Abstract
A 9-week feeding experiment was conducted to evaluate the effects of dietary vitamin E (VE) supplementation on growth performance, liver fatty acid composition, lipid peroxidation and peroxisome proliferator-activated receptors (PPAR) genes expressions in blunt snout bream juveniles. Fish (average initial weight: 0.59 g) were fed diet supplemented with 0, 50, 100, 300 and 500 mg α-tocopherol acetate/kg in triplicates, which were found to, respectively, contain 11.2, 56.3, 114.6, 306.5 and 588.4 mg α-tocopherol/kg diet. Results showed that final weight, body weight gain and specific growth rate significantly increased with increasing dietary VE supplemented level from 11.2 to 56.3 mg/kg. When the broken-line model was employed to estimate the adequate requirement of vitamin E based on body weight gain, the optimal level was 55.5 mg/kg in diet. Hepatosomatic index value significantly decreased with incremental dietary VE levels. However, liver VE concentration showed a direct relationship with the dietary VE level. The percentages of 20:5n-3, 22:6n-3 and total n-3 long chain polyunsaturated fatty acids in liver increased with increasing dietary VE supplementation. Meanwhile, the expressions of PPAR-α, PPAR-β and PPAR-γ in liver were down-regulated by supplementation of dietary VE level from 56.3 to 588.4 mg/kg. In conclusion, supplementation of more than 55.5 mg/kg vitamin E may improve growth and increase n-3 LC-PUFA content in blunt snout bream, which is beneficial to human consumer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vitamin E (α-tocopherol) is an effective lipid-soluble, chain-breaking antioxidant that could protect cell membranes from oxidative damage (Mourente et al. 2007). Fish have no capacity to synthesize vitamin E and must rely on a dietary supply. A dietary requirement for vitamin E in fish has been reported in a number of studies including channel catfish (Ictalurus punctatus) (Bai and Gatlin 1993), rohu (Labeo rohita) fry (Sau et al. 2004), grouper (Epinephelus malabaricus) (Lin and Shiau 2005), eel (Anguilla japonica) (Bae et al. 2013) and cobia (Rachycentron canadum) (Zhou et al. 2013c).
Long chain (LC) n-3 polyunsaturated fatty acids (PUFA) are most commonly defined as PUFA with ≥C20 and ≥3 double bonds, particularly eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3). Fish naturally contains high levels of the n-3 LC-PUFA. Recent studies have shown that n-3 LC-PUFAs play a vital role in prevention and treatment of cardiovascular disease, inflammation, aggression, depression, hypertension, autoimmune disorders and cancer in human. However, LC-PUFAs are potentially susceptible to lipid peroxidation (Hamre 2011), which leads to development of undesirable influence on fish metabolism in several ways like decreased growth, increased mortality and lost vitamins concentrations of fish. It has been shown that vitamin E can protect biological membranes containing LC-PUFA against free radicals formation and effectively minimize lipid peroxidation in biological systems in fish (Tocher et al. 2003; Mourente et al. 2000; Gao et al. 2012a). Moreover, Gao et al. (2012b) reported that the contents of n-3 LC-PUFA such as EPA, DHA in fish tissues were increased by supplementation of dietary vitamin E. Therefore, the vitamin E not only prevents increases in lipid peroxidation as antioxidant, but also plays a major role in lipid metabolism in fish.
Functions of the lipid metabolism of peroxisome proliferator-activated receptors (PPARs) have caused a great deal of attention. PPARs have three isotypes, termed PPAR-α, PPAR-β and PPAR-γ. These three isotypes exhibit distinct patterns of tissue expression, differ in their ligand-binding domains and are involved in multiple, distinctive but often complementary physiological pathways and functions (Kuenzli and Saurat 2003). Ruyter et al. (1997) reported PPARs in fish for the first time and determined their responses to peroxisome proliferators and fatty acids. It has been proved that PPARs are ligand-dependent transcription factors which regulate several of the genes involved in lipid metabolism (Qi et al. 2000; Kliewer et al. 2001; Lee et al. 2003). Although the effects of vitamin E on expressions of PPARs in mammals have been reported (Fang et al. 2010), there is no information regarding the effects of vitamin E on the expression levels of PPARs in fish.
