Abstract
Lipoic acid (LA) is an antioxidant that also favors glucose uptake in mammals. Until now, there are no studies evaluating the potential effect of this molecule on glycemic control in fish. It was evaluated LA effects on glucose uptake in common carp Cyprinus carpio fed with carbohydrate diets from two carbohydrate sources: glucose (GLU) and starch (STA), and supplemented or not with LA, being the diets: +GLU/−LA (GLU); +GLU/+LA (GLU + LA); +STA/−LA (STA); and +STA/+LA (STA + LA). Carp juveniles (6.5 ± 0.1 g) were fed with each diet ad libitum 4 times a day, during 68 days. Muscle glycogen concentration was higher (p < 0.05) in GLU and GLU + LA than in STA and STA + LA groups. On fish fed with starch, muscle cholesterol and triglyceride concentrations were higher (p < 0.05) in fish fed diets supplemented with LA. Muscle protein levels were higher in fish fed with LA, independent of the diet carbohydrate source. Lipid peroxidation was significantly reduced (p < 0.05) in fish muscle on fish fed the STA + LA diets when compared with the STA diet. Our findings indicate that LA modulates lipid, proteins and carbohydrate metabolism together with the well-known antioxidant effect. Also, LA showed to enhance starch utilization taking into account muscle cholesterol and triglyceride levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the last decade, human diet showed an increased consumption of simple carbohydrates, while consumption of complex carbohydrates has decreased (Henry et al. 1991). Robert et al. (2008) reported that rats fed with potato, a source of complex carbohydrate and antioxidant molecules, had enhanced the antioxidant defenses and improved lipid metabolism, when compared with rats fed with sucrose, a simple sugar. In humans or in laboratory animals, diets with high levels of monosaccharides (glucose, fructose) or disaccharides (sucrose) have been associated with several metabolic disorders (Henry et al. 1991; Busserolles et al. 2002; Robert et al. 2008). One of the most important of these metabolic disorders is diabetes, which is characterized by a poor ability to control blood glucose homeostasis, thus resulting in persistent hyperglycemia (Brownlee 2001; Rains and Jain 2011). This disease is due to decreased insulin secretions or increased insulin resistance. Although diabetes is associated to mammals, fish have a similar persistent hyperglycemia after a meal and the reasons of this remain under debate (Moon 2001; Polakof et al. 2010). It is known that fish show plasma insulin levels similar or higher than those observed in mammals (Mommsen and Plisetskaya 1991). Also, unlike mammals, insulin-like growth factor-I (IGF-I) receptors are more abundant than insulin receptors in all fish tissues studied (Párrizas et al. 1995) and IGF-I appears to be more relevant than insulin on regulation of carbohydrate metabolism in fish (Castillo et al. 2004). Further, fish have more skeletal muscle GLUT4 (glucose facilitative transporter type 4) on plasma membrane than mammals when both cells were exposed to insulin (Díaz et al. 2007). From the above, it seems that fish have a signaling cascade of carbohydrate metabolism different from mammals, and it is still difficult to understand the poor glucose utilization observed in this group of animals.
It is well established that hyperglycemia induces oxidative stress in several animals. A pro-oxidant environment may be achieved either directly, by generation of reactive oxygen species (ROS), especially through mitochondrial superoxide overproduction, or indirectly through the polyol pathway, by formation of advanced glycation end products (AGE), and activation of protein kinase C (Brownlee 2001). It has been questioned for long time whether oxidative stress has a primary role in insulin signaling cascade, resulting in insulin resistance (Baynes and Thorpe 1999; Rains and Jain 2011). Baynes and Thorpe (1999) further questioned whether researches should focus on the application of antioxidant therapy or on the steps that generate chronically poor glycemic control. In this context, it is interesting to consider molecules that can simultaneously perform a glycemic and an antioxidant control.
