Abstract
The impact of replacing circa 70 % fish oil (FO) by a vegetable oil (VO) blend (rapeseed, linseed, palm oils; 20:50:30) in diets for European sea bass juveniles (IBW 96 ± 0.8 g) was evaluated in terms of activities of digestive enzymes (amylase, lipase, alkaline phosphatase, trypsin and total alkaline proteases) in the anterior (AI) and posterior (PI) intestine and tissue morphology (pyloric caeca—PC, AI, PI, distal intestine—DI and liver). For that purpose, fish were fed the experimental diets for 36 days and then liver and intestine were sampled at 2, 6 and 24 h after the last meal. Alkaline protease characterization was also done in AI and PI at 6 h post-feeding. Dietary VO promoted higher alkaline phosphatase activity at 2 h post-feeding in the AI and at all sampling points in the PI. Total alkaline protease activity was higher at 6 h post-feeding in the PI of fish fed the FO diet. Identical number of bands was observed in zymograms of alkaline proteases of fish fed both diets. No alterations in the histomorphology of PC, AI, PI or DI were noticed in fish fed the VO diets, while in the liver a tendency towards increased hepatocyte vacuolization due to lipid accumulation was observed. Overall, and with the exception of a higher intestine alkaline phosphatase activity, 70 % FO replacement by a VO blend in diets for European sea bass resulted in no distinctive alterations on the postprandial pattern of digestive enzyme activities and intestine histomorphology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the aquaculture sector, constrains in fish oil (FO) availability and price dictate an urgent need to replace it by more readily available, cheaper and sustainable alternatives such as vegetable oils (VO). However, a drawback of using VO in aquafeeds is their fatty acid (FA) profile, which has a different n3:n6 ratio comparatively to FO, and lacks n-3 long-chain polyunsaturated fatty acids (LC-PUFA), particularly eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids, which are essential FA for marine fish. Also, changes in dietary FA composition has been reported to affect FA composition of fish storage lipids and of cell membranes, including that of hepatic (Caballero et al. 2002; Kjaer et al. 2008; Fountoulaki et al. 2009; Castro et al. 2015b) and intestinal (Caballero et al. 2003; Ruyter et al. 2006) cells, the histomorphology of the liver (Caballero et al. 2002; Kjaer et al. 2008; Fountoulaki et al. 2009) and digestive tract (Olsen et al. 1999; Moldal et al. 2014). Dietary FO replacement by a single source or by blends of VOs was shown to induce accumulation of lipid droplets in: hepatocytes of rainbow trout (Caballero et al. 2002), Atlantic salmon (Kjaer et al. 2008) and gilthead sea bream (Fountoulaki et al. 2009), and enterocytes of Arctic char (Olsen et al. 1999), rainbow trout (Caballero et al. 2002; Olsen et al. 2003), gilthead sea bream (Caballero et al. 2003; Santigosa et al. 2011) and pikeperch (Kowalska et al. 2010), and to affect gut integrity and induce tissue damage in Arctic char (Olsen et al. 1999). Additionally, reduction of mucosal fold height in the mid-intestine and reduction of wall thickness in the distal intestine were also reported in Atlantic salmon fed diets with olive, rapeseed or soybean oils (Moldal et al. 2014).These alterations may interfere with nutrient digestion, transport, diffusion and absorption efficiency or function and metabolism (Olsen et al. 1999; Caballero et al. 2002, 2003; Jutfelt et al. 2007; Wassef et al. 2007; Geurden et al. 2009; Santigosa et al. 2011).
Studies assessing the effect of dietary FO substitution by VOs on digestive enzyme activities in fish are scarce (Santigosa et al. 2011; Bowyer et al. 2012; Ribeiro et al. 2015). In yellowtail kingfish, dietary coconut oil promoted a decrease in trypsin and lipase activities but had no effect on amylase activity (Bowyer et al. 2012). In meagre, the dietary incorporation of a VO blend had no marked effects on the intestinal alkaline phosphatase activity (Ribeiro et al. 2015). In gilthead sea bream fed diets including a VO blend, lipase and amylase were unaffected, while total alkaline protease (TAP) activity increased in pyloric caeca and decreased in proximal intestine (Santigosa et al. 2011). Furthermore, zymogram determination of TAP composition revealed higher intensity of trypsin- and chymotrypsin-like proteolytic bands in the pyloric caeca of gilthead sea bream fed VO diet, concurring with the higher TAP activity observed in that tissue (Santigosa et al. 2011).
In fish, digestive enzyme activities were shown to be modulated by diet composition (Munilla-Morán and Saborido-Rey 1996a, b; Hidalgo et al. 1999; Fernández et al. 2001; Fountoulaki et al. 2005; Furné et al. 2005; Santigosa et al. 2008; Pérez-Jiménez et al. 2009; Castro et al. 2013) and by intestinal transit time, which determines the digesta arrival to each intestinal section (Einarsson et al. 1996; Fountoulaki et al. 2005; Caruso et al. 2008; Santigosa et al. 2008; Rodiles et al. 2012). The digesta transit time along the gastrointestinal tract is itself also affected by diet composition (Santigosa et al. 2008; Rodiles et al. 2012). All these conditions may influence the digestive and absorptive processes (Castro et al. 2015a).
European sea bass (Dicentrarchus labrax) is an economically important carnivorous fish species of aquaculture in Europe. In this species, data obtained so far demonstrate that FO can be replaced by a single source (soybean oil) or a blend (rapeseed, linseed palm and/or olive oils at different proportions) of VO up to 60 % without compromising growth performance and survival (Figueiredo-Silva et al. 2005; Mourente et al. 2005; Richard et al. 2006b; Castro et al. 2015b). While the impact of such substitutions at metabolic level, mainly in liver, has been assessed (Richard et al. 2006b; Castro et al. 2015b), little information is available on the potential effects in physiological functions of intestine (Castro et al. 2015a).Thus, this study aimed to evaluate the effect of replacing circa 70 % fish oil (FO) by a VO blend on the activities of digestive enzymes of pancreatic (total alkaline proteases, trypsin, amylase and lipase) and brush border membrane (alkaline phosphatase) origin and on the histomorphology of different sections of the digestive tract and liver of European sea bass juveniles.
