Abstract
Growth hormone (GH) is a single-chain polypeptide hormone mainly secreted by somatotropes of the anterior pituitary gland and is an important regulator of somatic growth in vertebrates including teleosts. In this study, a polyclonal antiserum against ricefield eel Gh was generated and the expression of Gh at the mRNA and protein levels was analyzed. Both RT-PCR and western blot analysis showed that Gh was predominantly expressed in the pituitary glands of ricefield eels. The immunoreactive Gh signals were localized to the multicellular layers of the adenohypophysis adjacent to the neurohypophysis in ricefield eels. Ontogenetic analysis showed that immunoreactive Gh signals could be detected in the pituitary glands of ricefield eel embryos as early as 3 days post-fertilization. During the sex change from female to male, the levels of the immunoreactive Gh signals in the pituitary glands of the ricefield eels peaked at the intersexual stage. These results suggest that Gh in the pituitary glands may be associated with embryonic development before hatching, as well as with the sex change in the adult ricefield eels, possibly via the classical endocrine manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Growth hormone (GH) is a single-chain pleiotropic polypeptide mainly expressed in the anterior pituitary gland of vertebrates. In addition to being produced in the pituitary gland, mammalian Gh has been shown to be produced in some extrapituitary tissues, including the placenta, mammary tissue, brain, and pineal gland (Butler and Le Roith 2001). In teleosts, gh transcripts were also detected in the testis of pejerrey (Odontesthes bonariensis) (Sciara et al. 2006), and in the brain, liver, kidney, spleen, heart, muscle, and ovary of the Prenant’s schizothoracin (Schizothorax prenanti) (Li et al. 2011). The extrapituitary Gh may act in a paracrine/autocrine manner (Vong et al. 2003; Eppler et al. 2007). To date, Gh in fish has been studied extensively for its potential application of growth enhancement in aquaculture.
In addition to its regulation of somatic growth and development (Yowe and Epping 1995; Reinecke et al. 2005), Gh is also associated with many other physiological functions, such as metabolism (Rousseau and Dufour 2007), reproduction (Le Gac et al. 1993; Hull and Harvey 2002), appetite (Zizzari et al. 2011), osmoregulation (Sangiao-Alvarellos et al. 2005; Varsamos et al. 2005; Sakamoto and McCormick 2006), social behavior (Canosa et al. 2007), and immunity (Yada et al. 2001) in both terrestrial and aquatic vertebrates. In mammals, GH participates in steroidogenesis, gametogenesis, and ovulation (Hull and Harvey 2000) as well as in gonadotropin responsiveness (Le Gac et al. 1993). In grass carp, a teleost fish, Gh was suggested to inhibit Lh secretion but at the same time to maintain Lh synthesis by activating lhβ gene expression (Zhou et al. 2004). Gh has also been shown to stimulate steroid production in the testis of several teleost fish in vivo and in vitro (Schulz et al. 2010). Moreover, Gh treatment promoted spermatogonial proliferation in catfish (Gopal et al. 2014) and in the in vitro cultured testes of Japanese eel (Miura et al. 2011).
The ricefield eel (Monopterus albus), a synbranchiform species with a great aquacultural value in China, is a protogynous hermaphroditic fish that changes sex naturally from female through intersex to male during its life cycle (Liu 1944; Liem 1963). The process of sex change in ricefield eels involves the rapid proliferation of male germ cells and the concomitant extensive development of interstitial cells in the gonadal lamellae (Chan and Phillips 1967; Chan et al. 1972). As Gh was implicated in the regulation of spermatogonial proliferation in teleosts (Gopal et al. 2014; Miura et al. 2011), an examination of the expression pattern of Gh in ricefield eels, particularly during the sex change, is of interest. Previously, the putative Gh cells were shown by histochemical methods to be localized to the thin cellular layers bordering the neurohypophysis interdigitations in the pituitary gland of ricefield eels (Wai-Sum and Chan 1974). However, the identity of these putative Gh cells needs to be further confirmed. The cDNA sequence encoding ricefield eel Gh has been reported (Zhang et al. 2005). In the present study, a specific antiserum against ricefield eel Gh was generated, and the expression and ontogeny of Gh were analyzed in ricefield eels.
Materials and methods
Experimental fish and sampling procedure
The adult ricefield eels (body length 20–45 cm and body weight 20–45 g) were purchased from a local dealer in Guangzhou, Guangdong, China. Fish were killed by decapitation, after which the pituitary gland and other tissues were removed by dissection and stored in a deep freezer (−80 °C) for tissue extraction or in Sample Protector (Takara Bio Inc., Shiga, Japan) for the isolation of RNA. The tissues for histology and immunohistochemistry were fixed in Bouin’s solution for 24 h and embedded in paraffin. Serial sections were cut at 4 μm (the pituitary) or 6 μm (the gonad) on a Leica microtome. The sexual stages were verified by histological examination of the gonadal sections, which were stained with hematoxylin and eosin (H&E).