Lipoic acid (1,2-dithiolane-3-pentanoic acid; LA) has been considered as “universal antioxidant,” since it meets all criteria considered in the evaluation of the antioxidant potential of a compound (Packer et al. 1995). LA and its reduced form, dihydrolipoic acid (DHLA), form a redox DHLA/LA couple with a very low redox potential (E 0 = −0.32 V). For comparison, reduced glutathione (GSH)/glutathione disulfide (GSSG) couple has an E 0 = −0.24 V. Thus, the DHLA/LA couple is capable of reducing GSSG to GSH, but the inverse is not possible (Packer et al. 1995). In fish, LA improves glutamate–cysteine ligase (GCL) and glutathione-S-transferase (GST) activities in brain and liver, pointing to an enhanced capacity to synthesize reduced glutathione (GCL is the rate-limiting enzyme) and cellular detoxification (Monserrat et al. 2008; Kütter et al. 2014). Also in fish, LA was shown to reduce muscle lipid peroxidation (Kütter et al. 2012), and to reverse ascorbic acid depletion in liver and gill (Park et al. 2006).
Moreover, LA is a cofactor of mitochondrial enzymes involved in nutrient metabolism and in the tricarboxylic acid cycle (Packer et al. 1995). Additionally, since 1970, several studies indicate that LA stimulates glucose uptake (Haugaard and Haugaard 1970; Ziegler et al. 1995; Estrada et al. 1996; Henriksen et al. 1997; Yaworsky et al. 2000; Coleman et al. 2001; Shay et al. 2009). The signaling pathway where LA acts is still unclear, but in mammal adipocytes were verified that LA improved glucose uptake through rapid translocation of glucose transporters (GLUT1 and GLUT4) from cytosol to plasma membrane, thus mimicking some actions of insulin (Yaworsky et al. 2000). To our knowledge, there are not studies evaluating the effects of LA on glucose uptake in fish.
One of the worldwide aquaculture concerns is the replacement of fish meal by sustainable ingredients on aquafeeds, due to their environmental and economic impacts (FAO 2014). Often, dietary fish meal is replaced with plant feedstuffs that are rich in carbohydrates (Salze et al. 2010; Bauer et al. 2012). Although fish do not use carbohydrates efficiently (Wilson 1994; Moon 2001), dietary carbohydrates may have protein-sparing effects, minimizing the use of protein for energy purposes and for glucogenesis, thus increasing feed efficiency and reducing nitrogen release to the environment (Wilson 1994; Erfanullah and Jafri 1995; Hemre et al. 2002; Zamora-Sillero et al. 2013). Therefore, improving the use of carbohydrates on aquafeeds is an important goal for aquaculture practices. In this study, it was evaluated the effects of dietary LA supplementation on carbohydrate diets on common carp growth performance, antioxidant status and muscle metabolites.
Materials and methods
Diet formulation
Diets were formulated to contain 30 % carbohydrate, 35 % crude protein brute and 10 % crude lipid, and to include or not 0.1 % of synthetic (±) α-lipoic acid. Thus, four experimental diets were defined: +glucose/−LA (GLU); +glucose/+LA (GLU + LA); +starch/−LA (STA); and +starch/+LA (STA + LA). The carbohydrate level was chosen to induce a hyperglycemic condition in experimental fish at each meal (Takeuchi et al. 2002; Tan et al. 2009). Two different carbohydrate sources (glucose and starch) were chosen to analyze differences on carbohydrate complexity. The nominal concentration of LA employed (1000 mg/kg of diet) was based on the study of Kütter et al. (2012). Dietary composition and proximate analysis is presented in Table 1. Dry ingredients were thoroughly mixed, 30 % water was added, and the mixture was extruded with a meat grinder to form 3-mm-diameter pellets. Pellets were then dried at 60 °C for 24 h and stored in hermetic plastic bags at −18 °C until use. Proximate composition of the diets was analyzed following procedures described in AOAC (1995): dry matter after drying at 105 °C until constant weight; ash by incineration in a muffle furnace at 450 °C for 6 h; protein content (N × 6.25) by the Kjeldahl method; and lipid by petroleum ether extraction. Effective LA concentration in ration was measured using liquid chromatography with mass spectrometric detection, using the same methodology of previous studies (Kütter et al. 2012; Martins et al. 2014).