Materials and methods
Experimental diets
Two isoproteic (45 % crude protein; CP) and isolipidic (18 % crude lipids; CL) diets were formulated to contain 20 % gelatinized maize starch and FO or VO blend as lipid sources (Table 1). The VO blend was composed of rapeseed, linseed and palm oils (20:50:30) and replaced circa 70 % of dietary lipids provided by cod liver oil and fish meal. The diets were similar in saturated fatty acids (SFA) content, but, comparatively to the VO diet, the FO diet had higher levels of eicosapentaenoic acid (EPA, 20:5n-3), docosahexaenoic acid (DHA, 22:6n-3) and the monounsaturated fatty acids (MUFA) 20:1 and 22:1 and lower levels of linoleic acid (LA, 18:2n-6), linolenic acid (LNA, 18:3n-3) and oleic acid (OA, 18:1n-9) (Table 2). Details on diet preparation and analysis are given in Castro et al. (2015a).
Animals, experimental conditions and sampling
The experiment was directed by accredited scientists (following FELASA category C recommendations) and conducted according to the European Union Directive (2010/63/EU) on the protection of animals for scientific purposes, at the Marine Zoological Station, University of Porto, Portugal. European sea bass (D. labrax) juveniles with a mean initial body mass of 96 ± 0.8 g were placed in a thermoregulated recirculation water system equipped with a battery of twelve fibreglass tanks of 60 L capacity and supplied with continuous flow of filtered seawater. After 2 weeks of adaptation to the experimental conditions, 16 fish were randomly distributed to each tank and each diet was randomly assigned to triplicate groups of fish. The trial lasted 36 days, and fish were hand-fed to satiation twice a day. During the trial, water temperature averaged 24.1 ± 0.9 °C, salinity averaged 34.7 ± 0.8 g L−1 and dissolved oxygen was kept near saturation. The photoperiod was the natural one for May to June. This study is part of a bigger study, and further information on nutrient digestibility, serum metabolites and hepatic and intestinal gene expression analysis of key genes involved in lipid transport and lipoprotein metabolism is presented elsewhere (Castro et al. 2015a).
At the end of the trial, two fish from each tank were randomly sampled at 2, 6 and 24 h after the last meal and euthanized by a sharp blow to the head. Fish were dissected on chilled trays, and the liver and digestive tract were excised and freed from adherent adipose and connective tissues. For digestive enzyme analyses, the intestine was divided in two sections: anterior intestine (AI, section between the last pyloric caeca and the mid-line of the intestinal length) and posterior intestine (PI, section from the mid-line of the intestinal to distal intestine). Each section with intestinal content was immediately frozen in liquid nitrogen and stored at −80 °C until analysis.
For histological analyses, liver and intestine samples were collected from the fish euthanized at 6 h post-feeding. For intestinal evaluation, two pyloric caeca, and sections with approximately 0.5 cm of AI, PI and distal intestine (DI, collected from the last part of the posterior intestine, visually distinguished by a darker and thicker mucosa) were collected. Tissue samples were rinsed in phosphate-buffered saline (PBS), blotted dry with a paper towel, immediately fixed in phosphate-buffered formalin (4 %, pH 7.4) for 24 h and then transferred to ethanol (70 %) until further processing. An additional section of the liver was collected to cryotubes containing 10 % formol + 30 % saccharose + 1 % CaCl2, left at 4 °C for 48 h, then transferred to frozen embedding medium (Thermo Scientific™ Shandon Cryomatrix™) and stored at −20 °C until processing.
Digestive enzyme activities
Each section of the digestive tract was homogenized (dilution 1:9, w/v) in ice-cold buffer (100 mM Tris–HCl, 0.1 mM EDTA and 0.1 %Triton-X-100 (v/v), pH 7.8) and centrifuged at 30,000g for 30 min at 4 °C. The resultant supernatants were collected, and aliquots were stored at −80 °C until digestive enzyme analysis.
For each enzyme activity, assay dilution tests were previously done to ensure optimum ratio between enzyme and substrate. All enzyme activities were measured at 37 °C in a microplate reader (ELx808; Bio-Tek Instruments). The specific assay conditions for each enzyme were as follows:
α-Amylase (EC 3.2.1.1) activity was determined with a commercial kit (ref. 41201, Spinreact, Girona, Spain), with modification in the proportion of supernatants and assay buffer (200 µL of the assay buffer with 10 µL of supernatants). The rate of product formation (2-chloro-4-nitrophenol) was quantified at 405 nm.
Lipase (EC 3.1.1.3) activity was determined using a commercial kit (ref. 1001275, Spinreact, Girona, Spain) with modification in the proportion of supernatants and assay reactives (200 µL of the assay buffer, 40 µL substrate with 10 µL of supernatants). 1-2-O-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin)-ester was used as substrate, and the formation rate of methylresorufin was followed at 580 nm.
Trypsin (EC 3.4.21.4) activity was determined according to Faulk et al. (2007), using 1 mM Nα-Benzoyl-l-arginine 4-nitroanilide hydrochloride (BAPNA) as substrate combined with 50 mM Tris–HCl, 20 mM CaCl2, pH 8.2 buffer. Production of p-nitroaniline was monitored at 410 nm.
Alkaline phosphatase activity was determined using a commercial kit from Sigma (Sigma procedure no. 104) as previously described by Krogdahl et al. (2003). The reaction was measured at 420 nm using p-nitrophenyl phosphate as substrate and p-nitrophenol as standard.
Total alkaline protease activity was determined by the casein-hydrolysis method described by Walter (1984) and adapted by Hidalgo et al. (1999). A reaction mixture containing 0.25 mL casein at 1 % (w/v), 0.25 mL buffer (0.1 M Tris HCl, pH 9) and supernatant from the homogenates (0.1 mL) was incubated for 1 h at 37 °C. A control blank for each sample was assayed adding the supernatant from the homogenates after the incubation time. The reaction was stopped by the addition of 0.6 mL 8 % (w/v) trichloroacetic acid solution in blanks and reaction samples. After being kept for 1 h at 4 °C, blanks and reaction samples were centrifuged at 1800g for 10 min and the absorbance of supernatants measured at 280 nm. Tyrosine solution was used as standard.
Unit (U) of enzyme activity was defined as μmol of product generated per minute under the measurement conditions described above and expressed per mg soluble protein (specific activity). Protein concentration was determined according to Bradford (1976) using Sigma protein assay kit and bovine serum albumin as standard.