The ricefield eel embryos and larvae were obtained from Dazhong Breeding Co. Ltd., Jianyang, Sichuan, China, and raised in our laboratory under natural photoperiod and temperature. The embryogenesis of ricefield eels takes approximately 7 days. After hatching, animals were fed Chironomus larvae. Samples were collected at different time points, including 2 and 3 days post-fertilization (dpf), and 0, 1, 3, 5, and 7 days post-hatching (dph). The whole body of each fish was fixed in Bouin’s solution, and the heads of the ricefield eel larvae were dissected from the trunk before histological processing as above. All procedures and investigations were reviewed and approved by the Center for Laboratory Animals of Sun Yat-Sen University and were performed in accordance with the guiding principles for the care and use of laboratory animals.
RT-PCR analysis of gh mRNA expression in tissues
Total RNA isolated from tissues was first treated with DNase I (1 U/μl) to remove any genomic DNA contamination. Then, 1 μg of total RNA was reverse transcribed with oligo(dT)18 primers using the RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania) according to the manufacturer’s instructions. The integrity of all RNA samples was verified by the successful amplification of bactin (AY647143.1). The absence of genomic DNA contamination in the DNase I-treated RNA sample was verified by the amplification of target genes from the reverse transcription reaction mixture without the addition of the reverse transcriptase (designated as the RT control).
The first-strand cDNA synthesized above (0.25 μl) was amplified for each target gene using the Biometra Tgradient thermal cycler. PCR was performed in a 25 μl final volume containing 2.5 μl 10× Taq buffer, 2.0 mM MgCl2, 0.2 mM dNTP, 0.4 μM of each primer, and 1.25 U Taq DNA polymerase (Fermentas). Water was used as a negative control in the RT-PCR. The reaction mixture was heated at 94 °C for 3 min followed by 32 or 36 cycles for gh (AY265351.1) and 28 cycles for bactin. The cycling conditions were 94 °C for 0.5 min, 56 °C for 0.5 min, and 72 °C for 1 min, with a final extension at 72 °C for 5 min. The primers were eGh-F1 and eGh-R1 for gh and bactin-F and bactin-R for bactin, which generated PCR products of 557 and 484 bp, respectively. These primers are listed in Table 1. The PCR products were separated on a 1.5 % agarose gel and stained with ethidium bromide (0.5 μg/ml). The gel image was captured on the Bio-Rad GelDoc 2000 (Bio-Rad Laboratories, Inc., CA, USA).
Production of recombinant proteins and the polyclonal antiserum against ricefield eel Gh
The cDNA sequence encoding the mature polypeptide of the ricefield eel Gh (AY265351.1) was amplified using gene-specific primers eGh-F2 and eGh-R2 (Table 1), subcloned into the prokaryotic expression vector pET-15b, and expressed in E. coli BL21 (DE3) cells upon induction with isopropyl β-d-1-thiogalactopyranoside (IPTG). The recombinant Gh polypeptide was gel purified from inclusion bodies and used to immunize New Zealand white rabbits as previously reported (Wu et al. 2012).
To examine the cross-reactivities of the anti-Gh antiserum generated in the present study, the recombinant ricefield eel follicle-stimulating hormone beta subunit (Fshb), luteinizing hormone beta subunit (Lhb), the common alpha-glycoprotein subunit (Cga), prolactin (Prl), and thyroid-stimulating hormone beta subunit (Tshb) were produced as previously reported (Wu et al. 2012). The cDNA sequence encoding the mature polypeptide of ricefield eel somatolactin (Sl) was amplified with primers eSl-F and eSl-R and processed in the same way as the production of recombinant Gh described above. The sequences of primers are listed in Table 1.
Western blot analysis
The recombinant proteins or tissue extracts were separated on 12 % SDS-PAGE gels and transferred onto methanol-activated polyvinylidenefluoride membranes (Roche, Mannheim, Germany) by electroblotting. The membrane was then blocked with 5 % nonfat milk powder in 0.01 M PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, and pH 7.4) at 4 °C overnight. The primary antiserum was pre-adsorbed overnight at 4 °C with extracts of BL21 (DE3) bacteria transformed with the empty expression vector (pET-15b) after IPTG induction. The blocked membrane was incubated with the pre-adsorbed primary antiserum at a dilution of 1:1000 in blocking solution (5 % nonfat milk in 0.01 M PBS) at room temperature for 1 h, washed three times with PBS for 5 min, and incubated with the horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H+L) (1:1000; catalog number: A0208, Beyotime, Jiangsu, China) for 1 h at room temperature. After three 5-min final washes with PBS, the membrane was exposed to a chemiluminescence substrate (BeyoECL Plus Kit, Beyotime) according to the manufacturer’s instructions.
