Abstract
Potamodromous teleosts that require migration to reproduce show dysfunctions that block ovulation and spawning while in captivity. To understand the physiological basis of these reproductive dysfunctions, follicle-stimulating hormone b subunit (fshb) and luteinizing hormone b subunit (lhb) gene expression analyses by real-time quantitative PCR, together with measurements of estradiol (E 2), 17α-hydroxyprogesterone (17α-OHP) and 17α,20β-dihydroxy-4-pregnen-3-one (17α,20β-DHP) levels, were carried out throughout the reproductive cycle of the potamodromous Salminus hilarii. The following reproductive stages were evaluated in captive and wild females: previtellogenic (PV), advanced maturation/mature (AM) and regression/spent (REG/SPENT). In the wild females, fshb expression decreased from the PV to the AM stage, and the opposite pattern was detected for E 2, which increased from the PV to the AM stage. fshb was expressed at lower levels in captive than in wild females, and this difference did not change during the reproductive cycle. lhb expression also increased from the PV to the AM stage in both groups, but the wild females at the AM and REG/SPENT stages showed higher lhb expression levels than the captive females. The concentrations of 17α-OHP did not change during the reproductive cycle, and the levels were higher in the captive than in the wild females at all reproductive stages. 17α,20β-DHP levels did not change between wild and captive females. However, in captive females, the transition from PV to AM stage was followed by an increase in 17α,20β-DHP levels. These data indicate that dysfunctions in the gonadotropins and steroids synthesis pathways cause the ovulation failure in captive S. hilarii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The physiological control of fish reproduction is dependent on the species’ reproductive strategy, the synchrony of oocyte development and the spawning frequency (Rocha and Rocha 2006). The impediment of reproductive migration by natural or human interference prevents females from ovulating and, consequently, spawning. Zohar and Mylonas (2001) and Mylonas et al. (2010) have stated that an impediment of reproductive migration is the most common reproductive dysfunction in fish, which involves the completion of vitellogenesis but a failure of vitellogenic oocytes to undergo final oocyte maturation (FOM). Methods for hormonal manipulation that interfere with the hypothalamic–pituitary–gonadal (HPG) axis are necessary to reach the final maturation and ovulation for artificial reproduction in fish.
A variety of hormones in the HPG axis have been successfully used to induce the final maturation of fish gonads, but the effects of confinement on the endocrine system that ultimately block FOM are still unclear (Zohar and Mylonas 2001). Hypophysation is a common method to induce spawning in rheophilic fish (von Ihering 1937; Fontenele 1955) and has been used extensively for fingerling production in fish farming (Zohar and Mylonas 2001). Currently, many other methods are also applied, such as gonadotropin-releasing hormone (GnRH) delivery systems (Mylonas and Zohar 2001; Marino et al. 2003) and treatment with human chorionic gonadotropin (hCG) either alone (Zohar and Mylonas 2001) or in combination with pituitary extracts (Leonardo et al. 2004; Caneppele et al. 2009).
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are glycoproteins produced by adenohypophyseal cells, have a shared α subunit and a specific β subunit (Levavi-Sivan et al. 2010). The expression patterns of these gonadotropins (GtHs) during the reproductive cycle of teleosts varies according to the ovarian development and, consequently, spawning frequency, which directly influences the pattern of gonadal steroid production. High levels of FSH b subunit (fshb) gene expression in teleost species are related to the beginning of the reproductive cycle, i.e., the early stages of gametogenesis, while LH b subunit (lhb) gene expression is higher at the end of the reproductive cycle, mainly during ovulation and spawning (Levavi-Sivan et al. 2010).
After synthesization, GtHs undergo a fast release and activate gonadotropin receptors present in the ovarian follicular cells (Maruska et al. 2011). The activation of such membrane receptors by these glycoproteins triggers the follicular steroidogenesis (Young et al. 2005). The primary sex steroids produced by teleost ovaries are 17β-estradiol (E 2), testosterone (T), 17α-hydroxyprogesterone (17α-OHP) and 17α,20β-dihydroxy-4-pregnen-3-one (17α,20β-DHP). During the vitellogenic stage of oocyte development, E 2 is produced in the follicular layers. Then, it acts through nuclear estrogen receptor (ER) signaling, which stimulates hepatocytes to synthesize vitellogenin (Vtg) for release into the bloodstream. Vitellogenin (Vtg) is a phospholipoglycoprotein that is incorporated into oocytes during vitellogenesis. At the final maturation stage, there is a shift in the steroidogenic pathway, from E 2 to 17α-OHP. Accordingly, progestogens are the main gonadal steroids that are produced to stimulate FOM, ovulation and spawning (Lubzens et al. 2010).
Factors influencing the success of fish reproduction are the synthesis and secretion rates of GtHs and gonadal steroids (Zohar and Mylonas 2001). Therefore, analyzing the expression pattern of these glycoproteins and steroids during the reproductive cycle is the first step in understanding the reproductive process and evaluating whether the dysfunction during the reproduction cycle of certain Neotropical fish species is due to any impairment in GtH synthesis or release.
The Neotropical Salminus hilarii, which is also known as tabarana, is an important migratory freshwater fish, of great ecological importance of the Brazilian ichthyofauna. This species inhabits the upper Paraná River basin and the São Francisco, Tocantins, Alto Amazonas and Alto Orinoco basins (Mirande 2010), and similar to many freshwater Neotropical fish, the tabarana has a migratory reproductive behavior and is considered a short distance migration species (Agostinho et al. 2003). S. hilarii is a group synchronous (total spawner) species that spawn from November to February in the upper regions of the Tietê River in the Alto Tietê River basin (Honji et al. 2009). These authors observed a lack of postovulatory follicles and ripe oocytes (with migration of the nucleus to the periphery of cytoplasm towards the micropyle) in captive S. hilarii broodstock, which produced fewer and larger eggs than wild animals. These findings clearly showed that an impediment of migration negatively impacted FOM in this species, and observations related to the hormones produced by the HPG axis are considered important for understanding the possible physiological causes of the reproduction failure.