Blunt snout bream Megalobrama amblycephala is an herbivorous freshwater fish native to China. In China, aquaculture of this fish has expanded rapidly during the last decade because of its fast growth, tender flesh and high diseases resistance. Although few previous studies (Zhou et al. 2013a, b; Liu et al. 2014) have shown the effects of vitamin E supplementation in diets on immune functions, fillet quality and disease resistance in this fish, limit studies regarded the effects of vitamin E supplementation on growth performance, fatty acid composition, lipid peroxidation and PPAR genes expression in the fish juveniles has been reported. Therefore, the purpose of this study was to evaluate the effects of vitamin E supplementation on the growth performance, fatty acid composition, lipid peroxidation and expressions of PPAR genes in blunt snout bream juveniles.
Materials and methods
Diet formulation and preparation
Five iso-nitrogenous (43.1 %) and iso-lipidic (9.1 %) semi-purified experimental diets were used in this study. The composition of the semi-purified basal diet is presented in Table 1. Graded levels of dl-α-tocopheryl acetate were added to the basal diet at the expense of cellulose to provide the desired levels of 0, 50, 100, 300 and 500 mg/kg diet, which were found to, respectively, contain 11.2 (E11.2), 56.3 (E56.3), 114.6 (E114.6), 306.5 (E306.5) and 588.4 (E588.4) mg α-tocopherol/kg diet, and E11.2 was regarded as the control. The diets were made as follows: all dry ingredients were thoroughly mixed in a blender (SM-168; Muren Corp, Shenzhen, China) for 15 min; the liquid-soluble vitamins which contained different levels of vitamin E were first completely mixed with oil before being poured into the blender and stirred with the dry ingredients for 15 min; Then, 25 % water was added into the mixture which was stirred for 15 min to give a stiff dough. The wet dough was extruded through a 3.0 mm die to form pellets. The moist pellets were dried in a dry-air mechanical convection oven at 60 °C for 2 h and then stored at −20 °C until use. At the time of feeding, the diets were broken into appropriate size. Proximate composition, vitamin E concentrations and thiobarbituric acid reactive substances (TBARS) of the experimental diets are presented in Table 2, and fatty acid compositions are shown in Table 3.
Fish and feeding experiment
Blunt snout bream (initial body weight: 0.59 ± 0.01 g, mean ± SE) juveniles were obtained from Haida Co., Ltd., Tuanfeng County, Hubei Province, China and acclimated to laboratory conditions for 2 weeks by feeding a commercial feed (35 % crude protein and 10 % crude lipid, Haida Co., Ltd.). The growth experiment for 9 weeks was conducted indoors in a system with flow-through freshwater at 1 l/min. Three tanks (50 l water/tank) were randomly assigned to each dietary treatment with 15 fish per tank. All fish were hand-fed to apparent satiation with the designated diets thrice daily (8:00, 14:00 and 20:00 h). Uneaten diets were collected, freeze dried and weighed for corrected feed intake calculation. Dead fish were removed and weighed daily, and the number of dead fish was recorded to calculate the mortality (%) at the end of the feeding trial. A diurnal cycle of 12 h/12 h light/dark was maintained with fluorescent lights. Water temperature ranged from 20 to 24 °C, pH fluctuated between 7.0 and 7.5 and dissolved oxygen was maintained at approximately 6.5 mg/l during the feeding trial.
Sample collection and analyses
At the end of the feeding trial, all fish were fasted for 24 h prior to final sampling and were killed in an ice-slurry. Two fish from each tank were randomly collected and stored at −20 °C for whole body composition analysis. Three fish from each tank were randomly chosen to dissect the liver without any excreta and were stored at −80 °C after having been stored in liquid nitrogen for 8 h for the PPAR genes expression level determinations. Livers of the other fish from each tank were dissected out and weighed individually to calculate the hepatosomatic index (HSI). The liver samples were collected, pooled and stored at −80 °C for the determination of the vitamin E concentration, fatty acid composition and TBARS value.