Protease inhibition, SDS-PAGE and casein zymography
Alkaline protease zymograms were obtained after resolving by SDS-PAGE the homogenates of AI and PI of fish sampled at 6 h post-feeding (Moyano et al. 1996). Prior to electrophoresis, a range of specific inhibition solutions were used and selected according to Alarcón et al. (1998). Thus, 10 mM TLCK (N-alpha-tosyl-l-lysinyl-chloromethylketone) was used to inhibit trypsin-like serine proteases; 10 mM ZPCK (N-carbobenzoxy-l-phenylalanine chloromethyl ketone) was used to inhibit chymotrypsin-like serine proteases, and 100 mM PMSF (phenylmethanesulfonyl fluoride) was used to inhibit serine proteases in general. Inhibition reactions were prepared by combining 40 µL of the intestinal homogenate containing 35 mg of soluble protein with 10 µL of each inhibitor (TLCK, ZPCK or PMSF) or with 10 µL of H2O (control). Following 45-min incubation at room temperature, each sample mixture (with or without inhibitor) was mixed with sample loading buffer (2 % SDS, 0.125 M Tris, 5 % glycerol, 0.025 % bromophenol blue) and loaded on 12.5 % polyacrylamide gel for protein resolution by SDS-PAGE, according to Laemmli (1970) and adapted by García-Carreño et al. (1993). The full-range Amersham Rainbow Marker (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) was used to estimate the molecular weight of proteins. Electrophoresis was performed on a Mini-PROTEAN® Tetra Cell (Bio-Rad Laboratories, Inc.) at 25 mA per gel during 120 min. After electrophoresis, the gels were washed 3 × 10 min with 2.5 % Triton-X-100 to remove SDS and then incubated for 30 min at 4 °C, under agitation, with a 2 % casein substrate solution prepared in 50 mM TrisHCl, pH 8.2. The gels were then kept for additional 90 min at room temperature with shaking. Following substrate incubation, the gels were stained for 50 min with staining solution (0.3 % Coomassie blue R-250, 50 % ethanol, 10 % acetic acid) and de-stained using a mixture of 30 % ethanol and 10 % acetic acid, for protein visualization. The effect of each inhibitor on digestive proteases was visualized by partial or total disappearance of one or more hydrolysis bands, when compared to the profile of enzyme extract pre-incubated with distilled water.
Histology
Samples were processed and sectioned using standard histological techniques and stained with haematoxylin and eosin (H–E). Cryopreserved liver samples were sectioned with cryostat (CM3050 S, Leica, Deerfield, IL), placed on slides, air-dried for 30 min and stored at −20 °C until Sundan black staining (SB). SB was performed as follows: slides were washed in water, passed through ethanol 70 %, stained in SB (Merck 1387, Darmstadt, Germany; 3 % w/v in ethanol 70 %), washed in ethanol 70 %, passed through water and mounted with aqueous mounting media (S3025-Dako).
Liver and intestinal sections were subjected to a double-blinded evaluation using a semi-quantitative scoring system ranging from 1 to 5. Score 1 was considered the normal tissue appearance, and subsequent scores were accounted for increasing alterations of normal tissue histomorphology. Intestinal samples were evaluated according to the criteria suggested by Krogdahl et al. (2003): widening and shortening of the intestinal folds, loss of the supranuclear vacuolization in the absorptive cells (enterocytes) in the intestinal epithelium, widening of the lamina propria in the intestinal folds and infiltration of mixed leucocyte population in the lamina propria and submucosa.
Liver samples were evaluated for general histomorphology, giving particular attention to cytoplasm vacuolization of the hepatocytes and any signs of inflammation. The SB sections were used to confirm the nature of hepatocellular vacuolization observed in H–E-stained preparations.
Statistical analysis
Digestive enzyme activity data were checked for normal distribution and homogeneity of variances (Shapiro–Wilk and Levene tests, respectively) and normalized when appropriate. The effects of dietary lipid source (LS) sampling time (TIME) and intestinal section (SECTION) on digestive enzyme activities were analysed by 3 × 3 factorial analysis (three-way ANOVA). Significant differences between means were evaluated by the Tukey multiple range test. In case of significant interaction, a one-way ANOVA was performed to evaluate differences between time and section. Histological data were neither normal nor homogeneous; thus, the Kruskal–Wallis nonparametric test was used. The significance level of 0.05 was used for rejection of the null hypothesis. All statistical analyses were done using the IBM SPSS 21 software package (SPSS® Inc, Chicago, IL, USA) for Windows.
Results
Dietary VO inclusion had no effect on amylase, lipase, total alkaline proteases and trypsin activities (mU mg protein−1) in the AI and PI. Contrarily, alkaline phosphatase activity was higher in both intestinal sections in fish fed the VO diet (Table 3).
Differences in digestive enzyme activities among AI and PI sections were mostly time-related (time × section interaction) (Table 3). At 2 h post-feeding, amylase, lipase, alkaline phosphatase and trypsin activities were higher in AI than in PI, while at 6 and 24 h after feeding, lipase and total alkaline protease activities were lower in AI than in PI (Fig. 1). At 6 and 24 h post-feeding, alkaline phosphatase and trypsin activities were still higher in AI than in PI, respectively (Fig. 1). Differences among AI and PI sections on amylase (at 6 and 24 h post-feeding), total alkaline proteases (at 2 h post-feeding), alkaline phosphatase (at 24 h post-feeding) and trypsin (at 6 h post-feeding) activities were not observed (Fig. 1). Time × LS confirmed that there were no significant differences in these enzymes among AI and PI.
Summary of the statistical analyses of the specific digestive enzymes (mU mg−1 protein) in European sea bass whose activities showed a significant sampling time (TIME) × intestinal section (SECTION) interaction* (means of the fish fed two experimental diets, n = 12). Anterior (AI) and posterior (PI) intestine. *Identified by three-way ANOVA. Values are mean ± SD. Significant differences (p < 0.05) among sampling times are indicated by different lower-case letters, while within each sampling times, significant differences (p < 0.05) between AI and PI are indicated by symbols (> or <), and the lack of differences (p > 0.05) between AI and IP is indicated by symbol (=)
In AI, digestive enzyme activities were similar at 2 and 6 h after feeding, and lowest values were observed at 24 h (Fig. 1). In PI, digestive enzyme activity tended to increase from 2 to 6 h post-feeding and lowest values were observed at 24 h (Fig. 1).