Artificial reproduction can be applied for most potamodromous fish species in captivity using pituitary extracts (Baldisserotto and Gomes 2005). This protocol was also successful for S. hilarii, a potamodromous species, in which females do not ovulate or spawn naturally in captivity, although the males do release sperm (Honji et al. 2011). This fact raises the hypothesis of dysfunctions in the production of pituitary gonadotropins and/or gonadal steroids in the captive females.
Our studies have focused on the changes that occur in gonadal steroids and the gonadotropin mRNA expression in wild and captive S. hilarii females during the reproductive cycle, highlighting this species as a model to better understand the reproductive physiology of this species and the impact of a migration impediment on a potamodromous Neotropical fish species.
Materials and methods
Fish collection
The S. hilarii wild female broodstock (20 females; total length: 40.67 ± 2.0 cm; body mass: 843.83 ± 266.0 g) that were used in this study were caught from April 2004 to August 2006 in the upper regions of the Tietê River between the towns of Mogi das Cruzes and Biritiba Mirim, São Paulo State, Brazil, which is a region of the Alto Tietê basin. This time period was considered based on the description of the reproductive cycle established for S. hilarii by Honji et al. (2009). This region was characterized by the lotic waters in the main channel of the Tietê River (23°32′S and 46°08′W).
The second group of fish (captive), which was also comprised of 20 animals (total length 35.23 ± 0.86 cm; body mass 534.22 ± 45.47 g), was maintained during the same period in two 100 m2 ponds at the Ponte Nova Fish Farm (23°35′S and 45°58′W) and also in the Alto Tietê basin in Salesópolis, São Paulo State, Brazil. During the experimental period, the animals in captivity were fed with commercial feed (40 % crude protein), and both the temperature and dissolved oxygen level were monitored daily with an oximeter (model 55; YSI, Yellow Springs, OH). Additionally, the experimental design, sampling frequency and fish measurements were identical to those described by Honji et al. (2009).
The females sampled in the river and in captivity (randomly selected from the ponds) were transported to the laboratory, anesthetized with tricaine methanesulfonate (MS-222; Sigma Diagnostics INS, St. Louis, MO) (1 g MS-222 per 10 l water) and neutralized with sodium bicarbonate (1 g per 10 l water). Blood samples were collected by a puncture of the caudal blood vessels using a heparinized syringe, and the blood was centrifuged for 5 min at 655.1 × g. The obtained plasma was frozen and kept at −80 °C until steroid analysis.
Animals were killed by decapitation at the level of the operculum (according to the Institutional Animal Care Protocols—CEMEA approval 0.28.04), and the pituitaries were collected, frozen in liquid nitrogen and kept at −80 °C until RNA extraction. Following this procedure, the animals were dissected, and the ovaries were removed and weighed to the nearest decigram. Both the gonadosomatic index (GSI), which is expressed as the percentage of body weight as related to the ovaries [GSI = (gonad weight/total weight) × 100] (Vazzoler 1996), and the histological results were used to determine the maturity stage.
The pattern of oocyte development in wild and captivity S. hilarii females was previously described by Honji et al. (2009) and adjusted herein in three stages: previtellogenic (PV), advanced maturation (AM) and regression/spent (REG/SPENT). Additionally, the oocyte development classification presented herein is accordingly by França et al. (2010) and Quagio-Grassiotto et al. (2011, 2014), based on the cellular events that drive oocyte development in the Ostariophysi group.
To confirm the maturation stage of the gonads, the ovaries were fixed in Bouin’s solution for 24 h, dehydrated in ethanol, cleared in dimethylbenzene and embedded in Paraplast (Erviegas, São Paulo, Brazil). Ovary sections (5 µm thick) were stained with hematoxylin–eosin, examined and documented using a computerized image analyzer (Leica DM1000 light microscope, Leica DFC 295 camera, and computer image capture, Leica Application Suite Professional, LAS v3.6). Histological methods were previously described by Honji et al. (2009).
Steroid analysis
The plasma levels of 17β-estradiol (E 2), 17α-hydroxyprogesterone (17α-OHP) and 17α, 20β-dihydroxy-4-pregnen-3-one (17α,20β-DHP) were quantified by an enzyme-linked immunosorbent assay (ELISA) (Diagnostic Systems Laboratories, DSL, Inc., Texas, USA, for E 2 and 17α-OHP and Cayman Chemical Company, Michigan, USA, for 17α,20β-DHP). The absorbance measurements were performed in a microplate reader (Tecan Sunrise, NC, USA).
Analyses were carried out strictly following the manufacturer’s instructions, and a standard curve was run for each ELISA plate. Also, pilot assays using three different dilutions of three samples were carried out in order to establish the appropriate working dilutions. Additionally, plasma samples were run in duplicate, and validations of kits were determined by calculating the intra- and inter-assay coefficients of variation (% CV). The acceptable limit for the intra- and inter-assay % CV was ≤20.0 (Sink et al. 2008). The detection limit of the assay was 10 pg/ml for E 2, 15 pg/ml for 17α,20β-DHP and 50 pg/ml for 17α-OHP. Plasma validation for S. hilarii was assessed by computing intra- and inter-assay coefficients of variation (CVs) that were (minimum–maximum) 0.69–14.76 and 3.32–16.54 % for E 2; 1.50–16.80 and 2.92–18.97 % for 17α-OHP; and 2.30–15.10 and 1.47–16.25 % for 17α,20β-DHP.
