Abstract
A 90-day feeding trial was conducted on the growth performance, feeding efficacy, body indices, various hematological and plasma biochemical parameters, and histopathological examination of the gonads from male and female Nile tilapia fingerlings when fed different crude plant extracts from Cinnamomum camphora, Euphorbia hirta, Azadirachta indica, or Carica papaya at 2 g kg−1 compared to a control diet. This was followed by a 14-day challenge to Streptococcus agalactiae. All treatments were triplicated, and each treatment consisted of 30 fish. Results showed that C. papaya extracts were the most effective at delaying gonadal maturation to both male and female tilapia, as well as significantly increasing (P < 0.05) growth performance compared to the control treatment. Similarly, dietary C. camphora and E. hirta extracts also significantly improved growth, while no significant growth effect was detected between the A. indica and control treatments (P > 0.05). Further, crude body lipid was lower in the C. camphora, E. hirta and C. papaya treatments, but was only significantly lower for the E. hirta treatment compared to the control. Meanwhile, none of the hematological or biochemical parameters were significantly affected, although plasma ALT was significantly lower for tilapia fed A. indica compared to the control. After the 14-day bacterial challenge, tilapia fed C. camphora supplementation had significantly higher survival, compared to the control, but was not significantly higher than the other supplemented diets. Results indicate that dietary C. papaya extract can significantly promote growth and delay gonadal maturation to both male and female tilapia, while C. camphora was the most effective prophylactic to S. agalactiae and may be a cost-effective and eco-friendly alternative to antibiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tilapia are currently the second most farmed fish group in the world due to many beneficial characteristics including fast growth, tolerance to adverse environmental conditions, and high market demand (Ng and Romano 2013). However, a major drawback is their precocious spawning since this can lead to uneven harvesting sizes, high stocking densities, resource wastage, and potentially energy channeled for reproduction, which could otherwise be for somatic growth. One method to mitigate unwanted reproduction is the farming of monosex male populations through manual sexing, direct sex reversal to males at the early fry stage or using “supermale” technology, by sexually reversing male broodstock to produce eggs (Beardmore et al. 2001; Singh 2013). While these methods have been shown to be successful, they can be time-consuming, expensive and, moreover, the use of hormones for food fish is increasingly becoming regulated in some areas. Subsequently, there has been increasing interest in the use of more environmentally friendly and inexpensive methods to induce sterility in fish which have included various plant meals and extracts (Jegede and Fagbenro 2008a, b; Obaroh and Nzeh 2013; Khalil et al. 2014).
It has been demonstrated that the gonadal maturation of tilapia was delayed from the ingestion of different plants including papaya, Carica papaya, seed powder (Jegede and Fagbenro 2008a; Abbas and Abbas 2011; Khalil et al. 2014), mango, Mangifera indica, leaf extract (Obaroh and Nzeh 2013) and neem, Azadirachta indica, leaf meal (Jegede and Fagbenro 2008b) and extract (Obaroh and Achionye-Nzeh 2011; 2013). Among these, it was shown that C. papaya seed powder decreased male and female sex hormones to tilapia, which was suggested as the cause to delaying gonadal maturation (Khalil et al. 2014). Meanwhile, sterility was induced in mammals when fed diets containing extracts of asthma weed, Euphorbia hirta (Mathur et al. 1995), or camphor bark, Cinnamomum camphora (Jamshidzadeh et al. 2006); however, to the best of our knowledge, the effects of these plants to fish reproduction are yet unknown.
Moreover, the benefits of certain plants and their extracts as dietary supplementations may also extend to promoting growth and/or acting as prophylactics to various pathogenic bacteria (Galina et al. 2009; Reverter et al. 2014). Generally, the results have been promising, and with antibiotic use becoming more restricted in the aquaculture industry, this area has been receiving increasing attention (Galina et al. 2009; Reverter et al. 2014). In an early study, Logambal et al. (2000) showed that the dietary supplementation of holy basil, Ocimum sanctum, increased antibody responses of Mozambique tilapia and their subsequent resistance to the bacterial pathogen Aeromonas hydrophila. More recently, E. hirta powder supplementations to the diets of Asian seabass, Lates calcarifer, acted as an immunostimulator and enhanced their resistance to Vibrio harveyi (Talpur and Ikhwanuddin 2013). Moreover, the inclusion of dietary Cinnamomum verum oil and C. kanehirae extract injections improved the resistance of Nile tilapia and the white shrimp, Litopenaeus vannamei, to pathogenic bacteria, respectively (Yeh et al. 2009; Rattanachaikunsopon and Phumkhachorn 2010).