Immunohistochemistry
The deparaffinized and hydrated sections of the pituitary gland (together with the brain) were incubated for 30 min in 3 % hydrogen peroxide at room temperature to quench the endogenous peroxidase activity. Then, the sections were boiled for 15 min in 10 mM sodium citrate buffer (pH 6.0) for antigen retrieval and blocked in 0.01 M PBS containing 10 % normal goat serum for 20 min at room temperature. The blocked sections were washed in PBS and incubated at room temperature for 3 h with 1:1000 dilution of the primary rabbit anti-Gh antiserum. After being rinsed with PBS, the sections were exposed to the secondary antibody, HRP-conjugated goat anti-rabbit IgG (H+L) (1:1000; catalog number: A0208, Beyotime). After rinsing with PBS, the sections were developed with 3,3′-diaminobenzidine (DAB), counterstained with hematoxylin, mounted, and digitally photographed with a microscope (E800; Nikon, Japan). To confirm the specificity of the immunostaining, control sections were incubated with the primary antiserum pre-adsorbed with an excess of recombinant ricefield eel Gh antigen. Additional negative controls included the replacement of the primary antiserum with PBS or the pre-immune serum and the omission of secondary antibody.
Quantification of Gh immunoreactivities in the pituitary glands of ricefield eels at different sexual stages
Serial consecutive sagittal sections of the pituitary glands from female, intersexual, and male ricefield eels were cut into 4-μm-thick slices, and Gh immunostaining was visualized with DAB as described as above. The quantitative analysis of Gh immunoreactivity in the pituitary section was performed with the Image-Pro Plus 6.0 software (Media Cybernetics, Inc., PA, USA) by a method similar to a previous report (Nyuji et al. 2012). All images for analysis were taken under the same conditions with a Nikon E800 microscope. The hematoxylin-counterstained image was used to delineate the boundary between the adenohypophysis (AH) and neurohypophysis (NH) because the latter is mostly devoid of stain. The areas of the whole pituitary, the NH, and the immunoreactive Gh signals were digitally measured by the Image-Pro Plus 6.0 software (Media Cybernetics, Inc.). The area of the NH was subtracted from the area of the whole pituitary to yield the area of the AH. The immunoreactive (ir) level of Gh was calculated by dividing the area of its corresponding immunoreactivity with the area of the AH and presented as a percentage of the AH area. Three sections (one presumably cut from the central position containing the pituitary stalk, and the other two cut from either side at a distance about half away from the central position) were examined for each fish, with 3–4 fish being analyzed for each sexual stage and a mean value being calculated and presented.
Statistical analysis
All values were expressed as mean ± SEM, and the data were analyzed by one-way analysis of variance (ANOVA) followed by the Tukey multiple comparison test using the SPSS 17.0 software (SPSS, Inc., IL, USA). Significance was set at p < 0.05.
Results
The expression of ricefield eel gh mRNA in tissues
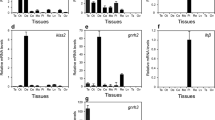
The tissue patterns of the gh mRNA expression were analyzed in both female (Fig. 1a) and male (Fig. 1b) ricefield eels using RT-PCR. When the PCR was performed for 32 cycles, the expression of the gh mRNA was detected only in the pituitary gland of both female and male ricefield eels. When the number of PCR cycles was increased to 36, faint expression of the gh mRNA started to be detected in some extrapituitary tissues, including the optic tectum–thalamus, ovary, liver, eyes in the female (Fig. 1a), and the testes in the male (Fig. 1b).
RT-PCR analysis of gh mRNA in tissues of female (a) and male (b) ricefield eels. The PCR was performed for 32 or 36 cycles for gh and 28 cycles for bactin. Ob olfactory bulb, Te telencephalon, Hy hypothalamus, Ot optic tectum–thalamus, Ce cerebellum, Mo medulla oblongata, Pi pituitary, Go gonad, Mu muscle, Sp spleen, Pa pancreas, He heart, Li liver, Ki kidney, In intestines, Bl blood, Ey eyes, Ub urinary bladder, RT- RT without reverse transcriptase from the pituitary gland RNA sample, NC negative control
The expression of immunoreactive ricefield eel Gh in tissues
A polyclonal antiserum against the ricefield eel Gh was generated, which was shown to react with the recombinant Gh antigen protein, but not the other recombinant pituitary hormonal polypeptides, including Fshb, Lhb, Cga, Prl, Tshb, and Sl (Fig. 2a), indicating that the anti-Gh antiserum was of high specificity. Therefore, western blot analysis was further employed to examine the expression of Gh in tissues of the ricefield eels. A specific band of approximately the expected size (23 kDa) was detected only in the pituitary gland, but not in extracts of other tissues, including the brain, testis, ovary, and liver (Fig. 2b). When the anti-Gh antiserum was pre-absorbed with an excess of recombinant ricefield eel Gh antigen, the above-detected specific band disappeared (Fig. 2, the lower panels), further confirming the specificity of the anti-Gh antiserum.
