days of deprivation (Fig. 2) may pose an immunological threat to fish as it can lead to an increase in phosphorylated proteins (Xu et al. 2000; Molina et al. 2005), though it did return to basal levels after 12 days. Feed and light deprivation triggered significant elevation of the antibacterial defences, lysozyme and esterase. Lysozyme activity is thought to increase during exposure to longer photoperiods in rainbow trout and other fish (Pickering and Pottinger 1989; Ángeles Esteban et al. 2006; Uribe et al. 2011), but it is suppressed at 24:0 h LD, likely due to chronic stress (Leonardi and Klempau 2003). There was a significant increase in serum levels despite the lack of light, suggesting that short-term feed deprivation may play a significant role on its own. The varied response of antioxidant enzymes is consistent with measurements in Atlantic salmon and rainbow trout where antioxidant capacity was measured in response to vitamin supplementation (Andersen et al. 1998; Kiron et al. 2004). Given the minimal changes revealed by the selected biomarkers following deprivation, it is plausible that the imposed conditions did not have far-reaching impacts in the immunological status of fish. The changes observed in the present study may also be a general adaptive response to cope with changes in photoperiod, similar to the responses of Atlantic cod (Giannetto et al. 2014) and Nile tilapia Oreochromis niloticus (Lazado et al. 2016), when exposed to constant illumination.
Fish are able to conserve energy during food deprivation through biochemical, physiological and behavioural adaptations which could include reductions in voluntary activity. The current study has demonstrated that there is a significant reduction in energy use between 1 and 12 days of feed and light deprivation. Furthermore, there is a significant difference in the degree of energy conservation between rainbow trout that have been deprived of feed and light for 12 days and those that have been deprived of feed but kept on a LD cycle. This agrees with the hypothesis and is most evident from the significant difference in weight loss between the two light regimes. The simplest explanation for this is that voluntary/spontaneous activity levels were reduced in the fish that were kept in DD which conserved energy stores and body mass. \( {M}_{{\mathrm{O}}_2} \) frequency distributions also become unimodal more quickly in the fish exposed to total darkness compared to those who were exposed to a LD regime (day 4, Fig. 3b, f). The distributions on days 8 and 12 also appear to have less pronounced “tails” into higher \( {M}_{{\mathrm{O}}_2} \) values in the fish exposed to DD, suggesting lower levels of spontaneous activity and energy consumption (Fig. 3c, d, g, h).
The absolute amount of oxygen consumed above SMR provides a measure of energy expenditure above the estimated minimum requirements, accounting for all voluntary and spontaneous activities (Fig. 4b). As expected, this value decreases significantly between 1 and 12 days of deprivation for both light regimes (Pandian and Vivekanandan 1976; van Dijk et al. 2002) and is what would be expected during confinement within a (large) respirometer (Steffensen 1989; Clark et al. 2013). SMR also decreases significantly over the 12 days of deprivation, as expected (Fig. 4a). On day 1 however, spontaneous activity is significantly lower in fish that were exposed to DD than those exposed to light, suggesting that darkness reduces activity quicker. The bimodal distribution of Fig. 3b may also be displaying a higher level of routine and spontaneous activity in fish exposed to light than the unimodal distribution of the fish exposed to DD after 4 days (Fig. 3f). Previous studies have shown that the highest proportional loss in body weight occurs in the first few days of food deprivation in fish (Einen et al. 1998), and the current data supports this. Changes in energy expenditure during this initial 2–3-day period may have important consequences as to how long the deprivation can be tolerated.
The current study has revealed that rainbow trout are not adversely affected by up to 12 days of feed and light deprivation and that their immunological states are not compromised. It also shows that weight loss is reduced as a result of reduced light exposure. In periods of controlled feed deprivation in aquaculture (e.g. prophylactic/disease treatment and preparation for slaughter), darkness may be an effective tool to limit weight and condition loss though reduced activity. Improved swimming performance over 12 days of deprivation is an interesting result and begs the question: When will U crit decrease due to metabolic fuel limitation? Potentially, improved performance with short-term food deprivation may have adaptive benefits in terms of securing prey items when food is scarce.