Total RNA isolation and reverse transcription
Total RNA from S. hilarii pituitaries was extracted using the SV Total RNA Isolation System (Promega) according to the manufacturer’s instructions. The RNA pellet was dissolved in DEPC-treated water and quantified by its absorbance at OD260/OD280. The quality of the RNA preparation was analyzed by electrophoresis on a 2.0 % agarose gel. Total RNA (1 µg) from each sample was reverse transcribed in the presence of the enzyme RT-MMLV (Invitrogen Life Technologies) and an RNase inhibitor (RNaseOUT, Invitrogen Life Technologies) according to the manufacturer’s instructions.
Polymerase chain reaction, cloning and sequence analysis
The polymerase chain reaction (PCR) for amplification of the fshb subunit cDNA from S. hilarii was performed using degenerated primers, which were based on the nucleotide sequences of Clarias gariepinus (Vischer et al. 2003) and Cyprinus carpio (Chang et al. 1992), and the PCR for lhb used degenerated primers that were based on the nucleotide sequence described by Parhar et al. (2003) (Table 1).
The PCR products were separated on a 2.0 % agarose gel, and the desired size of the bands was approximately 220 bp for both fshb and lhb. The amplified fragments of each gene were ligated into the pGEM-T-Easy plasmid (Promega Co., Madison, WI) and transformed into competent Escherichia coli DH5α cells (Invitrogen Life Technologies). Single colonies of cells that were grown on LB plates were selected, and the plasmidic DNA was extracted by alkaline lysis using the QIAprep Spin Miniprep Kit (Qiagen, Germany) and digested with the restriction enzyme EcoRI (Fermentas Life Science) according to the manufacturer’s instructions. The sizes of the inserts were checked by electrophoresis using a 1 % agarose gel with 10 % ethidium bromide and visualized in a UV transilluminator (Bio-Rad Gel-Doc-1000 Darkroom Documentation System).
Strands were sequenced with an ABI 3100 sequencer (Applied Biosystems) using the T7 or SP6 primers and the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) according to the manufacturer’s instructions. Nucleotide sequences were identified by the NCBI BLASTx program (GenBank, NCBI).
qPCR
The fshb and lhb mRNA levels in the pituitary glands of wild and captive females at different maturation stages were determined by qPCR. Specific primers were designed for qPCR (Table 2) at internal regions of the fshb and lhb partial sequences obtained for S. hilarii. Quantification and amplification were performed with 12.5 µl of Platinum SYBR® Green qPCR SuperMix-UDG (Invitrogen Life Technologies), 0.5 µl of each primer [10 µM] (sense and antisense) and 1 µl of cDNA, with water added to obtain a final volume of 25 µl. The samples were amplified using an iCycler iQ thermocycler (Bio-Rad) with the following conditions: 50 °C (2 min), 95 °C (1 min), 40 cycles at 95 °C (15 s) and 60 °C (30 s). For the melting curve, the temperature increased from 72 to 95 °C at a rate of 0.5 °C/30 s. For normalization of the cDNA loading, all samples were run in parallel using the reference gene elongation factor 1α (Table 2). This gene has been commonly used as a reference gene in pituitary research (Varsamos et al. 2006; Tam et al. 2011) and was the most stable reference gene in validation experiments (Olsvik et al. 2005; Infante et al. 2008). The relative mRNA expressions of fshb and lhb were determined using the \(2^{{ - (\Delta C_{\text{t}} )}}\) method. The CVs in the qPCR assay were (minimum–maximum) 13.5–18.3 % for FSH and 6.5–14.7 % for LH.
The partial sequences that were obtained in this work are available in the GenBank: Genetic sequence database at the National Center for Biotechnical Information (NCBI) for fshb (GenBank ID: JQ045707) and for lhb (GenBank ID: JQ045708).
Statistics
The results were expressed as the mean ± SEM (standard error of the mean). Comparisons between wild and captive females and among the maturation stages of the ovaries within each group were performed by a two-way analysis of variance (ANOVA), followed by the all pairwise multiple comparison procedures (Holm–Sidak method) for identification of statistically distinct groups. The significance level adopted was 95 % (P < 0.05). Statistical analyses were performed using the software SigmaStat for Windows version 3.10 (Systat Software, San Jose, CA, USA).
Results
GSI analysis and ovarian maturation stages
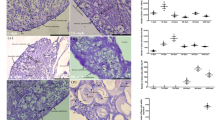
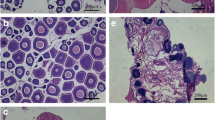
After assessing the oocyte development, the maturation stages were identified based on ovarian histological analyses of females from both environments. The classification of maturation stages was: PV stage (April–August), which comprises the animals before the beginning of exogenous vitellogenesis (perinucleolar and cortical alveolar oocytes in both environments) (Fig. 1a, b—for wild females, captive females); AM stage (September–December), which include the vitellogenic females (showing only vitellogenic oocytes) for captive females, and vitellogenic females (vitellogenic oocytes), and also those that showed vitellogenic oocytes with nuclear migration (for wild females) (Fig. 1c–f—for wild females, captive females); and REG/SPENT stage (January–March), which include the animals that shows atretic oocytes (in both environments), and for wild females, those that presented the postovulatory follicles (Fig. 1g–h—for wild females and Fig. 1i for captive females).