Western blot analysis of the specificity of the anti-Gh antiserum against recombinant pituitary gland hormonal polypeptides (a) and tissue homogenates (b) of ricefield eel. The recombinant polypeptides (20 ng) or tissue homogenates (10 μg) were separated on 12 % SDS-PAGE gels, transferred to a polyvinylidenefluoride membrane, and then allowed to immunoreact with the rabbit anti-Gh antiserum (1:1000). The lower panels the same preparations as in the upper panels allowed to immunoreact with the anti-Gh antiserum pre-absorbed by the recombinant Gh antigen. The secondary antibody was 1:1000 diluted horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H+L) (1:1000; catalog number: A0208, Beyotime). The blots were visualized using BeyoECL plus kit (Beyotime). Gh growth hormone, Fshb follicle-stimulating hormone beta subunit, Lhb luteinizing hormone beta subunit, Cga the common alpha-glycoprotein subunit, Prl prolactin, Tshb thyroid-stimulating hormone beta subunit, Sl somatolactin
The expression of Gh in the pituitary gland, brain, and gonads was further examined with immunohistochemical analysis, which detected immunoreactive Gh signals in the pituitary gland (Fig. 3a) but not the brain or ovary (Fig. 3c, d). Gh immunostaining in the pituitary gland sections of the female ricefield eels was predominantly localized to the multicellular layers of the adenohypophysis bordering along the highly convoluted neurohypophysis (Fig. 3a). Pre-adsorption of the primary antiserum with excessive recombinant Gh antigen abolished the immunoreactive Gh signals in the pituitary gland (Fig. 3b).
Gh immunostaining (brown) in the sections of the pituitary gland attached to the brain through the pituitary stalk (black arrowheads) (a), midbrain (c), and ovary (d) of female ricefield eels as visualized by the DAB chromogen and counterstained with hematoxylin. The control pituitary gland section adjacent to that shown in panel a was immunostained with the pre-adsorbed anti-Gh antiserum (b). Sagittal sections of ricefield eel pituitary glands were shown here with the rostral (anterior) part of the gland to the left. The black triangles and stars indicate the adenohypophysis and neurohypophysis tissues, respectively. HT hypothalamus, OT optic tectum–thalamus, VOC vitellogenic oocyte. Scale bar 100 μm. (Color figure online)
Ontogeny of immunoreactive Gh cells in the pituitary glands of ricefield eels
The immunoreactive Gh cells and pituitary placode were detected at 3 days post-fertilization (dpf) (Fig. 4a) but not at 2 dpf (data not shown). Gh cells were large in size and round in shape. Situated between the diencephalon and a thick layer of loose connective tissue above the roof of the primitive oral cavity, the pituitary anlage is separated from the diencephalon by a thin layer of connective tissue and bordered at its ventral portion with loose connective tissue (Fig. 4a). At 0 dph, the pituitary anlage became more distinguished and separated from the loose connective tissue, with the early development of the neurohypophysis in the posterior region of the gland. The nerve bundles started to penetrate the adenohypophysis tissue. An increase in the number of Gh cells was observed in the medial and ventral regions of the pituitary gland (Fig. 4b). At 3 dph, the pituitary gland became more elongated in shape and the pituitary stalk was formed. The nerves of the neurohypophysis were penetrating the adenohypophysis. The immunoreactive Gh signals were mainly detected in the regions of the adenohypophysis bordering the neurohypophysis (Fig. 4c). At 7 dph, the pituitary gland had a marked growth of all its components, with a large population of immunoreactive Gh cells localized in the regions of adenohypophysis bordering the neurohypophysis (Fig. 4d).
The analysis of immunoreactive Gh signals (brown) during the early development of the pituitary glands in ricefield eel embryos and larvae. Sagittal sections of the pituitary glands were developed with DAB and counterstained with hematoxylin. a The embryo at 3 dpf. The pituitary placode is delineated with a dashed line. The immunoreactive Gh cells were detected first. b The larvae at 0 dph. The pituitary anlage, separated from the diencephalon by a thin layer of connective tissue (red arrowhead), became more distinguished, with the early appearance of the putative neurohypophysis (green arrowhead) in the posterior region of the gland. c The larvae at 3 dph. The pituitary gland was detached from the diencephalon, with the formation of the pituitary stalk (black arrowheads). The black triangles and stars indicate the adenohypophysis and neurohypophysis tissues, respectively. d The larvae at 7 dph. The pituitary gland had a marked growth of all its components, containing Gh cells in the regions of adenohypophysis bordering along with the neurohypophysis. A anterior, P posterior, D diencephalon, V ventricle, CT connective tissue, BV blood vessel, PM primitive mouth, HT hypothalamus. Scale bar 25 μm. (Color figure online)
Immunoreactive Gh levels in the pituitary glands of ricefield eels during sex change
The distribution of immunoreactive Gh signals in the pituitary sections of ricefield eels at the intersexual and male stages exhibited a similar pattern as that at the female stage, with Gh signals predominantly localized to the areas of the adenohypophysis adjacent to the neurohypophysis (Fig. 5a, c, e). The quantitative analysis showed that the immunoreactive Gh levels in the pituitary glands of the ricefield eels were significantly higher at the intersexual stage than at either the female or male stage (Fig. 5g).