Fish and experimental design
The experiment was performed at the Marine Aquaculture Station of the Federal University of Rio Grande (FURG) in Rio Grande do Sul State, Brazil. All procedures and protocols were performed in accordance with the guidelines for animal uses and approved by the Animal Ethic Committee of FURG (CEUA, protocol number Pq031/2014). One hundred and sixty-eight (168) common carp Cyprinus carpio (Teleostei: Cyprinidae) juveniles were purchased from local fish farm, and after transportation to the experimental facilities, fish were acclimated into 1000-L tank connected to a recirculation water system for 1 week. The system was equipped with a biofilter, submersible heaters and a magnetic water pump (1950 L/h). During this period, fish were fed twice a day ad libitum with a commercial diet (28 % of protein and 5 % of lipid, manufactured by SUPRA, Carazinho, RS, Brasil). Photoperiod was 14 h light:10 h dark, and water quality parameters were routinely monitored to assure safety level to this species during acclimation. After acclimation, 14 fish were stocked into each 12 polystyrene tanks (300 L useful water volume) after fish were weighted (6.48 ± 0.13 g) and randomly distributed. These 12 tanks were sorted to contemplate the four treatments (GLU; GLU + LA; STA; STA + LA) in triplicate (14 fish/tank). The growth period lasted 68 days, and fish were daily fed to apparent visual satiety four times a day at 08:30; 11:00; 14:30; 17:00. The photoperiod was maintained in 14 h light:10 h dark, temperature averaged 25.5 ± 2.3 °C, pH 7.4 ± 0.3 and dissolved oxygen 6.6 ± 0.8 mg/L. Ammonium (NH3 < 0.014 ppt) and nitrate (NO2 < 0.15 mg/L) were kept within safe margins for the species.
Growth and feed utilization parameters
The following formulas were used to assess growth and feed utilization parameters:
Sample collection
At the end of the growth trial, fish were fasted for 24 h for gut clearance and then six random fish from each treatment were euthanized with an overdose of benzocaine (200 ppm). Fish were then dissected and muscle was collected and rapidly stored into liquid nitrogen before being kept at −80 °C until analysis.
Hematocrit, glycated hemoglobin and muscle metabolites
Red blood cells content was determined after centrifugation according to Lund et al. (2000). The blood concentration of glycated hemoglobin (Hb1Ac) was measured using a commercial colorimetric kit (Glicohemoglobina, Katal, Belo Horizonte, MG, Brazil).
Muscle samples were homogenized according to Laiz-Carrión et al. (2012) as modified by Zamora-Sillero et al. (2013). Muscle triglyceride and cholesterol were measured with commercial kits (Triglicerides Enzimático Líquido and Colesterol Enzimático Líquido, both from Doles, Goiânia, GO, Brazil). Muscle glycogen was assessed as described by Carr and Neff (1984) modified by Nery and Santos (1993), using amyloglucosidase from Aspergillus niger (Sigma-Aldrich Co.) to hydrolyze glycogen into glucose. The resultant glucose was measured with a commercial kit (Glicose Enzimática, Doles, Goiânia, GO, Brazil).
Determination of oxidative damage
Muscle tissues were homogenized as described by Amado et al. (2011). Lipid peroxidation on muscle was determined by a fluorometric method (520 and 580 nm for excitation and emission wavelength, respectively), according to Oakes and Van der Kraak (2003) and adapted to a microplate reader according to Da Rocha et al. (2009). The method measures the concentration of thiobarbituric acid-reactive substances (TBARS) using tetramethoxypropane (TMP, Acros Organics) as a standard.