One of the most common worldwide diseases during tilapia culture that leads to substantial mortalities is caused by Streptococcus spp. (Suanyuk et al. 2008; Li et al. 2014), and identifying supplements that can act as effective prophylactics to pathogenic bacteria would clearly benefit the aquaculture industry. However, to the best of our knowledge, no information is available on the use of extracts from C. papaya, E. hirta, A. indica, or C. camphora on disease resistance to tilapia. Assessing the prophylactic effectiveness, as well as any potential detrimental effects to productivity and overall health status, is an important criteria for the long-term practical use of dietary supplements particularly since A. indica, C. papaya, and E. hirta can induce toxic side effects or even mortalities to fish (Ayotunde et al. 2011; Mousa et al. 2008; Abbas and Abbas 2011; Saravanan et al. 2010; Sheikhlar et al. 2011). The aim of the current study was to evaluate the effects of dietary supplementations of C. camphora, E. hirta, A. indica, and C. papaya crude extracts to the growth performance, survival, feed efficiency, body indices, whole-body proximate composition, various blood and plasma parameters, and gonadal maturation of Nile tilapia after 90 days and their subsequent resistance to S. agalactiae challenge.
Materials and methods
Extract and diet preparation
Extracts from four different plants were examined which included camphor (Cinnamomum camphora) bark, asthma weed (Euphorbia hirta), neem leaf (Azadirachta indica), and papaya (Carica papaya) seed. The bark of C. camphora was collected from a forest in Melaka, Malaysia, while, for E. hirta, the flowers, leaves, and stems were harvested from a terrestrial farm in Melaka, Malaysia. The leaves of A. indica were collected from trees at the Universiti Putra Malaysia campus, while C. papaya seeds were purchased from a local grocery store (Serdang, Malaysia).
Soon after obtaining the plant materials, these were brought to the laboratory, thoroughly washed with sterile distilled water and air-dried until constant weight. The plant material was then ground to a fine powder using an electric grinder, and 200 g of each sample was then immersed in one liter of methanol for 3 days at room temperature (Lam et al. 2000). This was then filtered through a Whatman filter paper (Whatman™, Florham Park, NJ, USA), and the solution was evaporated using a rotary vacuum with reflux on a water bath at 50 °C (Eyela, N-1001; Tokyo Rikakikai co., Ltd). The residue was stored in screw tight glass vials at −20 °C until use.
All extracts were added to the diets of tilapia at 2 g kg−1, since a previous study showed that C. papaya seed at 2 g kg−1 was effective to delay gonadal maturation in tilapia (Jegede and Fagbenro 2008a; Abbas and Abbas 2011), while the other extracts were added in the same amount to compare their relative efficacy. To add these to the diets, 2 g of each extract was dissolved in 16 mL cyclohexane, which was then sprayed on one kilogram of a basal diet designed for tilapia fry, containing 40 % crude protein and 5 % crude lipid (Ding Ding, Malaysia), when being mixed at low speed in a Hobart mixer. For the control diet, 2 g of cellulose was added to 16 mL cyclohexane. The diets were then prepared according to Saccol et al. (2013) in which after mixing the diets with water, they were left to dry under forced air until they no longer smelled of the solvent, broken and stored in a freezer at −20 °C until use.
Experimental animals and design
Nile tilapia fingerlings were purchased from a local tilapia farm (Serdang, Malaysia) and acclimated for 15 days in a fiberglass 1000-L tank and fed on a commercially available diet to satiation. After acclimation, a total of 300 tilapia (4–4.2 g) were equally and randomly distributed into 15 glass aquaria (total of 20 fish/replicate). Each treatment was triplicated and organized randomly. The aquaria were filled with 100 L of filtered and de-chlorinated public utility water. All aquaria received gentle aeration via an airstone as well as an individual preconditioned biological filter to maintain water quality. The tilapia were hand-fed daily to satiation with their designated feed for a total of 90 days.
During the 90-day trial, each aquaria received a 75 % water exchange each week, and prior to and after the water exchange, the ammonia-N, temperature, pH, and dissolved oxygen were measured using a digital probe which were <0.4 mg L−1, 27–29 °C, 6.7–7.1, and >6 mg L−1 throughout the trial. At 30-day intervals, the fish were batched weighed in each treatment to calculate the relative growth rates. At the end of the trial, the tilapia mildly anesthetized using clove oil and all were individually measured for total wet weight (g) and total length (cm). A total of 150 fish from each treatment were later used for the bacterial challenge test in new aquaria, while the remaining fish were used to measure various body indices, immunological parameters, and whole-body proximate composition.