The diets and whole fish body were analyzed for moisture, crude protein, total lipid and ash, in triplicate, using standard AOAC methods (A.O.A.C 1990). The diet moisture level was determined by drying the sample at 105 °C to a constant weight. The fish samples were freeze dried (Freezone 2.5, Labconco, Co., Ltd., USA) prior to proximate composition analyses. Loss in weight represented whole body moisture content. Total lipid was determined following the method of Bligh and Dyer (1959). The measurement of TBARS was carried out using a method adapted from Yagi (1987). The vitamin E contents in fish and diets were determined by high-performance liquid chromatography (HPLC) with a UV detector (FLD) according to Sau et al. (2004). The fatty acid composition of test diets and livers was determined by a gas chromatography (Agilent Technologies Inc., Santa Clara, California, USA; column: Omegawax™ 320) according to the method of Gao et al. (2012a). The temperatures of the injector and flame ionizing detector (FID) were set at 200 and 240 °C, respectively. High-purity helium was used as the carrier gas at a flow rate of 1 ml/min. The samples (1.0 μl) were manually injected into an injection port, and the identified fatty acids were presented as area percentage of total fatty acids.
Total RNA was extracted from the liver using RNAiso Plus (Takara Co. Ltd., Japan). RNA samples were treated by RQ1 RNase-Free DNase prior to RT-PCR (Takara Co. Ltd., Japan) to avoid genomic DNA amplification. cDNA was generated from 500 ng DNase-treated RNA using ExScript™ RT-PCR kit (Takara Co. Ltd., Japan). Analyses of gene transcript levels were conducted by the real-time PCR method. Real-time PCR was employed to determine mRNA levels based on the SYBR Green I fluorescence kit. Primers for the real-time PCR analysis were designed using Primer5 Software, based on the cDNA sequences available in GenBank (PPAR-α, HM140628; PPAR-β, HM140629; PPAR-γ, HM140627). Real-time PCR was performed in a Mini Option real-time detector (Bio-Rad, USA). The fluorescent quantitative PCR solution consisted of 5 μl SYBR® premix Ex Taq™ (2×), 0.4 μl PCR forward primer (20 μM), 0.4 μl PCR reverse primer (10 μM) (primers sequences are given in Table 4), 1.0 μl RT reaction (cDNA solution) and 3.2 μl dH2O. The cDNA solution was previously diluted to 20 %. The reaction conditions were as follows: 95 °C for 30 s followed by 40 cycles consisting of 95 °C for 5 s and 57 °C for 30 s. The fluorescent flux was then recorded, and the reaction continued at 72 °C for 6 s and 95 °C for 5 s. All amplicons were initially separated by agarose gel electrophoresis to ensure that they were of correct sizes. The gene expression levels were normalized toward the mean of the two reference genes (β-actin and Rpl13a). Normalized gene expressions of the control group were set to 1, and the expression of each target gene for the groups with added vitamin E was expressed relative to the control group. The optimized comparative Ct (2−ΔΔCt) value method was used here to estimate gene expression levels.
Statistical analysis
All data were subjected to Levene’s test of equality of error variances and one-way ANOVA followed by Tukey’s test using SPSS 16.0 (SPSS 16.0, Michigan Avenue, Chicago, IL, USA). Probability values of P < 0.05 were considered statistically significant. Dietary vitamin E requirement of blunt snout bream juveniles was estimated by the broken-line regression (Robbins 1986).