Zymogram analysis of alkaline proteases present in AI and PI sections of fish fed the two diets showed that the number of detectable active proteases was not affected by the dietary lipid source. Five bands with proteolytic activity against a casein substrate were detected in both intestinal sections, with molecular weights ranging between 17 and 52 kDa (band numbers 1–5, Fig. 2). All these proteins are predicted to be serine proteases, since their hydrolytic activity was reduced upon addition of PMSF, an inhibitor of serine protease activity. An additional band with an estimated molecular weight of less than 17 kDa (band number 6, Fig. 2) was visible in PI section. This band was apparently not inhibited by any of the inhibitors used (TLCK, ZPCK or PMSF), indicating that it probably corresponds to a nonserine protease (different catalytic residue). The two bands with estimated molecular weight between 17 and 24 kDa (bands 4 and 5, Fig. 2) were identified as chymotrypsin-like serine enzymes, as observed by a reduction in the band intensity when using the chymotrypsin-specific inhibitor ZPCK. On opposite, the band immediately above (band 3, Fig. 2), with an estimated molecular weight higher than 24 kDa, corresponds most probably to a trypsin-like serine protease, since a reduction in its intensity could be observed with TLCK (trypsin inhibitor). The two uppermost bands (bands 1 and 2, Fig. 2), with highest molecular weights, could putatively be identified as serine protease enzymes (other than trypsin-like or chymotrypsin-like), since their intensity was only reduced with PMSF treatment.
Zymograms of alkaline proteases in anterior (AI) and posterior (PI) intestine homogenates of European sea bass fed the fish oil (FO) and the vegetable oil blend (VO) diets at 6 h post-feeding and treated with different specific inhibitors: chymotrypsin inhibitor (ZPCK), trypsin inhibitor (TLCK), general serine protease inhibitor (PMSF) or water (H2O) used as control. MW molecular weight in kDa
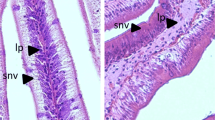
The inclusion of VO in the diets had no effects on the intestinal histomorphology (Table 4; Fig. 3). Fish from both dietary treatments showed similar histological features, with long, well-defined villus without fusion. Lamina propria and submucosa presented normal thickness without increased cellularity. The frequency of eosinophilic granulocytes and intraepithelial leucocytes was similar in fish from both dietary groups.
Histomorphological features of the pyloric ceca (a, b), anterior intestine (c, d), posterior intestine (e, f) and distal intestine (g, h) of European sea bass fed the fish oil—FO (a, c, e, g) or the vegetable oil—VO (b, d, f, h) diets. MF mucosal fold, SM submucosa, LP lamina propria, IELs intraepithelial leucocytes. Scale a, b, e, f, g, h 50 µm; c, d 100 µm; H–E staining
Both dietary treatments resulted in great hepatocyte vacuolization, and the group fed VO diets exhibited a tendency (p = 0.071) towards higher degree of vacuolization than the FO group (Fig. 4). The cytoplasmic vacuoles appeared clear and optically empty, often pushing the nucleus to the cell periphery without altering the shape of the nuclei. The Sudan black staining (Fig. 4c, d) confirmed the lipidic nature of the material contained in the cytoplasmatic vacuoles, showing that fish fed the VO diet had a tendency to higher hepatic lipid accumulation than fish fed FO diet.
Discussion
A few studies have already evaluated the effect of dietary protein source on the profile of digestive enzyme activities over time in fish (Santigosa et al. 2008; Rodiles et al. 2012). But, as far as we are aware, this is the first study addressing the effect of dietary lipid source on digestive enzyme activities over time carried out in fish. Accordingly, partial or total replacement of fish meal (FM) by plant protein sources in the diets for rainbow trout induced a drastic decrease in postprandial alkaline protease activity and a lack of enzyme activity peak when dietary FM was totally replaced by plant protein (Santigosa et al. 2008). On the contrary, the same authors observed in gilthead sea bream similar postprandial protease activity profiles, independently of diet composition, though a gradual decrease in alkaline protease activity peak was observed as dietary plant protein increased (Santigosa et al. 2008). The authors also observed a postprandial peak in amylase activity in both species when fed FM-based diets, but no clear peak in amylase activity was observed when fed plant-protein-based diets. In Senegalese sole, different postprandial profiles of total proteases, trypsin and chymotrypsin activities in fish fed FM-based diets or diets with FM partially replaced by plant protein sources were also reported (Rodiles et al. 2012).
It is reasonable to assume that differences in dietary fatty acid (FA) composition may induce changes in digesta residence time along the digestive tract and, consequently, distinctive digestive enzyme activity profiles over time. However, present results indicate a lack of time-related changes in digestive enzyme activities in response to dietary lipid source. On the other hand, the postprandial digestive enzyme activity profiles evidenced that enzyme secretion was stimulated by the presence of nutrients in the digestive tract. The similar digestive enzyme activities in the AI at 2 and 6 h post-feeding suggest that digesta was still arriving to the AI at 6 h post-feeding, while the higher enzyme activity in the PI at 6 h post-feeding suggests that at 2 h only a small amount of digesta was present in that region, while at 6 h post-feeding PI was full of digesta. The very low activity values of digestive enzymes at 24 h post-feeding in both sections of the intestine indicate that, by then, digestive processes were already accomplished and that pre-feeding enzyme activity values were achieved.
Digestive enzymes that are synthesized in fish exocrine pancreas are secreted into the anterior section of the digestive tract (Kuz’mina 2008; Bakke et al. 2010). Accordingly, this section seems to contribute more for nutrient digestion and absorption than more posterior sections, as demonstrated by a number of histomorphological, enzymatic and nutrient digestibility studies along the digestive tract of fish (Olsen et al. 1999; Nordrum et al. 2000; Chikwati et al. 2013; Hartviksen et al. 2014). In the present study, activities of digestive enzymes in both intestinal sections were in general high and differences among intestinal sections were mostly time-related (time × section interaction), reinforcing both the stimulation of pancreatic secretions by the feed ingestion and the evolution of digesta along the digestive tract. Activity of the selected digestive enzymes of pancreatic (total alkaline proteases, trypsin, amylase and lipase) and enterocyte brush border membrane (BBM) (alkaline phosphatase) origin in the present study has also been detected throughout the digestive tract in several fish species (Krogdahl et al. 2005; Pérez-Jiménez et al. 2009).