The quantification of immunoreactive Gh signals in the pituitary of ricefield eels at the female, intersexual, and male stages. The representative immunohistochemical images (Gh signals in brown) of pituitary sections (a, c, e) and the corresponding HE-stained images of gonadal sections (b, d, f) were shown above the histogram. Sagittal sections of ricefield eel pituitary glands are shown here with the rostral (anterior) part of the gland to the left. The inset in d is the magnified image of the boxed area. Scale bar 50 μm except where it is specifically designated otherwise. Gh-ir immunoreactive Gh, MOC mature oocyte, DGO degenerating oocyte, SG spermatogonia, SC spermatocyte, ST spermatid. Bars represent mean ± SEM of 3–4 fishes. Bars with different superscripts are significantly different (p < 0.05). (Color figure online)
Discussion
In addition to being expressed in the pituitary gland, Gh gene has been shown to be expressed in many extrapituitary tissues at both the mRNA and protein levels in mammals and birds (Harvey 2010). In teleosts, gh transcripts have also been detected in the extrapituitary tissues (Yang et al. 1999; Biga et al. 2004; Filby and Tyler 2007; Dong et al. 2010; Li et al. 2011), but the information on the immunoreactivities to Gh in the extrapituitary tissues seemed lacking. In the present study, a specific antiserum against ricefield eel Gh was generated, and immunoreactivities to Gh were detected in the pituitary gland but not in other tissues including the brain and gonads by western blot and/or immunohistochemical analysis. In agreement, high levels of gh expression in the pituitary gland could be detected by RT-PCR with the amplification in 32 cycles, but only faint expression was detected in the brain, ovary, and testis when PCR cycles were increased to 36. These results suggested that the expression of gh in ricefield eels is predominantly localized to the pituitary glands, with very low levels in the extrapituitary tissues. Whether the extrapituitary gh mRNA in ricefield eels and other teleosts is actually translated into functional Gh protein remains to be elucidated.
In the pituitary glands of some teleosts, Gh cells were shown to reside predominantly in the proximalis pars distalis (PPD) (Weltzien et al. 2003; Huang and Specker 1994; Laiz-Carrión et al. 2003; De Jesus et al. 2014). In ricefield eels, however, the present study showed that immunoreactive Gh signals seemed not to form a distinct zone within the pituitary gland, but rather to be localized to the multicellular layers of the adenohypophysis bordering the neurohypophysis interdigitations. In agreement, a previous study employing histochemical methods also showed that the putative Gh cells in the pituitary glands of ricefield eels formed a continuous layer of columnar cells, one or two cells thick, bordering much area of neurohypophysis interdigitations (Wai-Sum and Chan 1974). Interestingly, Gh cells were also found to be arranged in cords or multicellular layers adjacent to the neurohypophysis in the striped bass (Morone saxatilis) (Huang and Specker 1994) and Atlantic halibut (Hippoglossus hippoglossus L.) (Weltzien et al. 2003). Although Gh cells were restricted to the PPD within the pituitary gland of the American shad (Alosa sapidissima), those Gh cells located more dorsally contact branches of neurohypophyseal tissues (Laiz-Carrión et al. 2003). The close spatial contact between Gh cells and neurohypophyseal tissues observed in the pituitary of different teleosts above is suggestive of their functional relationship, which awaits further study.
The pituitary gland of vertebrates mainly consists of the adenohypophysis and neurohypophysis. Classically, the adenohypophysis was considered to have originated as an ectodermal upgrowth from the anterior roof of the embryonic oral cavity (Wingstrand 1966). Recent studies have demonstrated a neuroectodermal origin of the adenohypophysis in vertebrates from fish to mammals (Chapman et al. 2005; Kawamura et al. 2002). In the present study, the adenohypophysis, as indicated by the immunoreactive Gh signals, was observed in ricefield eel embryos at 3 dpf, but the earliest development of the neurohypophysis was identified later in the posterior region of the pituitary gland of ricefield eel larvae at 0 dph. Similarly, the earlier appearance of the adenohypophysis than the neurohypophysis was also observed in other teleosts such as the American shad (Alosa sapidissima) (Laiz-Carrión et al. 2003), zebrafish (Danio rerio) (Chapman et al. 2005), and a South American characiform species (Salminus brasiliensis) (De Jesus et al. 2014). The adenohypophyses of the ricefield eel embryos and larvae were closely adhered to the diencephalon at 3 dpf and 0 dph but separated from the roof of the embryonic cavity by a thick layer of connective tissues. Moreover, serial consecutive sections of ricefield eel embryos at 2 dpf did not reveal any trace of the pituitary placode or Gh immunoreactivity (data not shown). This early developmental pattern of the adenohypophysis in ricefield eels seems to be in line with the neuroectodermal view on its origin. Admittedly, more evidence such as cell fate mapping is needed to clarify the origin of the ricefield eel adenohypophysis. It is of great interest to note that immunoreactive Gh cells started to reorganize around the neurohypophyseal tissues when nerves grew into the adenohypophysis at 3 dph and assumed the pattern at 7 dph as observed in the adults. These results imply that interactions may exist between the Gh cells and the neurohypophyseal tissues during the early development of the pituitary gland in ricefield eels, which warrants further study.