Results
Growth performances of the fish fed diets contained different levels of vitamin E for 9 weeks are given in Table 5. Final weight, body weight gain and specific growth rate significantly increased with incremental dietary vitamin E levels from 11.2 to 56.3 mg/kg, but no significant differences were observed among E56.3, E114.6, E306.5 and E588.4 groups. There were no significant differences in survival and condition factor (CF) among treatments. HSI significantly decreased with incremental dietary vitamin E concentrations. The broken-line model was employed to determine the optimal point for the maximal body weight gain (Fig. 1). The result showed that the breakpoint at 55.5 mg/kg based on body weight gain, giving the least mean square error.
The whole body composition such as total lipid, moisture, ash and crude protein was not influenced by increasing levels of dietary vitamin E. Contents of total lipid content (dry mass), moisture, crude ash (dry mass) and crude protein (dry mass) were 25.7 ± 2.5, 58.9 ± 1.2, 9.8 ± 0.5 and 62.6 ± 1.5 %, respectively.
Liver fatty acid compositions of the fish fed test diets are shown in Table 6. The levels of 18:2n-6 and total n-6 fatty acids in liver decreased with increasing levels of dietary vitamin E. In contrast, the levels of EPA and DHA increased with increasing levels of vitamin E supplementation. Liver total n-3 fatty acid level in the fish fed the E588.4 diet was the highest, while the lowest in the fish fed the E11.2 diet.
Liver vitamin E concentrations increased with incremental levels of dietary vitamin E (Table 7). The liver vitamin E concentration in fish fed E306.5 diet increased significantly compared with the E11.2 group. The highest liver vitamin E concentration was detected in fish fed the E588.4 diet, which was significantly higher than that in E11.2, E56.3 and E114.6 group. Liver TBARS values are presented in Table 7. No significant differences were detected on the liver TBARS value, although the value trended a slight decrease with incremental dietary vitamin E levels.
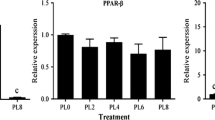
The expression levels of PPAR-α, PPAR-β and PPAR-γ in liver are shown in Fig. 2. The expressions of PPAR-α in E56.3, E114.6, E306.5 and E588.4 groups were significantly lower than that in E11.2 group. The highest expression of PPAR-β was found in fish fed E56.3 diet, and the expression of PPAR-β decreased with increasing dietary vitamin E levels from 56.3 to 588.4 mg/kg. Similarly, PPAR-γ expression in liver significantly decreased with increasing dietary vitamin E levels from 56.3 to 588.4 mg/kg.
Relative mRNA expression levels of PPARs (PPAR-α, PPAR-β, PPAR-γ) genes in liver (pooled livers of three fish per tank) of blunt snout bream fed different levels of vitamin E. Different letters above the bars showed significant difference among different treatments (P < 0.05), data with same alphabets are not significantly different (P > 0.05)
Discussion
In the present study, body weight gain of blunt snout bream markedly increased with increasing supplemented vitamin E levels from 11.2 to 56.3 mg/kg. Results of the broken-line model indicated that the vitamin E requirement of blunt snout bream juveniles for optimum growth was considered to be 55.53 mg/kg under the present experimental conditions, which was similar to most fish species, e.g., 50 mg/kg for rainbow trout (Salmo gairdneri) (Cowey et al. 1983), 50 mg/kg for channel catfish (I. punctatus) (Wilson et al. 1984) and 31 mg/kg for red drum (Sciaenops ocellatus) (Li and Gatlin 2009). However, Zhou et al. (2013b) found that dietary supplementation of 100–150 mg/kg vitamin E promoted M. amblycephala growth, improved meat quality and strengthened the immune system of the fish. It is well known that the dietary vitamin E requirement of fish is affected by several factors such as fish size, cultured condition, the dietary lipid level, the degree of unsaturation of the dietary lipid source and the oxidation of the dietary lipid. There were no significant differences in growth performance when supplementations of vitamin E exceed 56.3 mg/kg (even the vitamin E content in E588.4 group was about 10 times higher than that in E56.3 group). It indicated that an excess in vitamin E supplement did not further improve the growth performance of the fish. The similar cases also appeared in several previous studies (Chen et al. 2004; Yong et al. 2014; Hua et al. 2014). This suggested that 56.3 mg/kg vitamin E level reached a saturated requirement of blunt snout bream juvenile. In this study, the body weight gains in all groups were lower compared to our previous study using same fish species with similar initial weight (Li et al. 2015). This could be because the feeding trial in this study was conducted under a relatively low water temperature (20–24 °C).