Growth performance parameters, survival, and feeding efficiency
At 30-day intervals, the fish were batched weighed in each treatment to calculate the relative growth rates. At the end of the trial, the tilapia mildly anesthetized using clove oil and then all were individually counted and measured for total wet weight and total length in order to calculate the survival rate, weight gain (WG), specific growth rate (SGR), condition factor (K), and feed conversion ratio (FCR). The equations used to calculate the results of each parameter were as follows:
where W 1 = final weight, W 0 = initial weight, and T = time in days
where W 2 and W 1 are the final weight and initial weight, respectively.
Body indices and whole-body proximate composition
Six fish, three males and three females, from each tank were dissected for the liver, spleen, and gonads and weighed to calculate the hepatosomatic index (HSI), spleno-somatic index (SSI), and gonadosomatic index (GSI), respectively. The gonads were then fixed in formalin (10 % v/v) for later histopathological analysis. From different fish, the caudal vein was severed, from a total of six fish comprising three males and three females, in each treatment, and the blood was collected (>1 mL) into heparinized tubes, placed on ice, and later analyzed for plasma liver enzymes as well as blood and plasma biochemical and hematological parameters based on treatment and sex. The fish were then wrapped in paraffin, stored at −20 °C, and measured for the whole-body proximate composition according to standard AOAC methods (AOAC 1997).
Gonadal histopathology
After being fixed in formalin (10 % w/w) for 24 h, the gonads from males and females were processed at increasing concentrations of ethanol, cleared in xylene and then in paraffin wax. After embedding each sample, sections (5 μm) were made using a rotary microtome and then stained with hemotoxylin and eosin to examine the gonadal maturation. In order to quantify the gonadal stages of females in each treatment, the eggs were categorized as Stage I, Stage II, Stage III, and Stage IV oocytes which were counted from three different pictures from each of the three replicates. These percentages were then averaged for each replicate. Stage I were in the chromatin nucleolus or perinuclear stage (pre-vitellogenic state), Stage II were those that were in the cortical alveolar or early vitellogenesis stage, Stage III were those in the late or post-vitellogenic stage, and Stage IV were those that ovulated. These stages were classified based on slightly modified criteria from Srijunngam and Wattanasirmkit (2001).
Plasma and blood hematological, biochemical, and liver enzyme parameters
The blood of tilapia was briefly kept on ice and then centrifuged at 3000×g for 10 min at 4 °C to measure the packed cell volume (PCV), hemoglobin, red blood cells (RBC), white blood cells (WBC), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC) according to Sarder et al. (2001). Meanwhile, the plasma was then analyzed for protein, creatine, albumin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activity on a Hitachi 902 automated analyzer.
Challenge with Streptococcus agalactiae
After 90 days of the feeding trial, all remaining tilapia were then placed in new glass aquaria, in triplicate, in preparation for the bacterial challenge, and were continued to be fed their respective diets for 10 days. After 2 days, a minimum of three fish from each treatment was killed to verify whether they were free from Streptococcus agalactiae by killing three fish from each treatment, aseptically removing the kidneys and brain, streaked onto blood agar plates, and incubated at room temperature. This procedure also allowed the same amount of fish in each treatment (30 fish/treatment for a total of 150 fish) and was then continued to be fed their respective diets and every 3 days, each aquarium received a 100 % water exchange during acclimation and challenge test.
To prepare the bacteria for injection, all procedures were conducted under sterile conditions. A frozen (−80 °C) stock of S. agalactiae (strain no. ATCC 27956), containing glycerol, was previously isolated and identified from infected tilapia (Alsaid et al. 2010). The bacteria were then streak plated onto tryptic soy agar (TSA, USA) for 24 h at 31 °C, and then, three colony-forming units were then transferred into a tryptic soy broth (TSB, USA) and incubated at 31 °C in a shaker for another 24 h. This broth was then serially diluted in sterile distilled water to yield a bacterial concentration of 1 × 106 CFU mL−1, based on prior plating and optical densities, and was intramuscularly injected into all tilapia at 0.5 mL. All fish were daily observed for any clinical signs, abnormal behavior, or mortalities over the 14-day challenge test and were fed their respective diets. Throughout the acclimation and bacterial challenge test, the water temperature, DO, pH, and ammonia were 28–29 °C, 5.5–5.8 mg L−1, 7.2–7.6, and <0.5 mg L−1, respectively.
Statistical analysis
The WG, SGR, FCR, survival, and mortalities after bacterial challenge data were subjected to a one-way ANOVA after prior confirmation of data homogeneity, and any significant differences were identified using Duncan’s post hoc test. A two-way ANOVA was used to analyze the proximate composition, body indices (HSI, SSI, and GSI), and the various blood parameters, after prior confirmation of data homogeneity, and the main effects were treatment, sex, and any interactions. If a significant interaction was detected, a simple main effects analysis was then performed. All statistical analysis was performed using Statistical Analysis System (SAS).