The digestive enzyme activity profiles in response to a single meal fit well with the postprandial profiles of serum metabolites (reported in Castro et al. 2015a). However, present results do not allow linking the distinctive postprandial profile of serum lipids observed in fish fed the two experimental diets to different specificities of lipases in relation to FA profile of the tested diets. Lipase specificity is known to change in function of the unsaturation degree and of the chain length of dietary FA (Koven et al. 1994; Olsen and Ringø 1997; Tocher 2003). However, present results did not evidence a lipase activity modulation in relation to dietary lipids. This was not unexpected, as FA chain length and unsaturation degree of the dietary lipids were not much different. These results are also in accordance with the small differences recorded in apparent lipid digestibility of the tested diets (see Castro et al. 2015a). Similarly, no difference on lipase activity was observed in gilthead sea bream juveniles fed diets including FO or a blend of VO (Santigosa et al. 2011). On the other hand, in yellowtail kingfish, lower lipase activity was observed in fish fed diets with canola oil than with FO (Bowyer et al. 2012). On the contrary, in European sea bass larvae, an increment in lipase activity was observed with the ingestion of coconut oil (Morais et al. 2004).
In the present study, no differences in the number of alkaline proteases present in AI or PI sections of intestine were observed between dietary treatments. Also, and consistent with zymogram results, no effect on trypsin activity was detected with the dietary replacement of FO by VO. On the contrary, a decrease in trypsin activity was observed in yellowtail kingfish juveniles fed a diet with canola oil (Bowyer et al. 2012) and in Senegalese sole larvae fed a diet with soybean oil (Morais et al. 2006). The presence of anti-nutritional factors (ANFs), in particular trypsin inhibitors, which may be fat soluble in some types of oils, could explain the differences between the two previous studies and the present results. However, in gilthead sea bream, Santigosa et al. (2011) reported higher total alkaline protease activity in the pyloric ceca of fish fed VO than FO diets, while the opposite was observed in the proximal intestine. Inhibition of luminal proteases due to the presence of ANFs in VO diets was at first hypothesized as the possible cause for the observed differences. However, the authors concluded that the increment in proteolytic activity in pyloric caeca and the higher intensity of trypsin- and chymotrypsin-like proteolytic bands in zymograms promoted by the VO were mainly linked to a slower release of proteases into the intestinal lumen in the VO group due to a decrease in digesta transit rate.
Due to its close nature with the hydrophobic core of intestinal microvillus membrane, the activity of alkaline phosphatase is often used as indicator of intestinal membrane integrity. Available data on the effect of dietary plant protein (Krogdahl et al. 2003) or lipid level (Cahu et al. 2000) on alkaline phosphatase indicate a decline in activity as indicator of malnutrition. According to Cahu et al. (2000), the decline in alkaline phosphatase activity in fish fed high-lipid diets was due to modifications of FA composition of brush border membrane and, consequently, modification in membrane fluidity. In rainbow trout, Ducasse-Cabanot et al. (2007) found that alkaline phosphatase activity in the AI decreased with the removal of FO from the diet. The authors pointed that the lower digestive capacity in the enterocyte membrane of fish fed low-lipid diets was related to the lower levels of dietary lipids rather than to a modification in the enterocyte brush border membrane FA composition, which remained unchanged. Contrarily to the present data, a recent study from Ribeiro et al. (2015) reported that alkaline phosphatase activity in intestine of meagre was not affected when dietary FO was replaced by a VO blend (rapeseed, linseed and palm oils). Although increased alkaline phosphatase activity in our study may suggest some modification at the enterocyte level in response to the presence of VO in the diet, no histopathological alterations were observed (see below). Even so, and considering that alkaline phosphatase is involved in several other cellular functions, further studies are required to understand potential mechanisms of action of dietary VO.
Although accumulation of lipid droplets in the enterocytes due to dietary VO has been reported in previous studies in other fish species (Caballero et al. 2003; Kowalska et al. 2010), in the present study, dietary VO did not affect intestinal histomorphology of European sea bass. A previous study in this species (Mourente et al. 2007) using identical VO blend and dietary inclusion levels also found no major histomorphological alterations on mid- or distal intestine, just increased absorptive vacuolization in the proximal intestine. Apart from species differences, the degree of lipid droplet accumulation in the enterocytes was also shown to vary with the level and type of dietary FA composition (Caballero et al. 2002, 2003; Santigosa et al. 2011). Accordingly, Caballero et al. (2003) found high accumulation of lipid droplets in gilthead sea bream fed diets containing linseed or soybean oils replacing 60 % FO, and even higher accumulation when fish were fed the same VO replacing 80 % FO, or with rapeseed oil at 60 %. Also in gilthead sea bream, Santigosa et al. (2011) found increased lipid droplet content in proximal intestine as the percentage of dietary VO blend increased, replacing from 33 to 100 % of dietary FO. These histomorphological alterations are thought to occur due to modifications in lipid transport mechanisms, more precisely with interferences of dietary lipid source on the intestinal lipoprotein assembly, namely in the reacylation mechanisms of the absorbed lipids (Caballero et al. 2003, 2006). The lack of alterations on tissue histomorphology due to dietary treatments in the present study is in accordance with the lack of transcriptional modifications of proteins related to lipid transport and lipoprotein metabolism (FABP, DGAT, ApoB, ApoA4, ApoA1, MTP) (see Castro et al. 2015a).
The effects of dietary VO on fish liver histomorphology are very diverse. In European sea bass fed diets with dietary soybean oil replacing 50 % of FO (Figueiredo-Silva et al. 2005) or a blend of VO replacing 60 % of FO (Mourente et al. 2007), no histomorphological effects were observed. Similarly, Ribeiro et al. (2015) observed in meagre that histological structure of liver was little affected by the 60 % replacement of FO by a VO blend. Contrarily, occurrence of variable size vacuoles and large amounts of lipid droplets in the liver of European sea bass fed rapeseed and linseed oils replacing 60 % of FO was described by Mourente et al. (2005). In the liver of rainbow trout (Caballero et al. 2002), gilthead sea bream (Caballero et al. 2004; Wassef et al. 2007; Fountoulaki et al. 2009) and Atlantic salmon (Kjaer et al. 2008), increased lipid accumulation was detected when fish were fed VO-rich diets. In the present study, the tendency towards higher hepatocellular vacuolization caused by an accumulation of lipid droplets in the VO group may be related to an induction of lipogenesis. In several fish species, VO administration promoted an increase (Jordal et al. 2007; Morais et al. 2011; Peng et al. 2014) in the transcript or enzymatic activity of lipogenic-related enzymes. In addition, and even though no changes were reported on the hepatic gene expression level in our fish (Castro et al. 2015a), it is also possible that factors involved in the assembly and secretion of the lipoprotein particles may also be involved. However, these hypotheses need further investigation, as available literature on the effects of FO replacement by VO on lipogenesis (Regost et al. 2003; Menoyo et al. 2004; Torstensen et al. 2004; Richard et al. 2006a, b; Castro et al. 2015b) and on the mechanisms of lipoprotein assembly and secretion (Richard et al. 2006a, b; Jordal et al. 2007; Kjaer et al. 2008; Leaver et al. 2008) is not clear and contradictory. Nevertheless, this kind of tissue histomorphological alteration at the hepatic level seems to be nonpathological and reversible when fish are re-fed with a balanced diet (Caballero et al. 2004).