Hepatosomatic index (HSI) value has been reported to be correlated with the amount of fat deposition (Gao et al. 2012b), who reported that supplementation of vitamin E significantly reduced whole body fat content and HSI value in red sea bream (Pagrus major). Similarly, in this study, the highest HIS value was found in fish fed the E11.2 diet. But the value decreased with incremental dietary supplementation of vitamin level, indicating that dietary vitamin E promoted liver fat metabolism. Vitamin E functions as a lipid-soluble antioxidant, protecting biological membranes and lipoproteins against oxidation. Baker and Davies (1996) reported that when fish were fed a diet without a vitamin E supplement, the lipid radicals could not be digested, which probably resulted in fatty infiltration into liver cells and enlarging the live size. Similar results were also found in halibut (Hippoglossus hippoglossus) (Tocher et al. 2003) and gilthead sea bream (Sparus aurata) (Mourente et al. 2002).
LC-PUFA, particularly EPA and DHA, are particularly sensitive to lipid peroxidation. Vitamin E plays an antioxidant role in cell membranes by preventing fatty acids and cholesterol against lipid oxidation. Therefore, it is believed that LC-PUFA must combine with vitamin E to protect against physiological oxidation (Hamre and Lie 1995; Gao et al. 2012b). In the present study, the percentages of liver EPA, DHA and total n-3 LC-PUFA significantly increased with increasing levels of dietary vitamin E from 11.2 to 588.4 mg/kg meanwhile DHA and total n-3 LC-PUFA had no significant differences among E11.2, E56.3, E114.6 and E306.5. In addition, it was interesting that DHA in the liver was 2.5-fold increased when vitamin E level increased from 11.2 to 588.4 mg/kg and hepatic n-3 LC-PUFA was more than doubled between the fish fed the extreme diets. The positive correlation between liver n-3 PUFA and dietary vitamin E level was also observed in channel catfish (I. punctatus) (Lim et al. 2010). Moreover, Lebold et al. (2011) reported that feeding zebrafish (Danio rerio) a vitamin E-deficient diet resulted in decreasing the percentage of n-6 and n-3 LC-PUFA of fish viscera. Thus, the protective effect of vitamin E could be a factor contributing to the increase in liver n-3 LC-PUFA content (Lim et al. 2010). Several previous studies have reported that LC-PUFA synthesis is affected by the concentration of vitamin E. Lebold et al. (2011) observed that zebrafish fed a vitamin E-deficient diet had a higher expression of the genes responsible for LC-PUFA synthesis like fads2 and elovl2 than those fed a diet with adequate vitamin E.
Results from the present study showed that liver deposition of vitamin E increased directly in response to elevated dietary supplementation of vitamin E in blunt snout bream as reported for other fish species including rainbow trout (S. gairdneri) (Jumroensri et al. 2005), coho salmon (Oncorhynchus kisutch). (Chen et al. 2004), channel catfish (I. punctatus) (Bai and Gatlin 1993) and red sea bream (Pagrus major) (Gao et al. 2012b). Hepatic TBARS analysis is one of the most popular and widely used methods for assessing tissue peroxidation. Furthermore, malondialdehyde (MDA), as a secondary oxidation product of polyunsaturated fatty acids, is an important indicator of the oxidative changes of lipid (Mourente et al. 2000). Supplementation of dietary vitamin E could reduce the liver TBARS value in fish has been demonstrated in a number of studies (Gao et al. 2012b; Qi et al. 2000).