Micrographs of the ovarian maturation stage of Salminus hilarii in both groups (wild and captive females). a, b Previtellogenic stage (PV) [wild females (a); captive females (b)], showing the perinucleolar oocytes in both groups; c–f advanced maturation stage (AM) [wild females (c–e); captive females (f)], showing the vitellogenic oocytes (c) (arrowhead), and vitellogenic oocyte with nuclear migration (d) (arrowhead), and details of nuclear migration (e) (arrowhead) to the periphery of the oocyte cytoplasm (arrow). These characteristics were observed only in wild females (vitellogenic oocyte with nuclear migration). Captive females showing only vitellogenic oocytes (f) (arrowhead) without nuclear migration; g–i regression/spent stage (REG/SPENT) [wild females (g, h); captive females (i)], showing the postovulatory follicles (g) (arrowhead) and atretic oocytes (arrowhead) (h). These characteristics were observed in wild females (postovulatory follicles). Captive females showing only atretic oocytes (i) (arrowhead). Hematoxylin–eosin staining. Bars 300 µm (a–d, f, i); 100 µm (e, g, h)
GSI increased from the PV to the AM stage (P < 0.001 for both groups), and while the values were maintained at a similar level at the REG/SPENT stage in the captive females, they decreased from the AM to the REG/SPENT stage in the wild females (P < 0.001). Thus, captive females had a higher GSI at the REG/SPENT stage compared to the wild females (P < 0.001) (Fig. 2).
Gonadosomatic index (%) during the reproductive cycle of wild and captive Salminus hilarii females. Data are presented as the mean ± standard error of the mean (mean ± SEM). Values followed by capital letters (A, B) are significantly different between the environments, and small letters (a, b) indicate significantly different mean values among the maturation stages (P < 0.05)
Plasma steroids
Plasma E 2 concentrations increased from the PV to the AM stage and decreased in the REG/SPENT stage in wild females (P = 0.004) but was not altered in captive females throughout the reproductive cycle (Fig. 3a). When comparing the same reproductive stage in both environments, the data showed that captive females presented lower plasma E 2 concentrations than the wild females in AM stage (P = 0.004) (Fig. 3a).
Plasma sex steroids levels in wild and captive Salminus hilarii females during the reproductive cycle. a Plasma 17β-estradiol (E 2). b Plasma 17α-hydroxyprogesterone (17α-OHP). c Plasma 17α,20β-dihydroxy-4-pregnen-3-one (17α,20β-DHP). Data are presented as the mean ± standard error of the mean (mean ± SEM). Values followed by capital letters (A, B) are significantly different between the environments, and small letters (a, b) indicate significantly different mean values among the maturation stages (P < 0.05)
The plasma 17α-OHP concentrations were not altered during the reproductive cycle in either the captive or wild animals (P = 0.8989 and P = 0.069, respectively) (Fig. 3b). However, the captive females presented higher plasma 17α-OHP concentrations than the wild females throughout the reproductive cycle (P = 0.029 in the PV stage; P = 0.008 in the AM stage; and P = 0.004 in the REG/SPENT stage) (Fig. 3b).
Plasma 17α,20β-DHP levels did not change during the reproductive cycle in wild females and were also not different between captive and wild females. However, in captive females, 17α,20β-DHP increased from PV to AM stage (P = 0.005) (Fig. 3c).
fshb and lhb mRNA expression
The PV females showed a higher fshb expression than the AM females from the wild, and the expression levels subsequently increased at the REG/SPENT stage (P < 0.01); however, this profile was not observed in the captive females (Fig. 4a). Wild females at the PV and REG/SPENT stages also showed higher fshb expression levels than the captive females (P < 0.001) (Fig. 4a).
Pituitary mRNA levels of fshb (a) and lhb (b) subunits in wild versus captive Salminus hilarii females during the reproductive cycle, analyzed by qRT-PCR. Levels were normalized to elongation factor 1α (EF1α) and represented as the mean ± standard error of the mean (mean ± SEM). Values followed by capital letters (A, B) are significantly different between the environments, and small letters (a, b) indicate significantly different mean values among the maturation stages (P < 0.05)
The pituitary lhb expression was increased in the wild and captive females at the AM stage, and the expression levels were maintained during the REG/SPENT stage (Fig. 4b). Additionally, the wild females at the AM and REG/SPENT stages showed higher lhb expression levels than the captive females (P < 0.001) (Fig. 4b).
Discussion
The reproductive cycle based on the morphology of S. hilarii ovaries (Honji et al. 2009) and the classification of the stages underwent some modifications to allow a comparison between the animals from the assessed environments. Based on the histological analyses, the morphology of the ovaries was similar between the two groups (wild and captive females), with difference only at the final maturation stage of oocyte and postovulatory follicles in wild animals. This corroborates previous findings that S. hilarii is a group synchronous species, with total spawn and a marked seasonality in the ovarian development (Honji et al. 2009). The specimens from both environments were grouped into different stages: PV (exogenous vitellogenesis), AM (vitellogenic oocytes and vitellogenic oocytes in nuclear migration) and REG/SPENT (atretic oocytes and postovulatory follicles). This classification derived from new knowledge about oocyte development in perciform species from marine (Grier et al. 2009) and freshwater environments, such as Ostariophysi, Gymnotus sylvius (França et al. 2010), Pimelodus maculatus (Quagio-Grassiotto et al. 2011) and Serrasalmus maculatus (Quagio-Grassiotto et al. 2014).