GH is not classically considered as a reproductive hormone. Nevertheless, an increasing body of evidence is relating GH to reproductive function in teleosts (Björnsson et al. 1994; Gomez et al. 1999; Weber and Grau 1999; Einarsdottir et al. 2002). Plasma GH levels were reported to elevate in association with the gonadal maturation in several teleost species, including the carp (Cyprinus carpio) (Lin et al. 1995), tilapia hybrid (Oreochromis niloticus x O. aureus) (Melamed et al. 1995), rainbow trout (Oncorhynchus mykiss) (Sumpter et al. 1991), and chum salmon (Oncorhynchus keta) (Kakizawa et al. 1995). GH stimulated gonadal steroidogenesis in mummichog (Fundulus heteroclitus) (Singh et al. 1988). Moreover, Gh receptors were shown to be expressed in the ovary of tilapia (Oreochromis mossambicus) (Kajimura et al. 2004) and the testis of seabream (Acanthopagrus schlegeli) (Tse et al. 2003), and Gh treatment promoted spermatogonial proliferation in catfish (Gopal et al. 2014) and in the in vitro cultured testes of Japanese eel (Miura et al. 2011). The ricefield eel changes sex from female through intersex to male in its life cycle and exhibits the rapid proliferation of male germ cells and the concomitant extensive development of interstitial cells in the gonadal lamellae at the intersexual stage (Chan and Phillips 1967; Chan et al. 1972). During the sex change, immunoreactive Gh levels in the pituitary of ricefield eels peaked at the intersexual stage, significantly higher than those at the female or male stage. These results imply that the pituitary Gh is possibly associated with the gonadal transformation in ricefield eels via the classical endocrine manner, which awaits further elucidation.
In summary, the ricefield eel gh gene is predominantly expressed in the pituitary. The immunoreactive Gh cells in the adenohypophysis emerged before hatching and the appearance of the neurohypophysis tissues and later reorganized in close contact with the neurohypophyseal interdigitations. The immunoreactive Gh levels in the pituitary of ricefield eels peaked at the intersexual stage. These results suggest that the pituitary Gh may play important roles in the embryonic development and gonadal transformation during sex change in ricefield eels.
References
Biga PR, Schelling GT, Hardy RW, Cain KD, Overturf K, Ott TL (2004) The effects of recombinant bovine somatotropin (rbST) on tissue IGF-I, IGF-I receptor, and GH mRNA levels in rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 135:324–333
Björnsson BT, Taranger GL, Hansen T, Stefansson SO, Haux C (1994) The interrelation between photoperiod, growth hormone, and sexual maturation of adult Atlantic salmon (Salmo salar). Gen Comp Endocrinol 93:70–81
Butler AA, Le Roith D (2001) Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu Rev Physiol 63:141–164
Canosa LF, Chang JP, Peter RE (2007) Neuroendocrine control of growth hormone in fish. Gen Comp Endocrinol 151:1–26
Chan STH, Phillips JG (1967) The structure of the gonad during natural sex reversal in Monopterus albus (Pisces: Teleostei). J Zool 151:129–141
Chan STH, Tang F, Lofts B (1972) Biopsy studies on the natural sex reversal in Monopterus albus (Pisces: Teleostei). J Zool 167:415–421
Chapman SC, Sawitzke AL, Campbell DS, Schoenwolf GC (2005) A three dimensional atlas of pituitary gland development in the zebrafish. J Comp Neurol 487:428–440
De Jesus LW, Chehade C, Costa FG, Borella MI (2014) Pituitary gland morphogenesis and ontogeny of adenohypophyseal cells of Salminus brasiliensis (Teleostei, Characiformes). Fish Physiol Biochem 40:897–909
Dong HY, Zeng LX, Duan D, Zhang HF, Wang YX, Li WS, Lin HR (2010) Growth hormone and two forms of insulin-like growth factors I in the giant grouper (Epinephelus lanceolatus): molecular cloning and characterization of tissue distribution. Fish Physiol Biochem 36:201–212
Einarsdottir IE, Sakata S, Björnsson BT (2002) Atlantic halibut growth hormone: structure and plasma levels of sexually mature males and females during photoperiod-regulated annual cycles. Gen Comp Endocrinol 127:94–104
Eppler E, Caelers A, Shved N, Hwang G, Rahman AM, Maclean N, Zapf J, Reinecke M (2007) Insulin-like growth factor I (IGF-I) in a growth-enhanced transgenic (GH-overexpressing) bony fish, the tilapia (Oreochromis niloticus): indication for a higher impact of autocrine/paracrine than of endocrine IGF-I. Transgenic Res 16(4):479–489