References
Alsop D, Wood C (1997) The interactive effects of feeding and exercise on oxygen consumption, swimming performance and protein usage in juvenile rainbow trout (Oncorhynchus mykiss). J Exp Biol 200:2337–2346
Álvarez A, García BG, Garrido M, Hernández M (2008) The influence of starvation time prior to slaughter on the quality of commercial-sized gilthead seabream (Sparus aurata) during ice storage. Aquaculture 284:106–114
Andersen F, Lygren B, Maage A, Waagbø R (1998) Interaction between two dietary levels of iron and two forms of ascorbic acid and the effect on growth, antioxidant status and some non-specific immune parameters in Atlantic salmon (Salmo salar) smolts. Aquaculture 161:437–451
Ángeles Esteban M, Cuesta A, Rodríguez A, Meseguer J (2006) Effect of photoperiod on the fish innate immune system: a link between fish pineal gland and the immune system. J Pineal Res 41:261–266
Auer SK, Salin K, Rudolf AM, Anderson GJ, Metcalfe NB (2016) Differential effects of food availability on minimum and maximum rates of metabolism. Biol Lett 12:20160586
Beauchamp DA, Stewart DJ, Thomas GL (1989) Corroboration of a bioenergetic model for sockeye salmon. Trans Am Fish Soc 118:597–607
Binuramesh C, Michael RD (2011) Diel variations in the selected serum immune parameters in Oreochromis mossambicus. Fish & Shellfish Immunol 30:824–829
Boeuf G, Le Bail P-Y (1999) Does light have an influence on fish growth? Aquaculture 177:129–152
Bradford R (1993) Differential utilization of storage lipids and storage proteins by Northwest Atlantic herring (Clupea harengus harengus). J Fish Biol 43:811–824
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fisheries Board Can 21:1183–1226
Brown E, Bruce M, Pether S, Herbert N (2011) Do swimming fish always grow fast? Investigating the magnitude and physiological basis of exercise-induced growth in juvenile New Zealand yellowtail kingfish, Seriola lalandi. Fish Physiol Biochem 37:327–336
Caipang CMA, Berg I, Brinchmann MF, Kiron V (2009) Short-term crowding stress in Atlantic cod, Gadus morhua L. modulates the humoral immune response. Aquaculture 295:110–115
Caipang CMA, Fatira E, Lazado CC, Pavlidis M (2014) Short-term handling stress affects the humoral immune responses of juvenile Atlantic cod, Gadus morhua. Aquac Int 22:1283–1293
Caraux G, Pinloche S (2005) PermutMatrix: a graphical environment to arrange gene expression profiles in optimal linear order. Bioinformatics 21:1280–1281
Chabot D, Claireaux G (2008) Environmental hypoxia as a metabolic constraint on fish: the case of the Atlantic cod Gadus morhua. Mar Pollut Bull 57:287–294
Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216:2771–2782
Coulson RA, Herbert JD, Thomas H (1978) Energy for amino acid absorption, transport and protein synthesis in vivo. Comp Biochem Physiol A 60:13–20
Einen O, Waagan B, Thomassen MS (1998) Starvation prior to slaughter in Atlantic salmon (Salmo salar): I. Effects on weight loss, body shape, slaughter- and fillet-yield, proximate and fatty acid composition. Aquaculture 166:85–104
Fjelldal PG, Hansen T, Huang T-s (2011) Continuous light and elevated temperature can trigger maturation both during and immediately after smoltification in male Atlantic salmon (Salmo salar). Aquaculture 321:93–100
Franklin CE, Farrell AP, Altimiras J, Axelsson M (2013) Thermal dependence of cardiac function in arctic fish: implications of a warming world. J Exp Biol 216:4251–4255
Frisk M, Skov PV, Steffensen JF (2012) Thermal optimum for pikeperch (Sander lucioperca) and the use of ventilation frequency as a predictor of metabolic rate. Aquaculture 324–325:151–157
Frisk M, Steffensen JF, Skov PV (2013) The effects of temperature on specific dynamic action and ammonia excretion in pikeperch (Sander lucioperca). Aquaculture 404–405:65–70
Froese R (2006) Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22:241–253
Giannetto A, Fernandes JMO, Nagasawa K, Mauceri A, Maisano M, De Domenico E, Cappello T, Oliva S, Fasulo S (2014) Influence of continuous light treatment on expression of stress biomarkers in Atlantic cod. Dev Comp Immunol 44:30–34
Hansen T, Stefansson S, Taranger G (1992) Growth and sexual maturation in Atlantic salmon, Salmo salar L., reared in sea cages at two different light regimes. Aquac Res 23:275–280
Henderson NE (1963) Influence of light and temperature on the reproductive cycle of the eastern brook trout, Salvelinus fontinalis (Mitchill). J Fisheries Board Can 20:859–897
Herbert NA, Kadri S, Huntingford FA (2011) A moving light stimulus elicits a sustained swimming response in farmed Atlantic salmon, Salmo salar L. Fish Physiol Biochem 37:317–325