Statistical analysis
All data are presented as mean ± standard deviation. Differences between the four treatments defined in “Diet formulation” section were determined by one-way analysis of variance after accessing normality by Shapiro–Wills test and for homogeneity of variance by the Levene’s test (Zar 1984). Differences between means were determined by the Newman–Keuls post hoc test. The significance level adopted in all tests was 5 % (α = 0.05).
Results
The mean measured concentration of lipoic acid (LA) in the diet supplemented with LA was 385.6 ± 71.82 mg LA/kg. At the end of trial, body weight and FCR were significantly different among carbohydrates groups, being higher (p < 0.05) on fish fed with starch (Table 2). Considering growth parameters, STA + LA showed a lower final weight, SGR and weight gain (p < 0.05) than the STA. The average values of final weight, weight gain, SGR, feed intake, FCR and PER are presented in Table 2.
Hematrocrit and glycated hemoglobin (HbA1c) presented no differences among treatments, with the only exception of GLU group, which presented a significantly lower red cell volume (p < 0.05) (Table 3). Regarding muscle metabolites (Table 3), glycogen concentration was higher in fish fed with glucose but no effect due to LA. Mainly on fish fed with starch, LA showed a significant increase (p < 0.05) in muscle cholesterol and triglyceride concentrations. LA treatment, both in GLU and STA groups, increased significantly (p < 0.05) the levels of muscle protein.
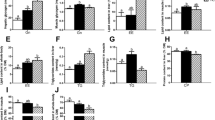
Levels of lipid peroxidation (TBARS) in muscle were significantly reduced (p < 0.05) in fish from STA + LA when compared with STA (Fig. 1).
Muscle concentration of thiobarbituric acid-reactive substances (TBARS, expressed as nmol of TMP equivalents/mg of wet tissue) of C. carpio after fed with carbohydrate diet from different sources, glucose (GLU) and starch (STA) supplemented with lipoic acid (+LA) or not during 68 days. Bars are shown as means ± standard deviations. Asterisk indicates significant (p < 0.05) differences of STA group with all other treatments. TMP tetramethoxypropane, the standard employed in TBARS measurements
Discussion
Cyprinids are the most cultivated teleost fish around the world, frequently through familiar aquaculture under semi-intensive pond culture (Kaushik 1995; FAO 2014). One of the reasons of its success is due to the feeding habits of this group that tolerates relatively higher quantities of carbohydrates, resulting in the use of low-cost artificial diets. Ufodike and Matty (1983) showed both rice starch and tapioca starch at levels of 45 % which are well utilized by common carp. Moreover, Murai et al. (1983) reported that the increase in the feeding frequency (from 2 to 6 meals per day) of juvenile common carp improved the utilization of glucose, dextrose, maltose or starch, at 30 % level. This study was carried with a diet containing 30 % of starch or glucose, and the fish were fed four times a day. Thus, it was expected that juvenile carp accepted well-trial conditions in present study. During the experiment, differences in growing were evident in fish fed with different carbohydrates sources. At the end of the experiment, fish fed starch grew approximately fourfold than those fed glucose. Reflex of this, other growth parameters (SGR, FCR and PER) were different between fish fed with glucose and fish fed with starch. Differences on growth between carbohydrate types have already been observed for herbivorous, omnivorous and carnivorous fish (Furuichi and Yone 1982; Furuichi et al. 1986; Erfanullah and Jafri 1995; Lee et al. 2003). However, the growth of fish fed glucose was much lower than starch group, although co-treatment with lipoic acid induced a significant weight gain (Table 2), even when the starch groups presented a clear better performance. High level of glycosuria 6 h after meal was found in 100 % of yellowtail, a carnivorous fish, when fed with a high level of glucose (20 %; Furuichi et al. 1986). Furuichi et al. (1986) suggested that glucose is absorbed without great interactions with the cells and this molecule is excreted before rise of hepatic enzyme activities related to carbohydrate metabolism, and thus fish fed with glucose show a slow growth. Ours findings seem to corroborate this assertion.