Results
Growth performance, survival, and feeding efficiency
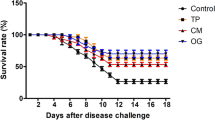
For the growth performance, tilapia fed C. papaya, C. camphora, and E. hirta extracts had significantly higher (P < 0.05) weight gain and specific growth rates compared to those in the A. indica or control treatment (no extract added). Survival remained high in all treatments and ranged from 91.6 to 95 %, and no significant differences among treatments were detected (P > 0.05). Meanwhile, the feed conversion ratio and condition factor were significantly better (P < 0.05) in the C. papaya, C. camphora, and E. hirta treatments compared to the A. indica and control treatment (Table 1). The relative growth rates increased month after month; however, by the final month, tilapia in the control and A. indica treatments were significantly lower (P < 0.05) than the other groups (Fig. 1).
Body indices and whole-body proximate composition
The HSI, SSI, and GSI of tilapia fed different plant extracts are presented in Fig. 2. There were no significant treatment, sex, or interaction effects on the SSI; however, both treatment and sex had a significant effect on the HSI and GSI (Table 2). The HSI was significantly lower (P < 0.01) in the E. hirta treatment, for both males and females, and A. indica treatment, for only females, compared to the others. Meanwhile, regardless of sex, the HSI among the C. camphora, C. papaya, and control treatments was not significantly different from each other (P > 0.05). For the GSI, there was a significant interaction (P < 0.05) between the treatment and sex and a simple main effect analysis showed the GSI of both males and females was significantly lower (P < 0.01) in the C. papaya treatment compared to all other treatments. The A. indica treatment also had significantly lower GSI, for both males and females, compared to the C. camphora, E. hirta, and control treatments.
The proximate composition from the whole body of tilapia in different treatments is shown in Table 3, and only crude lipid was significantly different among the treatments. Tilapia in the C. camphora and C. papaya treatments had slightly (but not significantly) reduced crude whole-body lipid, while only those fed diets with E. hirta extract had significantly lower (P < 0.05) whole-body crude lipid compared to the control treatment.
Blood/plasma biochemical and hematological parameters
The plasma protein, creatine, albumin, and AST were not significantly different among the treatments (Table 4). However, for ALT, this was significantly lower (P < 0.01) in the A. indica treatment compared to the control, while ALT was significantly higher (P < 0.01) in the C. camphora treatment compared to tilapia fed E. hirta extracts (Table 4). For the various blood and plasma hematological parameters, no significant differences among treatments were detected (P > 0.05) (Table 5).
Gonadal histopathology
Histopathological examination of the control ovaries appeared normal, with no pathological lesions and the follicle size increased with maturation (Fig. 3a). The ovaries reached the post-vitellogenic stage, and there were some instances of collapsed follicles indicating post-ovulation. Similar observations were also found for tilapia in the E. hirta extract treatment. For tilapia fed the C. camphora- and A. indica-treated diet, there were mature follicles but none that reached the post-ovulatory stage. Meanwhile, tilapia fed diets with the C. payaya extract were observed to have numerous groups of oogonia and small germ cells embedded within ovigerous lamellae in the ovary as well as pre-vitellogenic follicles (Fig. 3b). Quantification of the ovarian maturation is presented in Table 6. Results showed significantly more (P < 0.05) Stage I oocytes in the C. papaya treatment compared to all others, while those fed the control diet had significantly more (P < 0.05) Stage III eggs than all other treatments. Tilapia fed the C. camphora or A. indica had a similar pattern of oocyte maturation, while only tilapia fed the control or the E. hirta extract diet had ovaries that reached Stage IV.
Histopathology of ovaries from tilapia fed the control diet (a) or diets supplemented with C. papaya extract (b). Note the prevalence of mature follicles (MF) and post-ovulatory follicle (POF) in the control treatment, while there was a higher abundance of pre-vitellogenic (PreV) and vitellogenic (Vit) oocytes for tilapia in the C. papaya extract treatment. Magnification ×20; H&E stain
For the males, the histopathology of the testes for tilapia fed the control diet (Fig. 4a) as well as diets with E. hirta and C. camphora extracts showed advanced stages of maturation and normal testicular tissue architecture with open cyst walls containing spermatozoa. However, tilapia in the A. indica treatment showed signs of cystic seminiferous tubules, testicular structural alterations, and atrophy, while tilapia from the C. payaya treatment showed the presence of secondary and primary spermatocytes as well as spermatozoa distributed throughout the tubules (Fig. 4b).