The analysis of the S. hilarii gonadotropins revealed the occurrence of two cDNAs encoding fshb and lhb, which were cloned. Their expression levels were investigated in the pituitaries of wild and captive females throughout the reproductive cycle. Firstly, when PCR amplification of the GtHs cDNA was performed using degenerated primers of salmon, no amplification was observed, corroborating the previous immunohistochemistry (IHC) results (Honji et al. 2013). IHC test outcomes showed that the pituitary gonadotropes were weakly immunoreactive to anti-chum salmon antisera. S. hilarii GtHs cells were more immunoreactive to β-LH than to β-FSH antibodies (Honji et al. 2013), and due to lack of strong positive immunoreactions, the study of GtHs expression was more appropriate. Furthermore, plasma estrogens and progestogens (E 2, 17α,20β-DHP and 17α-OHP) were also measured. These assessments allowed an investigation of the changes in the pituitary–gonadal axis during the reproductive cycle of wild and captive S. hilarii females.
The fshb and lhb gene sequences have been extensively studied in many teleost species (Elizur et al. 1996; Gomez et al. 1999; Jackson et al. 1999; Saito et al. 2002; Mateos et al. 2003; Kim et al. 2005; Olsvik et al. 2005; So et al. 2005; Jeng et al. 2007; Cerdà et al. 2008; Guzmán et al. 2009; Zhou et al. 2010). These assays were reviewed by Levavi-Sivan et al. (2010). However, investigations in potamodromous, such as Characiformes, which is a large order comprising approximately 1674 species (Nelson 2006), have not yet been performed. Our results showed that the expression of the fshb subunit gene in wild females decreased at the AM stage and increased at the REG/SPENT stage. However, this pattern was not observed in the captive females, while the fshb gene was expressed at the same level during the entire reproductive cycle. There was a decrease in fshb expression in wild females at the AM stage. Subsequently, an increase occurred in the lhb expression, which remained throughout the REG/SPENT stage. The captive females also presented this lhb expression pattern. In Oncorhynchus mykiss, the fshb expression also increased in females at the early vitellogenic stage. Nevertheless, lhb was markedly increased at the later stage of vitellogenesis stage and persisted throughout oocyte maturation and ovulation, corroborating that lhb is a final-stage maturation gonadotropin (Gomez et al. 1999).
The same fshb and lhb profile was observed during the reproductive cycle of Carassius auratus (Yoshiura et al. 1997), Sebastes schlegeli (Kim et al. 2005), Gasterosteus aculeatus (Hellquist et al. 2006) and Micropogonias undulatus (Banerjee and Khan 2008). In most of these species, the fshb and lhb expression decreased after the vitellogenic stage. However, this profile expression was not observed in S. hilarii. Furthermore, the fshb plasma levels in O. mykiss increased immediately after the release of oocytes from the female body cavity. The FSH levels would have been maintained if the oocytes had not been released (Breton et al. 1998).
The captive Senegalese sole broodstock (Solea senegalensis) exhibited a lower fsh gene expression during the spawning season compared to the wild animals (Guzmán et al. 2009). This corroborates the data on S. hilarii at this time of the reproductive cycle. The abovementioned authors substantiated that the higher fsh expression on wild fish suggests an increase in FSH secretion from the pituitary, which results in higher levels of this glycoprotein in the bloodstream (Swanson et al. 2003). However, in S. senegalensis, the lhb transcript levels were similar in the wild and captive animals (Guzmán et al. 2009). Therefore, the dysfunction in ovulation in captive female of S. senegalensis could be associated with a lack of LH release into the bloodstream of this species. Mylonas et al. (2010) also suggested this lack of LH release in several fish species. Lower fsh expression in S. hilarii captivity females reflected the lower E 2 plasma levels in these animals, mainly at the AM stage, when compared to wild animals. Even with lower E 2 levels, oocytes can develop and become vitellogenic. Notwithstanding, as previously described, the absolute fecundity in S. hilarii in captivity is lower than in wild females (Honji et al. 2009). Therefore, the data found herein suggest that low fsh expression and, consequently, E 2 synthesis, can explain a lower oocyte recruitment in captivity females.
Contrastively, the lhb expression pattern in captive S. hilarii females was similar to that found in wild females all through the reproductive cycle. The expression levels in the wild animals were higher though. The responsiveness of the gonadotrophs to the hypothalamic gonadotropin-releasing hormone (GnRH) is highly dependent on the reproductive stage. In this case, gonadal steroid hormones can be the primary modulators of GnRH action on FSH and/or LH release (Ando and Urano 2005) through kisspeptin neurons (Oka 2009). In Sparus aurata captive females, the levels of lhb in the pituitary increased with the progression of vitellogenesis and the arrival of the spawning season (Meiri et al. 2004). However, after completion of vitellogenesis, pituitary LH reached its maximal levels. Consequently, the LH level in plasma remained undetectable, and the oocytes underwent atresia. These findings suggest that lh expression and release may be affected in captive females. Differences in the lhb gene expression can be the result of either a downregulation or an upregulation of genes in the steroid synthesis pathway, particularly considering that domestication causes broad effects on gene expression in fish (Tymchuk et al. 2009).
During the AM maturation stage, plasma levels of E 2 reached the highest level throughout the wild female reproductive cycles. The increase in E 2 plasma levels may have contributed to the decrease in fsh expression at this stage, particularly considering that estrogen has a negative feedback on fsh gene expression, as previously described in the literature (Levavi-Sivan et al. 2010). This pattern could be explained by a direct action of E 2 on fsh gene transcription via interactions with estrogen receptors (Dickey and Swanson 1998; Melamed et al. 2000; Mateos et al. 2002; Aroua et al. 2007; Banerjee and Khan 2008). It could also be ascribable to the presence of estrogen receptors in the gonadotrophs (Banerjee and Khan 2008; Levavi-Sivan et al. 2010). In captive females, the small amount of E 2 concentration was sufficient to promote vitellogenesis, which was confirmed by the high GSI values that were reached at the AM stage.