To our knowledge, the study of Zamora-Sillero et al. (2013) was the first that reported levels of glycated hemoglobin in fish. In present study, values were around 59 %, not much higher than the almost 47 % found by Zamora-Sillero et al. (2013). Those values are higher than the observed in humans without diabetes (4–5.9 %) or in people with poorly controlled diabetes (8.0 %; Silink and Mbanya 2007). However, there were no differences between treatments neither in the present nor in Zamora-Sillero et al. (2013) studies.
In muscle tissue, animals can synthesize reserve metabolites such as glycogen, cholesterol and triglyceride. In addition, unlike glycogen, large quantities of lipids may be stored to be used as fuel, and this pattern was similar in the present study. Muscle glycogen concentration was not affected by LA treatment although it was higher in glucose group (Table 3). This last result fits closely to those presented by Li et al. (2016). These authors fed during 60 days juvenile C. carpio with low (25 %, L) or high (50 %, H) levels of glucose (G) or starch (S), and their main results showed that:
-
1.
The highest liver enzyme activity of glucokinase (GK) was observed in HG group, indicating a sustainable capacity to convert excess of glucose in reserve molecules as glycogen. In fact, glycogen levels were higher in HG when compared with starch groups (HS and LS), as we observed in present study (Table 3).
-
2.
Carps fed with HS showed significant higher weight gain (%) than HG group, a result very similar to that observed in our study (Table 2).
-
3.
Carps fed with HS showed a significant higher PER (protein efficiency ratio) than that HG group, again a result similar to our data (Table 2).
Furthermore, there were differences on fat storage in accordance with the presence or not of LA in both carbohydrate groups. In both GLU and STA groups, LA showed to increase cholesterol levels, and the group STA + LA presented the highest levels of triglycerides (Table 3), suggesting that LA induces fat synthesis. Products of glucose oxidation are precursors of lipogenesis, and this is an indirect evidence that LA improves glucose uptake in muscle of common carp. It is important to remember that both triglycerides and cholesterol syntheses are regulated by hormonal control. Insulin stimulates the conversion of carbohydrate to triglycerides and also promotes covalent modification of Hydroxy-metyl-glutaryl-CoA (HMG-CoA) reductase, thus, activating this enzyme and favor cholesterol synthesis. The present results regarding muscle metabolites indicate that LA triggers effects similar to those of insulin.
The literature reports that dietary carbohydrates also induce protein-sparing effects in fish comparing different levels of these macronutrients (Erfanullah and Jafri 1995; Sá et al. 2008; Satpathy and Ray 2009; Zamora-Sillero et al. 2013). In this study, it was expected that LA could induce a protein-sparing effect in fish fed with carbohydrate diet due to its effects on glucose metabolism (Coleman et al. 2001; Shay et al. 2009). In fact, this happened since protein concentration rose on muscle, suggesting that carbohydrates are utilized as energy source. However, more studies are needed to solve the apparent contradiction of LA inducing lipogenesis and increasing protein concentration without any improvement on growth.
Regarding lipid peroxidation, an event of oxidative damage, reduced TBARS on muscle were observed on fish fed STA + LA when compared with STA group. It is important to remember that fish fed starch grew approximately fourfold than those fed glucose. Consequently, fish fed starch were submitted to more pro-oxidant environmental due to higher metabolism in comparison with fish fed glucose. Thus, LA was effective to reduce oxidative damage on muscle in fish fed starch. Previously, LA was registered to diminish lipid peroxidation on muscle of fish Trachinotus marginatus (Kütter et al. 2012) when they were fed with LA supplemented on meal during 42 days. As a consequence, LA long-term administration reduces lipid damage induced by reactive species in fish muscle.