Overall, FO replacement by circa 70 % of a balanced VO blend in European sea bass juveniles did not substantially modulate digestive enzyme activities, suggesting that luminal digestive processes were not affected by dietary lipids tested. The kinetics of digestive enzyme activity response to a single meal reflected feed ingestion and was not affected by dietary oil source. VO blend promoted no hepatic or intestinal histomorphological alterations. Nevertheless, a trend towards higher lipid accumulation in the hepatocytes was observed in the fish fed the VO diet, which may result in effects at long term, and requires to be further investigated.
In the context of the development of more sustainable and economic aquafeeds, the present data seem promising as it suggests that FO can be replaced up to 70 % with VO in diets for European sea bass without major alterations in the digestive function and intestinal histomorphology.
References
Alarcón FJ, Díaz M, Moyano FJ, Abellán E (1998) Characterization and functional properties of digestive proteases in two sparids; gilthead sea bream (Sparus aurata) and common dentex (Dentex dentex). Fish Physiol Biochem 19:257–267
Bakke AM, Glover C, Krogdahl Å (2010) Feeding, digestion and absorption of nutrients. In: Grosell M, Farrell AP, Brauner CJ (eds) Fish physiology. Academic Press, London, pp 57–110
Bowyer JN, Qin JG, Adams LR, Thomson MJS, Stone DAJ (2012) The response of digestive enzyme activities and gut histology in yellowtail kingfish (Seriola lalandi) to dietary fish oil substitution at different temperatures. Aquaculture 368–369:19–28
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye-binding. Anal Biochem 72:248–254
Caballero MJ, Obach A, Rosenlund G, Montero D, Gisvold M, Izquierdo MS (2002) Impact of different lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 214:253–271
Caballero MJ, Izquierdo MS, Kjørsvik E, Montero D, Socorro J, Fernández AJ, Rosenlund G (2003) Morphological aspects of intestinal cells from gilthead seabream (Sparus aurata) fed diets containing different lipid sources. Aquaculture 225:325–340
Caballero MJ, Izquierdo MS, Kjørsvik E, Fernández E, Rosenlund G (2004) Histological alterations in the liver of sea bream, Sparus aurata L., caused by short- or long-term feeding with vegetable oils. Recovery of normal morphology after feeding fish oil as the sole lipid source. J Fish Dis 27:531–541
Caballero MJ, Gallardo G, Robaina L, Montero D, Fernández A, Izquierdo MS (2006) Vegetable lipid sources affect in vitro biosynthesis of triacylglycerols and phospholipids in the intestine of seabream (Sparus aurata). Br J Nutr 95:448–454
Cahu CL, Infante JLZ, Corraze G, Coves D (2000) Dietary lipid level affects fatty acid composition and hydrolase activities of intestinal brush border membrane in sea bass. Fish Physiol Biochem 23:165–172
Caruso G, Denaro MG, Genovese L (2008) Temporal changes in digestive enzyme activities in the gastrointestinal tract of European eel (Anguilla anguilla) (Linneo 1758) following feeding. Mar Freshw Behav Phys 41:215–228
Castro C, Pérez-Jiménez A, Coutinho F, Pousão-Ferreira P, Brandão TM, Oliva-Teles A, Peres H (2013) Digestive enzymes of meagre (Argyrosomus regius) and white seabream (Diplodus sargus). Effects of dietary brewer’s spent yeast supplementation. Aquaculture 416–417:322–327
Castro C, Corraze G, Panserat S, Oliva-Teles A (2015a) Effects of fish oil replacement by a vegetable oil blend on digestibility, postprandial serum metabolite profile, lipid and glucose metabolism of European sea bass (Dicentrarchus labrax) juveniles. Nutr, Aquac. doi:10.1111/anu.12184
Castro C, Corraze G, Pérez-Jiménez A, Larroquet L, Cluzeaud M, Panserat S, Oliva-Teles A (2015b) Dietary carbohydrate and lipid source affect cholesterol metabolism of European sea bass (Dicentrarchus labrax) juveniles. Br J Nutr 1–14. doi:10.1017/S0007114515002731
Chikwati EM, Sahlmann C, Holm H, Penn MH, Krogdahl Å, Bakke AM (2013) Alterations in digestive enzyme activities during the development of diet-induced enteritis in Atlantic salmon Salmo salar L. Aquaculture 402–403:28–37
Ducasse-Cabanot S, Zambonino-Infante J, Richard N, Médale F, Corraze G, Mambrini M, Robin J, Cahu C, Kaushik S, Panserat S (2007) Reduced lipid intake leads to changes in lipid digestive enzymes in the intestine but has minor effects on key enzymes of hepatic intermediary metabolism in rainbow trout (Oncorhynchus mykiss). Animal 1:1272–1282
Einarsson S, Davies PS, Talbot C (1996) The effect of feeding on the secretion of pepsin, trypsin and chymotrypsin in the Atlantic salmon, Salmo salar L. Fish Physiol Biochem 15:439–446
Faulk CK, Benninghoff AD, Holt GJ (2007) Ontogeny of the gastrointestinal tract and selected digestive enzymes in cobia Rachycentron canadum (L.). J Fish Biol 70:567–583
Fernández I, Moyano FJ, Díaz M, Martínez TF (2001) Characterization of α-amylase activity in five species of Mediterranean sparid fishes (Sparidae, Teleostei). J Exp Mar Biol Ecol 262:1–12
Figueiredo-Silva A, Rocha E, Dias J, Silva P, Rema P, Gomes E, Valente LMP (2005) Partial replacement of fish oil by soybean oil on lipid distribution and liver histology in European sea bass (Dicentrarchus labrax) and rainbow trout (Oncorhynchus mykiss) juveniles. Aquac Nutr 11:147–155
Fountoulaki E, Alexis MN, Nengas I, Venou B (2005) Effect of diet composition on nutrient digestibility and digestive enzyme levels of gilthead sea bream (Sparus aurata L.). Aquac Res 36:1243–1251