Histopathology of the testes from tilapia fed the control diet (a) and those fed diets supplemented with C. papaya extract (b). Testes in both treatments show both secondary (SS) and primary spermatocytes (PS), although there was a higher prevalence of PS in the C. papaya extract treatment. Magnification ×20 (a) and magnification × 40 (b); H&E stain
Resistance to S. agalactiae challenge
After injecting tilapia with S. agalactiae, the majority of mortalities occurred from day 5–10 and then gradually stabilized over time, and after 14 days, tilapia fed C. camphora supplementations had significantly less (P < 0.05) cumulative mortality (20 %) compared to the A. indica (46.6 %) and control (43.3 %) treatments (Fig. 5). No significant differences were detected between the C. papaya (30 %) and E. hirta (30 %) treatments compared to the others (P > 0.05) regarding the level of protection against S. agalactiae (Fig. 5). The relative percent survival (RPS) showed that the tilapia fed C. camphora extract had the highest RPS (53.85 %) followed by C. papaya seed extract and E. hirta extract treatments (30.76 %). However, the lowest RPS was in the control and A. indica treatment at 0 %.
Discussion
The results of the current study showed that among the tested plant extracts in the diets of tilapia, C. papaya seed extract was the most effective at delaying gonadal maturation in both males and females, which was evident by significantly lower GSI as well as from histopathological observations and quantification of fewer mature gametes in the gonads. Meanwhile, dietary extracts of C camphora, E. hirta, and C. papaya seed, all acted as significant growth promoters to tilapia, with A. indica extract having no growth effect. Finally, while both dietary extracts of C. papaya seed and E. hirta provided some protection against S. agalactiae challenge, dietary C. camphora extract was shown to be the best prophylactic, which led to significantly less mortalities compared to the control treatment.
Several studies have shown that the use of dietary C. papaya seeds can delay the gonadal maturation of tilapia, which was evident by decreased breeding, reduced GSI, and/or reduced vitellogenic oocytes and secondary spermatids via histopathological observations (Jegede and Fagbenro 2008a; Abbas and Abbas 2011; Khalil et al. 2014). This is in agreement with the current study, since the GSI was significantly reduced and histopathological examination of the gonads revealed a much higher prevalence of immature gametes, which was significant. However, in these studies, C. papaya seeds were in powder form and were shown to have negative consequences. For example, while Farrag et al. (2013) showed that the inclusion of C. papaya seed powder at 6 g kg−1 improved tilapia growth within 45 days, this decreased by day 60. It appears possible this may be due to some toxic effects since a recent study showed that after 60 days, tilapia fed the lowest tested dietary level of C. papaya seed powder (at 2 g kg−1) led to increased plasma AST, which is an indicator of liver damage (Khalil et al. 2014). Meanwhile, Abbas and Abbas (2011) showed that 3 and 6 g kg−1 of dietary C. papaya seed powder caused histopathological liver damage, altered plasma ALT and AST as well as a reduction in some immunological parameters. However, in the current study, after 90 days of feeding C. papaya seed extract at 2 g kg−1 to tilapia, none of the selected parameters to indicate compromised health were different from the control. Moreover, dietary C. papaya extract acted as a significant growth promoter, in which the relative growth rate continued up to 90 days when the experiment ended. The discrepancy between this study and others may be related to the use of methanol extraction, rather than using seed powder, in which potentially some antinutritional factors may have been reduced/removed, although this requires further investigations.
The results for the other plant extracts that included, C. camphora, E. hirta and A. indica, to tilapia gonadal maturation and growth were mixed. In the C. camphora and A. indica treatments, there was a significant reduction in female GSI, compared to the control treatment, but these extracts were not as effective at delaying gonadal maturation as dietary C. papaya seed extract. In contrast, dietary E. hirta extract had no effect to GSI, but this extract as well as C. camphora acted as significant growth promoters to tilapia. The growth-promoting effects of some plants and their extracts as dietary supplements have been suggested to be related to increased feed intake or nutrient utilization (Shalaby et al. 2006; Ji et al. 2007; Abdelhamid and Soliman 2012; Putra et al. 2013; Munglue 2014). For example, 2 g kg−1 of C. camphora leaf meal significantly increased the growth performance, feeding efficiencies and feed intake of tilapia as well as whole-body crude protein and lipid (Abdelhamid and Soliman 2012). Moreover, it was shown that dietary additions of garlic, Allium sativum, at 30 g kg−1 significantly increased the growth performance of Nile tilapia as well as apparent protein, lipid, carbohydrate, and energy digestibility (Shalaby et al. 2006). More recently, it was also found that dietary extracts of lotus, Nelumbo nucifera, peduncle at 1 g kg−1 significantly increased the growth performance of tilapia (Munglue 2014). Finally, it was suggested that fatty acid utilization was improved when olive flounder, Paralichthys olivaceus, were fed a mixture of various herbs consisting of medicated leaven (Massa medicate fermentata), hawthorne (Crataegi fructus), virgate wormwood (Artemisia capillaris), and Cnidium officinale (2:2:1:1) (Ji et al. 2007). Although digestibility was not assessed in the current study, tilapia fed diets supplemented with E. hirta, C. camphora, or C. papaya had significantly better FCR and lower whole-body crude lipid, which may also indicate better lipid metabolism, but requires further investigations.