Filby AL, Tyler CR (2007) Cloning and characterization of cDNAs for hormones and/or receptors of growth hormone, insulin-like growth factor-1, thyroid hormone, and corticosteroid and the gender-, tissue-, and developmental-specific expression of their mRNA transcripts in fathead minnow (Pimephales promelas). Gen Comp Endocrinol 150:151–163
Gomez JM, Mourot B, Fostier A, Le Gac F (1999) Growth hormone receptors in ovary and liver during gametogenesis in female rainbow trout (Oncorhynchus mykiss). J Reprod Fertil 115:275–285
Gopal RN, Kumar P, Lal B (2014) Temperature dependent action of growth hormone on somatic growth and testicular activities of the catfish, Clarias batrachus. Gen Comp Endocrinol 195:125–131
Harvey S (2010) Extrapituitary growth hormone. Endocrine 38:335–359
Huang L, Specker JL (1994) Growth hormone-and prolactin-producing cells in the pituitary gland of striped bass (Morone saxatilis): immunocytochemical characterization at different life stages. Gen Comp Endocrinol 94:225–236
Hull KL, Harvey S (2000) Growth hormone: a reproductive endocrine-paracrine regulator. Rev Reprod 5:175–182
Hull KL, Harvey S (2002) GH as a co-gonadotropin: the relevance of correlative changes in GH secretion and reproductive state. J Endocrinol 172:1–19
Kajimura S, Kawaguchi N, Kaneko T, Kawazoe I, Hirano T, Visitacion N, Grau EG, Aida K (2004) Identification of the growth hormone receptor in an advanced teleost, the tilapia (Oreochromis mossambicus) with special reference to its distinct expression pattern in the ovary. J Endocrinol 181:65–76
Kakizawa S, Kaneko T, Ogasawara T, Hirano T (1995) Changes in plasma somatolactin levels during spawning migration of chum salmon (Oncorhynchus keta). Fish Physiol Biochem 14:93–101
Kawamura K, Kouki T, Kawahara G, Kikuyama S (2002) Hypophyseal development in vertebrates from amphibians to mammals. Gen Comp Endocrinol 126:130–135
Laiz-Carrión R, del Mar SNM, del Pilar MDRM, Mancera JM (2003) Ontogeny of adenohypophyseal cells in the pituitary of the American shad (Alosa sapidissima). Gen Comp Endocrinol 132:454–464
Le Gac F, Blaise O, Fostier A, Le Bail PY, Loir M, Mourot B, Weil C (1993) Growth hormone (GH) and reproduction: a review. Fish Physiol Biochem 11:219–232
Li CJ, Chen XH, Zhang Y, Ye H, Liu T (2011) Molecular and expression characterization of growth hormone/prolactin family genes in the Prenant’s schizothoracin. Mol Biol Rep 38:4595–4602
Liem KE (1963) Sex reversal as a natural process in the synbranchiform fish Monopterus albus. Copeia 2:303–312
Lin HR, Lu M, Lin XW, Zhang WM, Sun Y, Chen LX (1995) Effects of gonadotropin-releasing hormone (GnRH) analogs and sex steroids on growth hormone (GH) secretion and growth in common carp (Cyprinus carpio) and grass carp (Ctenopharyngodon idellus). Aquaculture 135:173–184
Liu CK (1944) Rudimentary hermaphroditism in the symbranchoid eel, Monopterus javanensis. Sinensia 15:1–8
Melamed P, Eliahu N, Ofir M, Levavi-Sivan B, Smal J, Rentier-Delrue F, Yaron Z (1995) The effects of gonadal development and sex steroids on growth hormone secretion in the male tilapia hybrid (Oreochromis niloticus × O. aureus). Fish Physiol Biochem 14:267–277
Miura C, Shimizu Y, Uehara M, Ozaki Y, Young G, Miura T (2011) Gh is produced by the testis of Japanese eel and stimulates proliferation of spermatogonia. Reproduction 142:869–877
Nyuji M, Selvaraj S, Kitano H, Shiraishi T, Yamaguchi A, Shimizu A, Matsuyama M (2012) Immunoreactivity of gonadotrophs (FSH and LH Cells) and gonadotropin subunit gene expression in the male chub mackerel Scomber japonicus pituitary during the reproductive cycle. Zool Sci 29:623–629
Reinecke M, Björnsson BT, Dickhoff WW, McCormick SD, Navarro I, Power DM, Gutiérrez J (2005) Growth hormone and insulin-like growth factors in fish: where we are and where to go. Gen Comp Endocrinol 142:20–24
Rousseau K, Dufour S (2007) Comparative aspects of GH and metabolic regulation in lower vertebrates. Neuroendocrinol 86:165–174
Sakamoto T, McCormick SD (2006) Prolactin and growth hormone in fish osmoregulation. Gen Comp Endocrinol 147:24–30
Sangiao-Alvarellos S, Miguez JM, Soengas JL (2005) Actions of growth hormone on carbohydrate metabolism and osmoregulation of rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 141:214–225