Fountoulaki E, Vasilaki A, Hurtado R, Grigorakis K, Karacostas I, Nengas I, Rigos G, Kotzamanis Y, Venou B, Alexis MN (2009) Fish oil substitution by vegetable oils in commercial diets for gilthead sea bream (Sparus aurata L.); effects on growth performance, flesh quality and fillet fatty acid profile: recovery of fatty acid profiles by a fish oil finishing diet under fluctuating water temperatures. Aquaculture 289:317–326
Furné M, Hidalgo MC, López A, García-Gallego M, Morales AE, Domenzain A, Domezain J, Sanz A (2005) Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture 250:391–398
García-Carreño FL, Dimes LE, Haard NF (1993) Substrate gel-electrophoresis for composition and molecular-weight of proteinases of proteinaceous proteinase-inhibitors. Anal Biochem 214:65–69
Geurden I, Jutfelt F, Olsen R, Sundell K (2009) A vegetable oil feeding history affects digestibility and intestinal fatty acid uptake in juvenile rainbow trout Oncorhynchus mykiss. Comp Biochem Physiol A 152:552–559
Hartviksen M, Bakke AM, Vecino JG, Ringo E, Krogdahl A (2014) Evaluation of the effect of commercially available plant and animal protein sources in diets for Atlantic salmon (Salmo salar L.): digestive and metabolic investigations. Fish Physiol Biochem 40:1621–1637
Hidalgo MC, Urea E, Sanz A (1999) Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture 170:267–283
Jordal AEO, Lie O, Torstensen BE (2007) Complete replacement of dietary fish oil with a vegetable oil blend effects liver and plasma lipoprotein levels in Atlantic salmon (Salmo salar). Aquac Nutr 13:114–130
Jutfelt F, Olsen RE, Björnsson BTh, Sundell K (2007) Parr–smolt transformation and dietary vegetable lipids affect intestinal nutrient uptake, barrier function and plasma cortisol levels in Atlantic salmon. Aquaculture 273:298–311
Kjaer MA, Vegusdal A, Gjøen T, Rustan AC, Todorčević M, Ruyter B (2008) Effect of rapeseed oil and dietary n-3 fatty acids on triacylglycerol synthesis and secretion in Atlantic salmon hepatocytes. Biochim Biophys Acta 1781:112–122
Koven WM, Henderson RJ, Sargent JR (1994) Lipid digestion in turbot (Scophtalmus maximus): I. Lipid class and fatty acid composition of digesta from different segments of the digestive tract. Fish Physiol Biochem 13:69–79
Kowalska A, Zakés Z, Jankowska B, Siwicki A (2010) Impact of diets with vegetable oils on the growth, histological structure of internal organs, biochemical blood parameters, and proximate composition of pikeperch Sander lucioperca (L.). Aquaculture 301:69–77
Krogdahl Å, Bakke-McKellep AM, Baeverfjord G (2003) Effects of graded levels of standard soybean meal on intestinal structure, mucosal enzyme activities, and pancreatic response in Atlantic salmon (Salmo salar L.). Aquac Nutr 9:361–371
Krogdahl Å, Hemre GI, Mommsen TP (2005) Carbohydrates in fish nutrition: digestion and absorption in postlarval stages. Aquac Nutr 11:103–122
Kuz’mina VV (2008) Classical and modern concepts in fish digestion. In: Cyrino JEP, Bureau DP, Kapoor BG (eds) Feeding and digestive functions of fishes. Science Publishers, Enfield, pp 85–154
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227:680–688
Leaver MJ, Villeneuve LAN, Obach A, Jensen L, Bron JE, Tocher DR, Taggart JB (2008) Functional genomics reveals increases in cholesterol biosynthetic genes and highly unsaturated fatty acid biosynthesis after dietary substitution of fish oil with vegetable oils in Atlantic salmon (Salmo salar). BMC Genom 9:299
Menoyo D, Izquierdo MS, Robaina L, Ginés R, Lopez-Bote CJ, Bautista JM (2004) Adaptation of lipid metabolism, tissue composition, and flesh quality in gilthead sea bream (Sparus aurata) to the replacement of dietary fish oil by linseed and soybean oils. Br J Nutr 92:41–52
Moldal T, Løkka G, Wiik-Nielsen J, Austbø L, Torstensen BE, Rosenlund G, Dale OB, Kaldhusdal M, Koppang EO (2014) Substitution of dietary fish oil with plant oils is associated with shortened mid intestinal folds in Atlantic salmon (Salmo salar). BMC Vet Res 10:60
Morais S, Cahu C, Zambonino-Infante J, Robin J, Rønnestad I, Dinis M, Conceição L (2004) Dietary TAG source and level affect performance and lipase expression in larval sea bass (Dicentrarchus labrax). Lipids 39:449–458
Morais S, Caballero MJ, Conceição LEC, Izquierdo MS, Dinis MT (2006) Dietary neutral lipid level and source in Senegalese sole (Solea senegalensis) larvae: effect on growth, lipid metabolism and digestive capacity. Comp Biochem Physiol B 144:57–69
Morais S, Pratoomyot J, Taggart JB, Bron JE, Guy DR, Bell JG, Tocher DR (2011) Genotype-specific responses in Atlantic salmon (Salmo salar) subject to dietary fish oil replacement by vegetable oil: a liver transcriptomic analysis. BMC Genom 12:255
Mourente G, Good JE, Bell JG (2005) Partial substitution of fish oil with rapeseed, linseed and olive oils in diet for European sea bass (Dicentrarchus labrax L.) effects on flesh fatty acid composition, plasma prostaglandins E2 and F2a, immune function and effectiveness of a fish oil finishing diet. Aquac Nutr 11:25–40
Mourente G, Good JE, Thompson KD, Bell JG (2007) Effects of partial substitution of dietary fish oil with blends of vegetable oils, on blood leucocyte fatty acid compositions, immune function and histology in European sea bass (Dicentrarchus labrax L.). Br J Nutr 98:770–779
Moyano FJ, Díaz M, Alarcón FJ, Sarasquete MC (1996) Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol Biochem 15:121–130
Munilla-Morán R, Saborido-Rey F (1996a) Digestive enzymes in marine species. I. Proteinase activities in gut from redfish (Sebastes mentella), seabream (Sparus aurata) and turbot (Scophthalmus maximus). Comp Biochem Physiol B 113:395–402