However, among the tested plant extracts, A. indica was the only treatment to have no effect on tilapia growth and may be related to possible damage to the liver, as evidenced by significantly lower and higher HSI and plasma ALT, respectively. Significant alterations to the ALT and AST were also detected for both Nile tilapia and African catfish, Clarias gariepinus, when A. indica was added to water (Mousa et al. 2008), and it is important to note that this plant is used to kill freshwater pests, including fish (Osuala and Okwuosa 1993; Winkaler et al. 2007). On the other hand, Obaroh and Achionye-Nzeh (2011) found that ethanol extracts of A. indica at 1 g kg−1 significantly improved tilapia growth performance, although higher amounts of 2 and 4–8 g kg−1 had no significant growth improvement and significantly decreased growth, respectively. Considering that the A. indica extract used in the current study had a twofold higher concentration than in the study by Obaroh and Achionye-Nzeh (2011) likely indicate the level used in the current study was excessive. On the other hand, Talpur and Ikhwanuddin (2013) found that dietary A. indica powder, added at 1–5 g kg−1, significantly increased the growth performance and various innate immunological responses of Asian seabass, Lates calcarifer, which may indicate species-specific host differences.
The in vitro antagonistic activities of plants and their extracts to pathogenic bacteria or as dietary prophylactics to various aquatic animals have been receiving increased attention, and to date, over 35 different plants and their extracts have been assessed (reviewed by Galina et al. 2009; Reverter et al. 2014). One of the contributing reasons for increasing research on this area is in response to greater restrictions and regulations on antibiotic use, and certain plants may provide a more environmentally friendly and cheaper alternative. However, to the best of our knowledge, among the tested plants in the current study, only A. indica has been investigated as a potential prophylactic to pathogenic bacteria (Talpur and Ikhwanuddin 2013). It was demonstrated that L. calcarifer fed diets with A. indica powder added from 1 to 5 g kg−1 significantly improved their resistance to Vibrio harveyi challenge, with 4 g kg−1 providing the best protection (Talpur and Ikhwanuddin 2013). Talpur and Ikhwanuddin (2013) suggested this was due to the immunostimulating role of A. indica and their potential antioxidant activities. However, in the current study, A. indica extract was shown to be an ineffective prophylactic for tilapia against S. agalactiae challenge, which may have been due to the harmful effects on the liver or potentially species-specific differences with the pathogen or host.
In contrast, the other tested plant extracts in the current study provided some prophylactic protection with C. camphora leading to significantly less mortalities to S. agalactiae compared to the control. This is consistent with findings from two closely related plant species, cinnamon, C. verum, and stout camphor tree, C. kanehirae (Yeh et al. 2009; Rattanachaikunsopon and Phumkhachorn 2010). It was shown that tilapia fed dietary C. verum oil at 0.4 g kg−1 had improved resistance to S. iniae challenge (Rattanachaikunsopon and Phumkhachorn 2010), while injecting a steam extract from C. kanehirae twigs, at 2 mg g shrimp−1 to L. vannamei, significantly increased their survival when challenged with V. alginolyticus (Yeh et al. 2009). In the later study, a subsequent in vitro analysis of C. kanehirae extracts was shown to enhance various immunological activities, which was suggested as a possible cause for improved disease resistance (Yeh et al. 2009). Meanwhile, it is likely worthy to note that methanol extracts of E. hirta of up to 7 g kg−1 in the diets of C. gariepinus enhanced some hematological parameters after 60 days (Sheikhlar et al. 2011). In the current study, immunological function of tilapia was not measured post-challenge, which points to further research directions.
Conclusions
To the best of our knowledge, this is the first experiment to compare the efficacy of various dietary plant extracts to tilapia gonadal maturation as well as implications to the growth performance, various health indicators, and prophylactic properties to a pathogen. Among the tested extracts, C. papaya seed was the most effective at delaying both male and female gonadal maturation as well as significantly improving growth, while dietary C. camphora bark extract acted as a strong prophylactic to S. agalactiae. It is yet unknown whether a blend of these extracts may offer a wider range of benefits or have any synergistic properties and certainly points to further research directions. Such research, along with the findings of the current study, clearly demonstrates a range of effectiveness that can improve tilapia productivity using cheap and available resources in an environmentally friendly way.