Houlihan DF, Carter CG, McCarthy ID (1995) Protein synthesis in fish. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes. Elsevier, London, pp 191–220
Jobling M (1983) Towards an explanation of specific dynamic action (SDA). J Fish Biol 23:549–555
Jobling M (1993) Bioenergetics: feed intake and energy partitioning. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Springer, London, pp 1–44
Jordan AD, Steffensen JF (2007) Effects of ration size and hypoxia on specific dynamic action in the cod. Physiol Biochem Zool 80:178–185
Khan JR, Pether S, Bruce M, Walker SP, Herbert NA (2014a) Optimum temperatures for growth and feed conversion in cultured hapuku (Polyprion oxygeneios)—is there a link to aerobic metabolic scope and final temperature preference? Aquaculture 430:107–113
Khan JR, Pether S, Bruce M, Walker SP, Herbert NA (2015) The effect of temperature and ration size on specific dynamic action and production performance in juvenile hapuku (Polyprion oxygeneios). Aquaculture 437:67–74
Khan JR, Trembath C, Pether S, Bruce M, Walker SP, Herbert NA (2014b) Accommodating the cost of growth and swimming in fish—the applicability of exercise-induced growth to juvenile hapuku (Polyprion oxygeneios). Front Physiol 5:1–9
Kiessling A, Johansson L, Kiessling K-H (1990) Effects of starvation on rainbow trout muscle: I. Histochemistry, metabolism and composition of white and red muscle in mature and immature fish. Acta Agric Scand 40:309–324
Kiron V, Puangkaew J, Ishizaka K, Satoh S, Watanabe T (2004) Antioxidant status and nonspecific immune responses in rainbow trout (Oncorhynchus mykiss) fed two levels of vitamin E along with three lipid sources. Aquaculture 234:361–379
Kissil GW, Lupatsch I, Elizur A, Zohar Y (2001) Long photoperiod delayed spawning and increased somatic growth in gilthead seabream (Sparus aurata). Aquaculture 200:363–379
Kolok AS (1991) Photoperiod alters the critical swimming speed of juvenile largemouth bass, Micropterus salmoides, acclimated to cold water. Copeia 1991:1085–1090
Korsmeyer KE, Steffensen JF, Herskin J (2002) Energetics of median and paired fin swimming, body and caudal fin swimming, and gait transition in parrotfish (Scarus schlegeli) and triggerfish (Rhinecanthus aculeatus). J Exp Biol 205:1253–1263
Lapointe D, Guderley H, Dutil JD (2006) Changes in the condition factor have an impact on metabolic rate and swimming performance relationships in Atlantic cod (Gadus morhua L.) Physiol Biochem Zool 79:109–119
Laurence GC (1972) Comparative swimming abilities of fed and starved larval largemouth bass (Micropterus salmoides). J Fish Biol 4:73–78
Lazado CC, Lund I, Pedersen PB, Nguyen HQ (2015) Humoral and mucosal defense molecules rhythmically oscillate during a light–dark cycle in permit, Trachinotus falcatus. Fish & Shellfish Immunol 47:902–912
Lazado CC, Skov PV, Pedersen PB (2016) Innate immune defenses exhibit circadian rhythmicity and differential temporal sensitivity to a bacterial endotoxin in Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunol 55:613–622
LeGrow SM, Beamish F (1986) Influence of dietary protein and lipid on apparent heat increment of rainbow trout, Salmo gairdneri. Can J Fish Aquat Sci 43:19–25
Leonardi M, Klempau A (2003) Artificial photoperiod influence on the immune system of juvenile rainbow trout (Oncorhynchus mykiss) in the Southern Hemisphere. Aquaculture 221:581–591
Li X-M, Yu L-J, Wang C, Zeng L-Q, Cao Z-D, Fu S-J, Zhang Y-G (2013) The effect of aerobic exercise training on growth performance, digestive enzyme activities and postprandial metabolic response in juvenile qingbo (Spinibarbus sinensis). Comp Biochem Physiol A 166:8–16
Loughna PT, Goldspink G (1984) The effects of starvation upon protein turnover in red and white myotomal muscle of rainbow trout, Salmo gairdneri Richardson. J Fish Biol 25:223–230
Love RM (1975) Variability in Atlantic cod (Gadus morhua) from the Northeast Atlantic: a review of seasonal and environmental influences on various attributes of the flesh. J Fisheries Board Can 32:2333–2342
Magnadóttir B (2006) Innate immunity of fish (overview). Fish & Shellfish Immunol 20:137–151
Martínez M, Bédard M, Dutil J-D, Guderley H (2004) Does condition of Atlantic cod (Gadus morhua) have a greater impact upon swimming performance at U crit or sprint speeds? J Exp Biol 207:2979–2990
Martínez M, Guderley H, Dutil J-D, Winger PD, He P, Walsh SJ (2003) Condition, prolonged swimming performance and muscle metabolic capacities of cod Gadus morhua. J Exp Biol 206:503–511