Analyzing both the lhb mRNA expression and the 17α,20β-DHP plasma levels, the data suggest that even when low levels of lhb are expressed, the concentration of 17α,20β-DHP in captive females compared to wild animals did not change. Thus, the lack of ovulation in S. hilarii captive females cannot be related only to dysfunctions in lhb synthesis and/or a modulation of progestogen production by gonadotropin. This assay results suggest that ovulation dysfunctions, which are common in captive fish, do not necessarily have a common physiological basis. In most teleost species, the ovulation dysfunction in captivity is attributed to the lack of LH release from the pituitary cells (Mylonas and Zohar 2001). However, this premise does not apply to the potamodromous S. hilarii, due to the lack of investigation of plasma LH in this species. The data acquired with this work revealed that genetic disturbances affecting different components of the pituitary–gonadal axis appear to influence the fshb and lhb gene expressions, as well as the steroidogenic pathway. Furthermore, we emphasize that there are differences in the synthesis and release of fshb and lhb correlation patterns. Therefore, we cannot address the role of dysfunctions only focusing on the alteration in the synthesis rate of those GtHs.
Despite the lack of measurements of GtH plasma levels in Characiformes species, Maruska et al. (2011) substantiated that pituitary gonadotropin mRNA levels in Astatotilapia burtoni (Perciformes) correlate positively to the plasma levels of these glycoproteins. Therefore, GtHs hormones are quickly released after synthesis. It is also known through extensive data analyses that GtHs are potential regulators of steroid synthesis (Blázquez et al. 1998; Zohar and Mylonas, 2001; Mylonas et al. 2010). This study enabled the observation of both the full regulation of the HPG axis and data on the GtHs gene expression and plasma steroids. These data, along with the previously published knowledge of oocyte development and fecundity (Honji et al. 2009), must be interpreted as a whole to solve the puzzle of reproductive dysfunction in females of S. hilarii in captivity.
This study constitutes the first approach to gonadotropin expression in a potamodromous fish. The results of this investigation will be useful in guiding more specific studies on induction protocols for the reproduction of S. hilarii, a target species for conservation actions in the Alto Tietê River basin. The development of spawning protocols requires thorough consideration of the complex function of the HPG axis, which includes the positive and negative feedback, which regulates hormone production (Dickey and Swanson 1998). Other HPG axis relevant functions are the key enzymes of the steroidogenic pathway (Tanaka et al. 2002; Sreenivasulu and Senthilkumaran 2009), the interrelation between the HPG axis and the hypothalamic–pituitary–interrenal axis (Milla et al. 2009), the interference of other peptides on gonadotropin expression, such as activins, follistatins and inhibins (Petrino et al. 2007; Aroua et al. 2012) and also the maturation promoting factor (MPF) (Nagahama and Yamashita 2008).
In conclusion, the fshb and lhb subunits are differentially expressed in wild and captive S. hilarii and could explain the success obtained by the hypophysation method that has been used for the assessment of this (Honji et al. 2011) and other potamodromous species applying artificial reproduction protocols. Additionally, plasma levels of E 2 and 17α-OHP, which are the main steroids produced by fish ovaries, are also differentially produced when reproductive migration is blocked. The development of immunoassays to measure plasma gonadotropins in Characiformes can be useful for determining the mechanisms underlying the dysfunction of the pituitary–gonadal axis in Neotropical migratory fish species in captivity. Finally, understanding the expression pattern of hormones in the HPG axis and the enzymes involved in the steroidogenic pathway will help improve the artificial reproduction methods, which are essential to successful fish farming of potamodromous migratory fish species.
References
Agostinho AA, Gomes LC, Suzuki HI, Júlio HF (2003) Migratory fish from the upper Paraná River Basin, Brazil. In: Carolsfeld J, Harvey B, Ross C, Baer A (eds) Migratory fishes of South America: biology, fisheries and conservation status. World Fisheries Trust. The World Bank and the International Development Research Centre, Victoria, pp 19–99
Ando H, Urano A (2005) Molecular regulation of gonadotropin secretion by gonadotropin-releasing hormone in salmonid fishes. Zool Sci 22:379–389. doi:10.2108/zsj.22.379
Aroua S, Weltzien FA, Le Belle N, Dufour S (2007) Development of real-time RT-PCR assays for eel gonadotropins and their application to the comparison of in vivo and in vitro effects of sex steroids. Gen Comp Endocrinol 153:333–343. doi:10.1016/j.ygcen.2007.02.027