References
Abbas HH, Abbas WT (2011) Assessment study on the use of pawpaw, Carica papaya seeds to control Oreochromis niloticus breeding. Pak J Biol Sci 14:1117–1123
Abdelhamid AM, Soliman AAA (2012) Possibility of using dried leaves of guava and camphor trees in tilapia diets. J Arab Aquac Soc 7:91–108
Alsaid M, Daud H, Bejo SK, Abuseliana A (2010) Antimicrobial activities of some culinary spice extracts against Streptococcus agalactiae and its prophylactic uses to prevent Streptococcal infection in red hybrid tilapia (Oreochromis sp.). World J Fish Mar Sci 2:532–538
Association of Official Analytical Chemists (AOAC) (1997) Official methods of analysis of AOAC international. In: Cunniff, PA (ed.) 16th edn. AOAC International, Arlington
Ayotunde EO, Offem BO, Bekeh AF (2011) Toxicity of Carica papaya Linn: haematological and piscicidal effect on adult catfish (Clarias gariepinus). J Fish Aquatic Sci 6:291–308
Beardmore JA, Mair GC, Lewis RI (2001) Monosex male production in finfish as exemplified by tilapia: applications, problems, and prospects. Aquaculture 197:283–301
Farrag FH, Khalil FF, Mehrim AI, Refaey MMA (2013) Papaw (Carica papaya) seeds powder in Nile tilapia (Oreochromis niloticus) diet. J Anim Poult Prod 4:363–379
Galina J, Yin G, Ardó L, Jeney Z (2009) The use of immunostimulating herbs in fish: an overview of research. Fish Physiol Biochem 35:669–676
Jamshidzadeh A, Sajedianfard J, Nekooeian AA, Tavakoli F, Omrani GH (2006) Effects of camphor on sexual behaviors in male rats. Iran J Pharm Sci 2:209–214
Jegede T, Fagbenro O (2008a) Histology of gonads in Oreochromis niloticus (Trewavas) fed pawpaw (Carica papaya) seed meal diets. In: 8th international symposium on Tilapia in aquaculture, pp 1135–1141
Jegede T, Fagbenro O (2008b) Dietary neem (Azadirachta indica) leaf meal as reproduction inhibitor in redbelly tilapia, Tilapia zillii. In: 8th international symposium on Tilapia in aquaculture, pp 365–373
Ji S-C, Jeong G-S, Gwang-Soon I, Lee S-W, Yoo J-H, Takii K (2007) Dietary medicinal herbs improve growth performance, fatty acid utilization, and stress recovery of Japanese flounder. Fish Sci 73:70–76
Khalil FF, Farrag FH, Mehrim AI, Refaey MMA (2014) Pawpaw (Carica papaya) seeds powder in Nile tilapia (Oreochromis niloticus) diets: 2 Liver status, sexual hormones and histological structure of the gonads. Egypt J Aquat Biol Fish 18:97–113
Lam TL, Lam ML, Au TK, Ip DT, Ng TB, Fong WP, Wan DC (2000) A comparison of human immunodeficiency virus type-1 protease inhibition activities by the aqueous and methanol extracts of Chinese medicinal herbs. Life Sci 67:2889–2896
Li YW, Liu L, Huang PR, Fang W, Luo ZP, Peng HL, Want YX, Li AX (2014) Chronic streptococcosis in Nile tilapia, Oreochromis niloticus (L.), caused by Streptococcus agalactiae. J Fish Dis 37:757–763
Logambal SM, Venkatalakshmi S, Michael RD (2000) Immunostimulatory effect of leaf extract of Ocimum sanctum Linn. in Oreochromis mossambicus (Peters). Hydrobiologia 430:113–120
Mathur A, Dixit VP, Dobal MP (1995) Antifertility plant product: Euphorbia hirta in males. In: Proceedings of the international symposium on male contraception: present and future
Mousa MAA, El-Ashram AMM, Hamed M (2008) Effect of neem leaf extract on freshwater fishes and zooplankton community. In: 8th international symposium on Tilapia in aquaculture, pp 307–318
Munglue P (2014) Effects of dietary Nelumbo nucifera (lotus) peduncle extract on growth performance of Nile tilapia (Oreochromis niloticus). The 1st environmental and natural resources international conference, 6–7th November, The Sukosol Hotel, Bangkok, Thailand, pp 279–310
Ng WK, Romano N (2013) A review of the nutrition and feeding management of farmed tilapia throughout the culture cycle. Rev Aquac 5:220–254
Obaroh IO, Achionye-Nzeh GC (2011) Effects of crude extract of Azadirachta indica leaves at controlling prolific breeding in Oreochromis niloticus (Linnaeus, 1758). Asian J Agric Res 5:277–282