Schulz RW, de França LR, Lareyre JJ, Le Gac F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Sciara AA, Rubiolo JA, Somoza GM, Arranz SE (2006) Molecular cloning, expression and immunological characterization of pejerrey (Odontesthes bonariensis) growth hormone. Comp Biochem Physiol C Toxicol Pharmacol 142:284–292
Singh H, Griffith RW, Takahashi A, Kawauchi H, Thomas P, Stegeman JJ (1988) Regulation of gonadal steroidogenesis in Fundulus heteroclitus by recombinant salmon growth hormone and purified salmon prolactin. Gen Comp Endocrinol 72:144–153
Sumpter JP, Lincoln RF, Bye VJ, Carragher JF, Le Bail PY (1991) Plasma growth hormone levels during sexual maturation in diploid and triploid rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 83:103–110
Tse DL, Tse MC, Chan CB, Deng L, Zhang WM, Lin HR, Cheng CH (2003) Seabream growth hormone receptor: molecular cloning and functional studies of the full-length cDNA, and tissue expression of two alternatively spliced forms. Biochim Biophys Acta 1625:64–76
Varsamos S, Nebel C, Charmantier G (2005) Ontogeny of osmoregulation in postembryonic fish: a review. Comp Biochem Physiol A Mol Integr Physiol 141:401–429
Vong QP, Chan KM, Leung K, Cheng CH (2003) Common carp insulin-like growth factor-I gene: complete nucleotide sequence and functional characterization of the 5′-flanking region. Gene 322:145–156
Wai-Sum O, Chan ST (1974) A cytological study on the structure of the pituitary gland of Monopterus albus (Zuiew). Gen Comp Endocrinol 24:208–222
Weber GM, Grau EG (1999) Changes in serum concentrations and pituitary content of the two prolactins and growth hormone during the reproductive cycle in female tilapia, Oreochromis mossambicus, compared with changes during fasting. Comp Biochem Physiol C 124:323–335
Weltzien FA, Norberg B, Helvik JV, Andersen Ø, Swanson P, Andersson E (2003) Identification and localization of eight distinct hormone-producing cell types in the pituitary of male Atlantic halibut (Hippoglossus hippoglossus L.). Comp Biochem Physiol A Mol Integr Physiol 134:315–327
Wingstrand KG (1966) Comparative anatomy and evolution of the hypophysis. In: Harris GW, Donovan BT (eds) The pituitary gland, vol 1. Butterworths, London, pp 58–126
Wu YS, He Z, Zhang LH, Jiang H, Zhang WM (2012) Ontogeny of immunoreactive Lh and Fsh cells in relation to early ovarian differentiation and development in protogynous hermaphroditic ricefield eel Monopterus albus. Biol Reprod 86(93):1–9
Yada T, Azuma T, Takagi Y (2001) Stimulation of non-specific immune functions in seawater-acclimated rainbow trout, Oncorhynchus mykiss, with reference to the role of growth hormone. Comp Biochem Physiol B Biochem Mol Biol 129:695–701
Yang BY, Greene M, Chen TT (1999) Early embryonic expression of the growth hormone family protein genes in the developing rainbow trout, Oncorhyncphus mykiss. Mol Reprod Dev 53:127–134
Yowe DL, Epping RJ (1995) Cloning of the barramundi growth hormone-encoding gene: a comparative analysis of higher and lower vertebrate GH genes. Gene 162:255–259
Zhang JN, Song P, Hu JR, Mo SJ, Peng MY, Zhou W, Zou JX, Hu YC (2005) Molecular cloning and sequence analysis of full-length growth hormone cDNAs from six important economic fishes. Yi Chuan Xue Bao 32:19–29
Zhou H, Wang X, Ko WK, Wong AO (2004) Evidence for a novel intrapituitary autocrine/paracrine feedback loop regulating growth hormone synthesis and secretion in grass carp pituitary cells by functional interactions between gonadotrophs and somatotrophs. Endocrinology 145:5548–5559
Zizzari P, Hassouna R, Grouselle D, Epelbaum J, Tolle V (2011) Physiological roles of preproghrelin-derived peptides in GH secretion and feeding. Peptides 32:2274–2282
Acknowledgments
The authors would like to thank Dazhong Breeding Co. Ltd., Jianyang, Sichuan, China, for providing ricefield eel embryos and larvae and Boyang Shi, Yize Zhang, and Xu Yang for their assistance in preparing the recombinant Prl, Sl, and Tshb proteins and tissue sections. This project was supported by Grants from the National Natural Science Foundation of China (31172088, 31372513) and by a undergraduate student training program from Sun Yat-Sen University (J1310025).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, D., Liu, J., Chen, W. et al. Expression and ontogeny of growth hormone (Gh) in the protogynous hermaphroditic ricefield eel (Monopterus albus). Fish Physiol Biochem 41, 1515–1525 (2015). https://doi.org/10.1007/s10695-015-0104-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0104-3