Aroua S, Maugars G, Jeng S, Chang C, Weltzien F, Rousseau K, Dufour S (2012) Pituitary gonadotropins FSH and LH are oppositely regulated by the activin/follistatin system in a basal teleost, the eel. Gen Comp Endocrinol 175:82–91. doi:10.1016/j.ygcen.2011.10.002
Baldisserotto B, Gomes LC (2005) Espécies nativas para pisciculturas no Brasil. Editoraufsm, Santa Maria
Banerjee A, Khan IA (2008) Molecular cloning of FSH and LH β subunits and their regulation by estrogen in Atlantic croaker. Gen Comp Endocrinol 155:827–837. doi:10.1016/j.ygcen.2007.09.016
Blázquez M, Bosma PT, Fraser EJ, Van Look KJW, Trudeau VL (1998) Fish as models for the neuroendocrine regulation of reproduction and growth. Comp Biochem Physiol (C) 119:345–364. doi:10.1016/S0742-8413(98)00023-1
Breton B, Govoroun M, Mikolajczyk T (1998) GTH I and GTH II secretion profiles during the reproductive cycle in female rainbow trout: relationship with pituitary responsiveness to GnRH-A stimulation. Gen Comp Endocrinol 111:38–50. doi:10.1006/gcen.1998.7088
Caneppele D, Honji RM, Hilsdorf AW, Moreira RG (2009) Induced spawning of the endangered Neotropical species Steindachneridion parahybae (Siluriformes: Pimelodidae). Neotrop Ichthyol 7(4):759–762. doi:10.1590/S1679-62252009000400026
Cerdà J, Chauvigne F, Agulleiro MJ, Marin E, Halm S, Martínez-Rodríguez G, Prat F (2008) Molecular cloning of Senegalese sole (Solea senegalensis) follicle-stimulating hormone and luteinizing hormone subunits and expression pattern during spermatogenesis. Gen Comp Endocrinol 156:470–481. doi:10.1016/j.ygcen.2008.02.006
Chang YS, Huang FL, Lo TB (1992) Isolation and sequence analysis of carp gonadotropin beta-subunit gene. Mol Mar Biol Biotechnol 1(2):97–105
Dickey JT, Swanson P (1998) Effects of sex steroids on gonadotropin (FSH and LH) regulation in coho salmon (Oncorhynchus kisutch). J Mol Endocrinol 21:291–306. doi:10.1677/jme.0.0210291
Elizur A, Zmora N, Rosenfeld H, Meiri I, Hassin S, Gordin H, Zohar Y (1996) Gonadotropin GtH-I and GtH-II from the gilthead seabream Sparus aurata. Gen Comp Endocrinol 102:39–46. doi:10.1006/gcen.1996.0044
Fontenele O (1955) Injecting pituitary (hypophyseal) hormones into fish to induce spawning. Prog Fish Cult 17:71–75. doi:10.1577/1548-8659(1955)17[71:IPHHIF]2.0.CO;2
França GF, Grier HJ, Quagio-Grassiotto I (2010) A new vision of the origin and the oocyte development in the ostariophysi applied to Gymnotus sylvius (Teleostei: Gymnotiformes). Neotrop Ichthyol 8(4):787–804. doi:10.1590/S1679-62252010000400008
Gomez JM, Weil C, Ollitrault M, Bail PL, Breton B, LeGac F (1999) Growth hormone (GH) and gonadotropin subunit gene expression and pituitary and plasma changes during spermatogenesis and oogenesis in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 113:413–428. doi:10.1006/gcen.1998.7222
Grier HJ, Uribe MC, Patiño R (2009) The ovary, folliculogenesis, and oogenesis in teleosts. In: Jamieson BGM (ed) Reproductive biology and phylogeny of fishes (agnathans and bony fishes). Science Publishers, Enfield, pp 25–84
Guzmán JM, Rubio M, Ortiz-Delgado JB, Klenke U, Kight K, Cross I, Sánchez-Ramos I, Riaza A, Rebordinos L, Sarasquete C, Zohar Y, Mañanós EL (2009) Comparative gene expression of gonadotropins (FSH and LH) and peptide levels of gonadotropin-releasing hormones (GnRHs) in the pituitary of wild and cultured Senegalese sole (Solea senegalensis) broodstocks. Comp Biochem Physiol (A) 153:266–277. doi:10.1016/j.cbpa.2009.02.032
Hellquist A, Schmitz M, Mayer I, Borg B (2006) Seasonal changes in expression of LH-β and FSH-β in male and female three-spined stickleback, Gasterosteus aculeatus. Gen Comp Endocrinol 145:263–269. doi:10.1016/j.ygcen.2005.09.012
Honji RM, Narcizo AM, Borella MI, Romagosa E, Moreira RG (2009) Patterns of oocyte development in natural habitat and captive Salminus hilarii Valenciennes, 1850 (Teleostei: Characidae). Fish Physiol Biochem 35:109–123. doi:10.1007/s10695-008-9239-9
Honji RM, Mello PH, Araújo BC, Almeida-Filho JA, Hilsdorf AWS, Moreira RG (2011) Influence of spawning procedure in gametes fertilization success in Salminus hilarii Valenciennes, 1850 (Teleostei: Characidae): implications for conservation of this species. Neotrop Icthyol 9(2):363–370. doi:10.1590/S1679-62252011005000012
Honji RM, Nóbrega RH, Pandolfi M, Shimizu A, Borella MI, Moreira RG (2013) Immunohistochemical study of pituitary cells in wild and captive Salminus hilarii (Characiformes: Characidae) females during the annual reproductive cycle. SpringerPlus 2:460–474. doi:10.1186/2193-1801-2-460
Infante C, Matsuoka MP, Asensio E, Cañavate JP, Reith M, Manchado M (2008) Selection of housekeeping genes for gene expression studies in larvae from flatfish using real-time PCR. BMC Mol Biol 9:28–40. doi:10.1186/1471-2199-9-28