Molina R, Moreno I, Pichardo S, Jos A, Moyano R, Monterde JG, Cameán A (2005) Acid and alkaline phosphatase activities and pathological changes induced in tilapia fish (Oreochromis sp.) exposed subchronically to microcystins from toxic cyanobacterial blooms under laboratory conditions. Toxicon 46:725–735
Mommsen TP (2004) Salmon spawning migration and muscle protein metabolism: the August Krogh principle at work. Comp Biochem Physiol B 139:383–400
Oppedal F, Dempster T, Stien LH (2011) Environmental drivers of Atlantic salmon behaviour in sea-cages: a review. Aquaculture 311:1–18
Oppedal F, Juell JE, Tarranger G, Hansen T (2001) Artificial light and season affects vertical distribution and swimming behaviour of post-smolt Atlantic salmon in sea cages. J Fish Biol 58:1570–1584
Pandian TJ, Vivekanandan E (1976) Effects of feeding and starvation on growth and swimming activity in an obligatory air-breathing fish. Hydrobiologia 49:33–39
Patterson S, Goldspink G (1973) The effect of starvation on the ultrastructure of the red and white myotomal muscles of the crucian carp (Carassius carassius). Z Zellforsch Mikrosk Anat 146:375–384
Pettersson LB, Hedenström A (2000) Energetics, cost reduction and functional consequences of fish morphology. Proc R Soc Lond B 267:759–764
Pickering AD, Pottinger TG (1989) Stress responses and disease resistance in salmonid fish: effects of chronic elevation of plasma cortisol. Fish Physiol Biochem 7:253–258
Plaut I (2001) Critical swimming speed: its ecological relevance. Comp Biochem Physiol A 131:41–50
Ross NW, Firth KJ, Wang A, Burka JF, Johnson SC (2000) Changes in hydrolytic enzyme activities of naive Atlantic salmon Salmo salar skin mucus due to infection with the salmon louse Lepeophtheirus salmonis and cortisol implantation. Dis Aquat Org 41:43–51
Secor SM (2009) Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol B 179:1–56
Sitjà-Bobadilla A, Palenzuela O, Alvarez-Pellitero P (2008) Immune response of turbot, Psetta maxima (L.) (Pisces: Teleostei), to formalin-killed scuticociliates (Ciliophora) and adjuvanted formulations. Fish & Shellfish Immunol 24:1–10
Smiley PC, Parsons GR (1997) Effects of photoperiod and temperature on swimming performance of white crappie. Trans Am Fish Soc 126:495–499
Smith RW, Houlihan DF (1995) Protein synthesis and oxygen consumption in fish cells. J Comp Physiol B 165:93–101
Solbakken VA, Hansen T, Stefansson SO (1994) Effects of photoperiod and temperature on growth and parr-smolt transformation in Atlantic salmon (Salmo salar L.) and subsequent performance in seawater. Aquaculture 121:13–27
Steffensen JF (1989) Some errors in respirometry of aquatic breathers: how to avoid and correct for them. Fish Physiol Biochem 6:49–59
Uribe C, Folch H, Enriquez R, Moran G (2011) Innate and adaptive immunity in teleost fish: a review. Veterinarni Medicina 56:486–503
Valdimarsson SK, Metcalfe NB (2001) Is the level of aggression and dispersion in territorial fish dependent on light intensity? Anim Behav 61:1143–1149
van Dijk P, Staaks G, Hardewig I (2002) The effect of fasting and refeeding on temperature preference, activity and growth of roach, Rutilus rutilus. Oecologia 130:496–504
Wang T, Hung CC, Randall DJ (2006) The comparative physiology of food deprivation: from feast to famine. Ann Rev Physiol 68:223–251
Xu L, Lam P, Chen J, Zhang Y, Harada K (2000) Comparative study on in vitro inhibition of grass carp (Ctenopharyngodon idellus) and mouse protein phosphatases by microcystins. Environ Toxicol 15:71–75
Yanase K, Herbert NA, Montgomery JC (2012) Disrupted flow sensing impairs hydrodynamic performance and increases the metabolic cost of swimming in the yellowtail kingfish, Seriola lalandi. J Exp Biol 215:3944–3954
Zapata AG, Varas A, Torroba M (1992) Seasonal variations in the immune system of lower vertebrates. Immunol Today 13:142–147
Acknowledgements
The present research was conducted as part of the project “Offshore Aquaculture” funded by the Danish Agrifish Agency through the Green Development and Demonstration Programme (Grant no. 3405-11-0293). The authors are grateful to the technical staff at the DTU Aqua, Section for Aquaculture, for the practical assistance with fish husbandry and laboratory analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, J.R., Lazado, C.C., Methling, C. et al. Short-term feed and light deprivation reduces voluntary activity but improves swimming performance in rainbow trout Oncorhynchus mykiss . Fish Physiol Biochem 44, 329–341 (2018). https://doi.org/10.1007/s10695-017-0438-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0438-0