PPARs play a critical role in regulating insulin sensitivity and lipid metabolism. PPAR-α gene plays an important role in the control of lipid metabolism, by regulating the expression of target genes encoding enzymes involved in the peroxisomal and mitochondrial β-oxidation of fatty acids of blunt snout bream (Lu et al. 2013). PPAR-β induced fatty acid oxidation by regulating genes involved in fatty acid transport, β-oxidation, and mitochondrial respiration in various tissues (Dressel et al. 2003; Yang et al. 2016). PPAR-γ plays an important role in lipid accumulation and adipocyte differentiation which are associated with insulin resistance and diabetes in humans (Stumvoll and Haring 2002). Overall, the expressions of PPAR-α, PPAR-β and PPAR-γ were down-regulated by supplementation of dietary vitamin E level from 56.3 to 588.4 mg/kg. The downregulation of three PPAR gene expressions in the fish fed diets with incremental vitamin E level might be associated with antioxidant effects of vitamin E. The n-6 and n-3 LC-PUFA have been shown to promote lipid oxidation by inducing PPAR-α (Jump 2002). Therefore, supplementation of dietary vitamin E increased tissues n-3 LC-PUFA against lipid oxidation through suppression expression of PPARs genes. To date, this was the first study to determine the effects of vitamin E supplementation on PPARs expressions in fish, and further studies are needed to investigate the reason for these effects.
The results of this study indicated that supplementation of dietary vitamin E could improve growth performance in blunt snout bream juveniles. When the broken-line model was employed to estimate the adequate requirement of vitamin E based on body weight gain, the optimal level was 55.5 mg/kg in diet. However, increasing the dietary vitamin E increased the percentages of liver EPA, DHA and total n-3 LC-PUFA and decreased the expression levels of PPARs in the liver of blunt snout bream. Reduced expressions of liver PPARs might be due to an increase of n-3 LC-PUFA by antioxidant effects of vitamin E. The mechanism of effects of dietary vitamin E on PPARs expression should be studied in future. In conclusion, supplementation of more than 55.5 mg/kg vitamin E may improve growth and increased n-3 LC-PUFA in blunt snout bream, which is beneficial to human consumer.
References
A.O.A.C (1990) Official methods of analysis of the association of official analytical chemists, 15th edn. Association of Official Analytical Chemists, Arlington
Bae JY, Park GH, Yoo KY, Lee JY, Kim DJ, Bai SC (2013) Evaluation of optimum dietary vitamin E requirements using DL-alpha-tocopheryl acetate in the juvenile eel, Anguilla japonica. J Appl Ichthyol 29:213–217
Bai SC, Gatlin DM (1993) Dietary vitamin-E concentration and duration of feeding affect tissue alpha-tocopherol concentrations of channel catfish (Ictalurus punctatus). Aquaculture 113:129–135
Baker RTM, Davies SJ (1996) Oxidative nutritional stress associated with feeding rancid oils to African catfish, Clarias gariepinus (Burchell) and the protective role of α-tocopherol. Aquac Res 27:795–803
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Chen HH, David AH, Shannon KB, Robert HD (2004) Effect of dietary vitamin E on growth, tissue lipid peroxidation, and erythrocyte fragility of transgenic coho salmon, Oncorhynchus kisutch. Comp Biochem Physiol A 139:199–204
Cowey CB, Adron JW, Youngson A (1983) The vitamin E requirement of rainbow trout (Salmo gairdneri) given diets containing polyunsaturated fatty acids derived from fish oil. Aquaculture 30:85–93
Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE (2003) The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol 17:2477–2493