Obaroh IO, Nzeh GG (2013) Antifertility effect of some plant leaf extracts on the prolific breeding of Oreochromis niloticus. Acad J Interdiscip Stud 2:87–94
Osuala FO, Okwuosa VN (1993) Toxicity of Azadirachta indica to freshwater snails and fish, with reference to the physicochemical factor effect on potency. Appl Parasitol 34:63–68
Putra A, Santoso U, Lee M-C, Nan F-H (2013) Effects of dietary katuk leaf extract on growth performance, feeding behavior and water quality of grouper Epinephelus coioides. Aceh Int J Sci Technol 2:17–25
Rattanachaikunsopon P, Phumkhachorn P (2010) Potential of cinnamon (Cinnamomum verum) oil to control Streptococcus iniae infection in tilapia (Oreochromis niloticus). Fish Sci 76:287–293
Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433:50–61
Saccol EMH, Uczay J, Pês TS, Finamor IA, Ourique GM, Riffel APK, Schmidt D, Caron BO, Heinzmann BM, Llesuy SF, Lazzari R, Baldisserotto B, Pavanato MA (2013) Addition of Lippia alba (Mill) N. E. Brown essential oil to the diet of the silver catfish: an analysis of growth, metabolic and blood parameters and the antioxidant response. Aquaculture 416–417:244–254
Saravanan M, Kumar DV, Malarvizhi A, Ramesh M (2010) Biosafety of Azadirachta indica (A. Juss) leaves extracts on certain biochemical parameters of Labeo rohita. J Biopestic 3:227–231
Sarder MRI, Thompson KD, Penman DJ, McAndrew BJ (2001) Immune responses of the Nile tilapia, Oreochromis niloticus L. clones. Non-specific responses. Dev Comp Immunol 25:37–46
Shalaby AM, Khattab YA, Abdel Rahman AM (2006) Effects of garlic (Allium sativum) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus). J Venom Anim Toxins 12:172–201
Sheikhlar A, Alimon AR, Daud H, Saad CR, Ramezani-Fard E (2011) Effects of crude methanol extract of Euphorbia hirta on hematological and biochemical indices and histological changes of liver in African catfish Clarias gariepinus (Burchell, 1822). J Fish Aquat Sci 6:802–808
Singh AK (2013) Introduction of modern endocrine techniques for the production of monosex population of fishes. Gen Comp Endocrinol 181:146–155
Srijunngam J, Wattanasirmkit K (2001) Histological structures of Nile tilapia Oreochromis niloticus Linn. ovary. Nat Hist J Chulalongkorn Univ 1:53–59
Suanyuk N, Kong F, Ko D, Gilbert GL, Supamattaya K (2008) Occurrence of rare genotypes of Streptococcus agalactiae in culture red tilapia Oreochromis sp. and Nile tilapia O. niloticus in Thailand—Relationship to human isolates? Aquaculture 284:35–40
Talpur AD, Ikhwanuddin M (2013) Azadirachta indica (neem) leaf dietary effects on the immunity response and disease resistance of Asian seabass, Lates calcarifer challenged with Vibrio harveyi. Fish Shellfish Immunol 34:254–264
Winkaler EU, Santos TR, Machado-Neto JG, Martinez CB (2007) Acute lethal and sublethal effects of neem leaf extract on the neotropical freshwater fish Prochilodus lineatus. Comp Biochem Physiol 145C:236–244
Yeh R-Y, Shiu Y-L, Shei S-C, Cheng S-C, Huang S-Y, Lin J-C, Liu C-H (2009) Evaluation of the antibacterial activity of leaf and twig extracts of stout camphor tree, Cinnamomum kanehirae, and the effects on immunity and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 27:26–32
Acknowledgments
This study was sponsored under an E. Science Fund No. 02-01-04-SF1433 (UPM Vote No. 5450652), Ministry of Science, Technology and Innovation (MOSTI) in Malaysia, and grant from a Universiti Putra Malaysia (UPM); Project No Gp-1pm/2013/9423000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kareem, Z.H., Abdelhadi, Y.M., Christianus, A. et al. Effects of some dietary crude plant extracts on the growth and gonadal maturity of Nile tilapia (Oreochromis niloticus) and their resistance to Streptococcus agalactiae infection. Fish Physiol Biochem 42, 757–769 (2016). https://doi.org/10.1007/s10695-015-0173-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0173-3