Munilla-Morán R, Saborido-Rey F (1996b) Digestive enzymes in marine species. II. Amylase activities in gut from seabream (Sparus aurata), turbot (Scophthalmus maximus) and redfish (Sebastes mentella). Comp Biochem Physiol B 113:827–834
Nordrum S, Krogdahl Å, Røsjø C, Olli JJ, Holm H (2000) Effects of methionine, cysteine and medium chain triglycerides on nutrient digestibility, absorption of amino acids along the intestinal tract and nutrient retention in Atlantic salmon (Salmo salar L.) under pair-feeding regime. Aquaculture 186:341–360
Olsen RE, Ringø E (1997) Lipid digestibility in fish: a review. Recent Res Dev Lipid Res 1:199–265
Olsen RE, Myklebust R, Kaino T, Ringo E (1999) Lipid digestibility and ultrastructural changes in the enterocytes of Arctic charr (Salvelinus alpinus L.) fed linseed oil and soybean lecithin. Fish Physiol Biochem 21:35–44
Olsen RE, Dragnes BT, Myklebust R, Ringø E (2003) Effect of soybean oil and soybean lecithin on intestinal lipid composition and lipid droplet accumulation of rainbow trout, Oncorhynchus mykiss Walbaum. Fish Physiol Biochem 29:327–329
Peng M, Xu W, Mai K, Zhou H, Zhang Y, Liufu Z, Zhang K, Ai Q (2014) Growth performance, lipid deposition and hepatic lipid metabolism related gene expression in juvenile turbot (Scophthalmus maximus L.) fed diets with various fish oil substitution levels by soybean oil. Aquaculture 433:442–449
Pérez-Jiménez A, Cardenete G, Morales AE, García-Alcázar A, Abellán E, Hidalgo MC (2009) Digestive enzymatic profile of Dentex dentex and response to different dietary formulations. Comp Biochem Physiol A 154:157–164
Regost C, Arzel J, Robin J, Rosenlund G, Kaushik SJ (2003) Total replacement of fish oil by soybean or linseed oil with a return to fish oil in turbot (Psetta maxima). I. Growth performance, flesh fatty acid profile, and lipid metabolism. Aquaculture 217:465–482
Ribeiro L, Moura J, Santos M, Colen R, Rodrigues V, Bandarra N, Soares F, Ramalho P, BarataM Moura P, Pousão-Ferreira P, Dias J (2015) Effect of vegetable based diets on growth, intestinal morphology, activity of intestinal enzymes and haematological stress indicators in meagre (Argyrosomus regius). Aquaculture. doi:10.1016/j.aquaculture.2014.12.017
Richard N, Kaushik S, Larroquet L, Panserat S, Corraze G (2006a) Replacing dietary fish oil by vegetable oils has little effects on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Br J Nutr 96:299–309
Richard N, Mourente G, Kaushik S, Corraze G (2006b) Replacement of a large portion of fish oil by vegetable oils does not affect lipogenesis, lipid transport and tissue lipid uptake in European seabass (Dicentrachus labrax L.). Aquaculture 261:1077–1087
Rodiles A, Santigosa E, Herrera M, Hachero-Cruzado I, Cordero ML, Martínez-Llorens S, Lall SP, Alarcón FJ (2012) Effect of dietary protein level and source on digestive proteolytic enzyme activity in juvenile Senegalese sole, Solea senegalensis Kaup 1850. Aquacult Int 20:1053–1070
Ruyter B, Moya-Falcón C, Rosemlund G, Vegusdal A (2006) Fat content and morphology of liver and intestine of Atlantic salmon (Salmo salar): effects of temperature and soybean oil. Aquaculture 252:441–452
Santigosa E, Sánchez J, Médale F, Kaushik S, Pérez-Sánchez J, Gallardo MA (2008) Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and seabream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 282:68–74
Santigosa E, García-Meilán I, Valentín JM, Navarro I, Pérez-Sánchez J, Gallardo MÁ (2011) Plant oils’ inclusion in high fish meal-substituted diets: effect on digestion and nutrient absorption in gilthead sea bream (Sparus aurata L.). Aquac Res 42:962–974
Tocher DR (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci 11:107–184
Torstensen BE, Froyland L, Lie O (2004) Replacing dietary fish oil with increasing levels of rapeseed oil and olive oil-effects on Atlantic salmon (Salmo salar L.) tissue and lipoprotein lipid composition and lipogenic enzyme activities. Aquac Nutr 10:175–192
Walter HE (1984) Proteinases: methods with hemoglobin, casein and azocoll as substrates. In: Bergmeyer HJ (ed) Methods of enzymatic analysis, vol V. Verlag Chemie, Weinham, pp 270–277
Wassef EA, Wahby OM, Sakr EM (2007) Effect of dietary vegetable oils on health and liver histology of gilthead seabream (Sparus aurata) growers. Aquac Res 38:852–861
Acknowledgments
This work was partially supported by the FCT (Foundation for Science and Technology), Portugal (Project PTDC/MAR-BIO/4107/2012) and co-financed by the European Regional Development Fund (ERDF) through the COMPETE—Operational Competitiveness Programme and national funds through FCT—under the project “PEst-C/MAR/LA0015/2011”. CC was supported by a Grant (SFRH/BD/76297/2011), AC (SFRH/BD/47495/2008) and APJ (SFRH/BPD/64684/2009) from FCT, Portugal. PDR and CRS were supported by Grants (NORTE-07-0124-FEDER-000038-BPD-2013-07; NORTE-07-0124-FEDER-000038-BPD-2013-05, respectively). We express our thanks to Laurence Larroquet for her kind help in the fatty acid analyses of diets and to P. Correia for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, C., Couto, A., Pérez-Jiménez, A. et al. Effects of fish oil replacement by vegetable oil blend on digestive enzymes and tissue histomorphology of European sea bass (Dicentrarchus labrax) juveniles. Fish Physiol Biochem 42, 203–217 (2016). https://doi.org/10.1007/s10695-015-0130-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0130-1