Fang F, Kang ZF, Wong CW (2010) Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol Nutr Food Res 54:345–352
Gao J, Koshio S, Ishikawa M, Yokoyama S, Ren T, Komilus CF, Han Y (2012a) Effects of dietary palm oil supplements with oxidized and non-oxidized fish oil on growth performances and fatty acid compositions of juvenile Japanese sea bass, Laterolabrax japonicas. Aquaculture 324–325:97–103
Gao J, Koshio S, Ishikawa M, Yokoyama S, Mamauag REP, Han Y (2012b) Effects of dietary oxidized fish oil with vitamin E supplementation on growth performance and reduction of lipid peroxidation in tissues and blood of red sea bream Pagrus major. Aquaculture 356–357:73–79
Hamre K (2011) Metabolism, interaction, requirements and functions of vitamin E in fish. Aquac Nutr 17:98–115
Hamre K, Lie Ø (1995) α-Tocopherol levels in different organs of Atlantic salmon (Salmo salar L.)—Effect of smoltification, dietary levels of polyunsaturated fatty acids and vitamin E. Comp Biochem Physiol A 111:547–554
Hua XN, Yu DJ, Peng H, Zhen M, Ji LL (2014) Effect of dietary vitamin E on the growth performance and nonspecific immunity in sub-adult turbot (Scophthalmus maximus). Fish Shellfish Immunol 41:501–506
Jump DB (2002) Dietary polyunsaturated fatty acids and regulation of gene transcription. Curr Opin Lipidol 13:155–164
Jumroensri P, Kiront V, Satoh S, Watanabe T (2005) Antioxidant defense of rainbow trout (Oncorhynchus mykiss) in relation to dietary n-3 highly unsaturated fatty acids and vitamin E contents. Comp Biochem Physiol C 140:187–196
Kliewer SA, Xu HE, Lambert MH, Willson TM (2001) Peroxisome proliferator-activated receptors from genes to physiology. Recent Prog Horm Res 56:239–265
Kuenzli S, Saurat JH (2003) Peroxisome proliferator-activated receptors in cutaneous biology. Br J Dermatol 149:229–236
Lebold KM, Jump DB, Miller GW, Wright CL, Labut EM, Barton CL, Tanguay RL, Traber MG (2011) Vitamin E deficiency decreases long-chain PUFA in Zebrafish (Danio rerio). J Nutr 141:2113–2118
Lee CH, Olson P, Evans RM (2003) Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144:2201–2207
Li P, Gatlin DM (2009) Dietary vitamin E requirement of the red drum Sciaenops ocellatus. Aquac Nutr 15:313–319
Li Y, Gao J, Huang S (2015) Effects of different dietary phospholipid levels on growth performance, fatty acid composition, PPAR gene expressions and antioxidant responses of blunt snout bream Megalobrama amblycephala fingerlings. Fish Physiol Biochem 41:423–436
Lim C, Yildirim-Aksoy M, Shelby R, Li MH, Klesius PH (2010) Growth performance, vitamin E status, and proximate and fatty acid composition of channel catfish, Ictalurus punctatus, fed diets containing various levels of fish oil and vitamin E. Fish Physiol Biochem 36:855–866
Lin YH, Shiau SY (2005) Dietary vitamin E requirement for grouper, Epinephelus malabaricus, at two lipid levels, and their effects on immune responses. Aquaculture 248:235–244
Liu B, Xu P, Xie J, Ge XP, Xia SL, Song CY, Zhou QL, Miao LH, Ren MC, Pan LK, Chen RL (2014) Effects of emodin and vitamin E on the growth and crowding stress of Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol 40:595–602
Lu KL, Xu WN, Li XF, Liu WB, Wang LN, Zhang CN (2013) Hepatic triacylglycerol secretion, lipid transport and tissue lipid uptake in blunt snout bream (Megalobrama amblycephala) fed high-fat diet. Aquaculture 408–409:160–168
Mourente G, Diaz-Salvago E, Tocher DR, Bell JG (2000) Effects of dietary polyunsaturated fatty acid/vitamin E (PUFA/tocopherol) ratio on antioxidant defence mechanisms of juvenile gilthead sea bream (Sparus aurata L, Osteichthyes, Sparidae). Fish Physiol Biochem 23:337–351