Conclusions
Results above suggest that LA improves glycemic control in fish, supporting the idea that LA could be a promising molecule on aquafeeds. Moreover, the improvement on carbohydrate metabolism was paralleled by ameliorated oxidative stress in fish fed with starch. In this way, it can be considered that LA possesses not only an antioxidant role but also is important in fish metabolic regulations.
References
Amado LL, Garcia ML, Ramos PB, Yunes JS, Monserrat JM (2011) Influence of a toxic Microcystis aeruginosa strain on glutathione synthesis and glutathione-S-transferase activity in common carp Cyprinus carpio (Teleostei: Cyprinidae). Arch Environ Contam Toxicol 60:319–326
Association of the Official Analytical Chemists (AOAC) (1995) Official methods of analysis of the association of the official analytical chemists international, 16th edn. AOAC International, Arlington
Bauer W, Prentice-Hernandez C, Tesser MB, Wasielesky W, Poersch LHS (2012) Substitution of fish meal with microbial floc meal and soy protein concentrate in diets for the pacific white shrimp Litopenaeus vannamei. Aquaculture 342–343:112–116
Baynes JW, Thorpe SR (1999) Perspectives in diabetes—role of oxidative stress in diabetic complications. A new perspective on an old paradigm. Diabetes 48:1–10
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Busserolles J, Rock E, Gueux E, Mazur A, Grolier P, Rayssiguier Y (2002) Short-term consumption of a high-sucrose diet has a pro-oxidant effect in rats. Br J Nutr 87:337–342
Carr RS, Neff JM (1984) Quantitative semiautomated enzymatic assay for tissue glycogen. Comp Biochem Physiol B 77:447–449
Castillo J, Codina M, Martínez ML, Navarro I, Martı L (2004) Metabolic and mitogenic effects of IGF-I and insulin on muscle cells of rainbow trout. Am J Physiol Regul Integr Comp Physiol 286:R935–R941
Coleman MD, Eason RC, Bailey CJ (2001) The therapeutic use of lipoic acid in diabetes: a current perspective. Environ Toxicol Pharmacol 10:167–172
Da Rocha AM, De Freitas DPS, Burns M, Vieira JP, de La Torre FR, Monserrat JM (2009) Seasonal and organ variations in antioxidant capacity, detoxifying competence and oxidative damage in freshwater and estuarine fishes from Southern Brazil. Comp Biochem Physiol C 150:512–520
Díaz M, Antonescu CN, Capilla E, Klip A, Planas JV (2007) Fish glucose transporter (GLUT)-4 differs from rat GLUT4 in its traffic characteristics but can translocate to the cell surface in response to insulin in skeletal muscle cells. Endocrinology 148:5248–5257
Erfanullah, Jafri AK (1995) Protein-sparing effect of dietary carbohydrate in diets for fingerling Labeo rohita. Aquaculture 136:331–339
Estrada E, Ewart HH, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H, Klip A (1996) Stimulation of glucose uptake by the natural co-enzyme α-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes 45:1798–1804
Food and Agriculture Organization of the United Nations (2014) The state of word fisheries and aquaculture- Opportunities and challenges. FAO, Rome
Furuichi M, Yone Y (1982) Availability of carbohydrate in nutrition of carp and red sea bream. Bull Jpn Soc Sci 48:945–948
Furuichi M, Taira H, Yone Y (1986) Availability of carbohydrate in nutrition of yellowtail. Bull Jpn Soc Sci 52:99–102
Haugaard N, Haugaard ES (1970) Stimulation of glucose utilization by thioctic acid in rat diaphragm incubated in vitro. Biochim Biophys Acta 222:583–586
Hemre G-I, Mommsen TP, Krogdahl A (2002) Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquac Nutr 8:175–194