Mourente G, Diaz-Salvago E, Bell JG, Tocher DR (2002) Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: attenuation by dietary vitamin E. Aquaculture 214:343–361
Mourente G, Bell JG, Tocher DR (2007) Does dietary tocopherol level affect fatty acid metabolism in fish? Fish Physiol Biochem 33:269–280
Qi C, Zhu Y, Reddy JK (2000) Peroxisome proliferator-activated receptors, coactivators, and downstream targets. Cell Biochem Biophys 32:187–204
Robbins KR (1986) A method, SAS program, and example for fitting the broken line to growth data. University of Tennesse Agricultural Experiment Station Research Report. University of Tennesse, Knoxville
Ruyter B, Andersen O, Dehli A, Ostlund Farrants AK, Gjoen T, Thomassen MS (1997) Peroxisome proliferator activated receptors in Atlantic salmon (Salmo salar): effects on PPAR transcription and acyl-CoA oxidase activity in hepatocytes by peroxisome proliferators and fatty acids. Biochim Biophys Acta 1348:331–338
Sau SK, Paul BN, Mohanta KN, Mohanty SN (2004) Dietary vitamin E requirement, fish performance and carcass composition of rohu (Labeo rohita) fry. Aquaculture 240:359–368
Stumvoll M, Haring H (2002) The peroxisome proliferator-activated receptor-gamma 2 pro12Ala polymorphism. Diabetes 51:2341–2347
Tocher DR, Mourente G, Vander Eecken A, Evjemo JO, Diaz E, Wille M, Bell JG, Olsen Y (2003) Comparative study of antioxidant defence mechanisms in marine fish fed variable levels of oxidised oil and vitamin E. Aquacult Int 11:195–216
Wilson RP, Bowser PR, Poe WE (1984) Dietary vitamin E requirement of fingerling channel catfish. J Nutr 114:2053–2058
Yagi K (1987) Lipid peroxides and human disease. Chem Phys Lipids 45:337–351
Yang L, Xiao L, Yin Z, Jian G (2016) Effects of different dietary soybean oil levels on growth, lipid deposition, tissues fatty acid composition and hepatic lipid metabolism related gene expressions in blunt snout bream (Megalobrama amblycephala) juvenile. Aquaculture 451:16–23
Yong KZ, Ting TH, Yong HW, Lei P, Xiao JC, Wei MW, Jian G (2014) Effects of vitamin E supplementation on growth, lipid peroxidation and fatty acid composition of Dojo loach (Misgurnus anguillicaudatus Cantor) fingerlings. Aquac Res. doi:10.1111/are.12612
Zhou M, Liu B, Ge XP, Xie J, Chen RL, Cui YT, Wan JJ, Michael HH, Habte-Tsion MH (2013a) Effects of vitamin E on serum biochemical indexes and antioxidant capacity of Megalobrama amblycephala under acute high temperature stress and recovery. J Fish China 37:1369–1377 (in Chinese)
Zhou M, Liu B, Ge XP, Xie J, Wan JJ, Cui SL (2013b) Effects of vitamin E on growth, physiological and biochemical indexes of blood and muscle physiochemical indices in Wuchang bream Megalobrama amblycephala. Chin J Anim Nutr 25:1488–1496 (in Chinese)
Zhou QC, Wang LG, Wang HL, Wang T, Elmada CZ, Xie FJ (2013c) Dietary vitamin E could improve growth performance, lipid peroxidation and non-specific immune responses for juvenile cobia (Rachycentron canadum). Aquac Nutr 19:421–429
Acknowledgments
This study was supported by the Fundamental Research Funds for the Central Universities of China (Project 2013PY074).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, Y., Liang, X. et al. Effects of dietary vitamin E supplementation on growth performance, fatty acid composition, lipid peroxidation and peroxisome proliferator-activated receptors (PPAR) expressions in juvenile blunt snout bream Megalobrama amblycephala . Fish Physiol Biochem 43, 913–922 (2017). https://doi.org/10.1007/s10695-016-0224-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0224-4