Henriksen EJ, Jacob S, Streeper RS, Fogtl DL, Hokama JY, Tritschler HJ (1997) Stimulation by alpha-lipoic acid of glucose transport activity in sketetal muscle of lean and obese zucker rats. Life Sci 61:805–812
Henry RR, Crapo PA, Thorburn AW (1991) Current issues in fructose metabolism. Ann Rev Nutr 11:21–39
Kaushik SJ (1995) Nutrient requirements, supply and utilization in the context of carp culture. Aquaculture 129:225–241
Kütter MT, Monserrat JM, Primel EG, Caldas SS, Tesser MB (2012) Effects of dietary α -lipoic acid on growth, body composition and antioxidant status in the Plata pompano Trachinotus marginatus (Pisces, Carangidae). Aquaculture 368–369:29–35
Kütter MT, Romano LA, Ventura-Lima J, Tesser MB, Monserrat JM (2014) Antioxidant and toxicological effects elicited by alpha-lipoic acid in aquatic organisms. Comp Biochem Physiol C 162:70–76
Laiz-Carrión R, Viana IR, Cejas JR, Ruiz-Jarabo I, Jerez S, Martos JA, Eduardo AB, Mancera JM (2012) Influence of food deprivation and high stocking density on energetic metabolism and stress response in red porgy, Pagrus pagrus L. Aquac Int 20:585–599
Lee S, Kim K, Lall SP (2003) Utilization of glucose, maltose, dextrin and cellulose by juvenile flounder (Paralichthys olivaceus). Aquaculture 221:427–438
Li JN, Xu CA, Wang LS, Wang ZG, Zhao ZG, Luo L (2016) Effects of dietary glucose and starch levels on the growth, haemotological indices and hepatic hexoquinase and glucokinase mRNA expression of juvenile mirror carp (Cyprinus carpio). Aquac Nutr 22:550–558
Lund SG, Phillips MCL, Moyes CD, Tufts BL (2000) The effects of cell ageing on protein synthesis in rainbow trout (Oncorhynchus mykiss) red blood cells. J Exp Biol 203:2219–2228
Martins ACS, Flores JA, Wasiliesky W Jr, Zanette J, Primel EG, Caldas SS, Monserrat JM (2014) Modulation of antioxidant and detoxification responses induced by lipoic acid in the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) subjected to hypoxia and re-oxygenation. Mar Freshw Behav Physiol 47:335–348
Mommsen TP, Plisetskaya EM (1991) Insulin in fishes and agnathans: history, structure and metabolic regulation. Rev Aquat Sci 4:225–259
Monserrat JM, Ventura JL, Ferreira R, Acosta D, Garcia ML, Ramos PB, Moraes TB, Dos-Santos LC, Amado LL (2008) Modulation of antioxidant and detoxification responses mediated by lipoic acid in the fish Corydoras paleatus (Callychthyidae). Comp Biochem Physiol C 148:287–292
Moon TW (2001) Glucose intolerance in teleost fish: fact or fiction? Comp Biochem Physiol B 129:243–249
Murai T, Akiyama T, Nose T (1983) Effects of glucose chain length of various carbohydrates and frequency of feeding on their utilization by fingerling carp. Bull Jpn Soc Sci Fish 49:1607–1611
Nery LEM, Santos EA (1993) Carbohydrate metabolism during osmoregulation in Chasmagnathus granulata Dana, 1851 (Crustacea, Decapoda). Comp Biochem Physiol B 106:747–753
Oakes KD, Van der Kraak GJ (2003) Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat Toxicol 63:447–463
Packer L, Witt EH, Tritschler HJ (1995) Alpha-lipoic acid as a biological antioxidant. Free Rad Biol Med 19:227–250
Park KH, Terjesen BF, Tesser MB, Portella MC, Dabrowski K (2006) α-Lipoic acid-enrichment partially reverses tissue ascorbic acid depletion in pacu (Piaractus mesopotamicus) fed vitamin C-devoid diets. Fish Physiol Biochem 32:329–338
Párrizas M, Plisetskaya EM, Planas J, Gutiérrez J (1995) Abundant insulin-like growth factor-1 (IGF-1) receptor binding in fish skeletal muscle. Gen Comp Endocrinol 98:16–25
Polakof S, Moon TW, Aguirre P, Skiba-Cassy S, Panserat S (2010) Effects of insulin infusion on glucose homeostasis and glucose metabolism in rainbow trout fed a high-carbohydrate diet. J Exp Biol 213:4151–4157
Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Rad Biol Med 50:567–575
Robert L, Narcy A, Rayssiguier Y, Mazur A, Rémésy C (2008) Lipid metabolism and antioxidant status in sucrose vs. potato-fed rats. J Am Coll Nutr 27:109–116
Sá R, Pousão P, Oliva-Teles A (2008) Effect of dietary starch source (normal versus waxy) and protein levels on the performance of white sea bream Diplodus sargus (Linnaeus) juveniles. Aquac Res 39:1069–1076
Salze G, Mclean E, Battle PR, Schwarz MH, Craig SR (2010) Use of soy protein concentrate and novel ingredients in the total elimination of fish meal and fish oil in diets for juvenile cobia, Rachycentron canadum. Aquaculture 298:294–299
Satpathy BB, Ray AK (2009) Effect of dietary protein and carbohydrate levels on growth, nutrient utilization and body composition in fingerling rohu, Labeo rohita (Hamilton). J Appl Ichthyol 25:728–733
Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM (2009) Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta 1790:1149–1160
Silink M, Mbanya J (2007) Global standardization of the HbA 1c assay: the consensus committee recommendations. Diabetes Voice 52:33–34
Takeuchi T, Satoh S, Kiron V (2002) Common carp, Cyprinus carpio. In: Webster CD, Lim C (eds) Nutrient requirements and feeding of finfish for aquaculture. CAB, New York, pp 245–261
Tan Q, Wang F, Xie S, Zhu X, Lei W, Shen J (2009) Effect of high dietary starch levels on the growth performance, blood chemistry and body composition of gibel carp (Carassius auratus var. gibelio). Aquac Res 40:1011–1018
Ufodike EBC, Matty AJ (1983) Growth responses and nutrient digesibility in mirror carp (Cyprinus carpio) fed different levels of cassava and rice. Aquaculture 31:41–50
Wilson RP (1994) Utilization of dietary carbohydrate by fish. Aquaculture 124:67–80
Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A (2000) Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by a-lipoic acid in 3T3-L1 adipocytes. Diabetologia 43:294–303
Zamora-Sillero J, Ramos LRV, Romano LA, Monserrat JM, Tesser MB (2013) Effect of dietary dextrin levels on the growth performance, blood chemistry, body composition, hepatic triglicerides and glycogen of Lebranche mullet juveniles (Mugil liza Valenciennes 1836, Mugilidae). J Appl Ichthyol 29:1342–1347
Zar JH (1984) Biostatistical analysis, 2nd edn. Prentice Hall, New Jersey
Ziegler D, Hanfeld M, Ruhnau KJ, Meibner HP, Lobisch M, Schutte K, Gries FA (1995) Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant a-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 38:1425–1433
Acknowledgments
The authors would like to thank Dr. Ricardo Roubax Robaldo of Federal University of Pelotas (UFPel). The authors also thank Dr. Luis Alberto Romano, M.Sc. Paola Milena, MSc. Juan Zamora-Sillero, Dr. Eduardo Martins Silva and oxidative stress and toxicology research group (EAOx) for sampling and analysis contributions. J. M. Monserrat; E. Pimentel and Marcelo B. Tesser are research fellows from Brazilian agency CNPq (process numbers PQ 307880/2013-3, DT 311605/2009-5 and PQ 308680/2014-6, respectively). R. A. Santos received support from the Brazilian Council of Research, CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, R.A., Caldas, S., Primel, E.G. et al. Effects of lipoic acid on growth and biochemical responses of common carp fed with carbohydrate diets. Fish Physiol Biochem 42, 1699–1707 (2016). https://doi.org/10.1007/s10695-016-0250-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0250-2