Jackson K, Goldberg D, Ofir M, Abraham M, Degani G (1999) Blue gourami (Trichogaster trichopterus) gonadotropic β-subunits (I and II) cDNA sequences and expression during oogenesis. J Mol Endocrinol 23:177–187. doi:10.1677/jme.0.0230177
Jeng SR, Yueh WS, Chen GR, Lee YH, Dufour S, Chang CF (2007) Differential expression and regulation of gonadotropins and their receptors in the Japanese eel, Anguilla japonica. Gen Comp Endocrinol 154:161–173. doi:10.1016/j.ygcen.2007.05.026
Kim DJ, Cho YC, Sohn YC (2005) Molecular characterization of rockfish (Sebaster schlegeli) gonadotropin subunits and their mRNA expression profiles during oogenesis. Gen Comp Endocrinol 141:282–290. doi:10.1016/j.ygcen.2005.01.001
Leonardo AFG, Romagosa E, Borella MI, Batlouni SR (2004) Induced spawning of hatchery-raised Brazilian catfish, cachara Pseudoplatystoma fasciatum (Linnaeus, 1766). Aquaculture 240:451–461. doi:10.1016/j.aquaculture.2003.11.030
Levavi-Sivan B, Bogerd J, Mañanós EL, Gómez A, Lareyre JJ (2010) Perspectives on fish and their receptors. Gen Comp Endocrinol 165:412–437. doi:10.1016/j.ygcen.2009.07.019
Lubzens E, Young G, Bobe J, Cerdà J (2010) Oogenesis in teleost: how fish eggs are formed. Gen Comp Endocrinol 165:367–389. doi:10.1016/j.ygcen.2009.05.022
Marino G, Panini E, Longobardi A, Mandich A, Finoia MG, Zohar Y, Mylonas CC (2003) Induction of ovulation in captive-reared dusky grouper, Epinephelus marginatus (Lowe, 1834) with a sustained-release GnRHa implant. Aquaculture 219:841–858. doi:10.1016/S0044-8486(03)00036-X
Maruska KP, Levavi-Sivan B, Biran J, Fernald RD (2011) Plasticity of the reproductive axis caused by social status change in an African cichlid fish: I. Pituitary gonadotropins. Endocrinology 152:281–290. doi:10.1210/en.2010-0875
Mateos J, Mañanós E, Carrillo M, Zanuy S (2002) Regulation of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) gene expression by gonadotropin-releasing hormone (GnRH) and sexual steroids in the Mediterranean sea bass. Comp Biochem Physiol (B) 132:75–86. doi:10.1016/S1096-4959(01)00535-8
Mateos J, Mañanós E, Martínez-Rodríguez G, Carrillo M, Quérat B, Zanuy S (2003) Molecular characterization of sea bass gonadotropin subunits (FSH-β and LH-β) and their expression during the reproductive cycle. Gen Comp Endocrinol 133:216–232. doi:10.1016/S0016-6480(03)00164-3
Meiri I, Knibb WR, Zohar Y, Elizur A (2004) Temporal profile of β follicle-stimulating hormones, β luteinizing hormone, and growth hormone gene expression in the protandrous hermaphrodite, gilthead seabream, Sparus aurata. Gen Comp Endocrinol 137:288–299. doi:10.1016/j.ygcen.2004.04.002
Melamed P, Gur G, Rosenfeld H, Elizur A, Schulz RW, Yaron Z (2000) Reproductive development of male and female tilapia hybrids (Oreochromis niloticus × O. aureus) and changes in mRNA levels of gonadotropin (GtH) Iβ and IIβ subunits. J Exp Zool 286:64–75. doi:10.1002/(SICI)1097-010X(20000101)286:1<64:AID-JEZ6>3.0.CO;2-D
Milla S, Wang N, Mandiki SNM, Kestemont P (2009) Corticosteroids: friends or foes of teleost fish reproduction? Comp Biochem Physiol (A) 153:242–251. doi:10.1016/j.cbpa.2009.02.027
Mirande JM (2010) Phylogeny of the family Characidae (Teleostei: Characiformes): from characters to taxonomy. Neotrop Ichthyol 8(3):385–568. doi:10.1590/S1679-62252010000300001
Mylonas CC, Zohar Y (2001) Use of GnRHa-delivery systems for the control of reproduction in fish. Rev Fish Biol Fish 10:463–491. doi:10.1023/A:1012279814708
Mylonas CC, Fostier A, Zanuy S (2010) Broodstock management and hormonal manipulations of fish reproduction. Gen Comp Endocrinol 165:516–534. doi:10.1016/j.ygcen.2009.03.007
Nagahama Y, Yamashita M (2008) Regulation of oocyte maturation in fish. Dev Growth Differ 50:S195–S219. doi:10.1111/j.1440-169X.2008.01019.x
Nelson JS (2006) Fishes of the world, 4th edn. Wiley, Hoboken
Oka Y (2009) Three types of gonadotropin-releasing hormone neurones and steroid-sensitive sexually dimorphic kisspeptin neurones in teleosts. J Neuroendocrinol 21:334–338. doi:10.1111/j.1365-2826.2009.01850.x
Olsvik PA, Lie KK, Jordal AEO, Nilsen TO, Hordvik I (2005) Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol Biol 6:21–29. doi:10.1186/1471-2199-6-21
Parhar I, Soga T, Ogawa S, Sakuma Y (2003) FSH and LH-β subunits in the preoptic nucleus: ontogenic expression in teleost. Gen Comp Endocrinol 132:369–378. doi:10.1016/S0016-6480(03)00121-7
Petrino TR, Toussaint G, Lin YP (2007) Role of inhibin and activin in the modulation of gonadotropin- and steroid-induced oocyte maturation in the teleost Fundulus heteroclitus. Reprod Biol Endocrinol 5:21–30. doi:10.1186/1477-7827-5-21
Quagio-Grassiotto I, Grier H, Mazzoni TS, Nóbrega RH, Amorin JPA (2011) Activity of the ovarian germinal epithelium in the freshwater catfish, Pimelodus maculatus (Teleostei: Ostariophysi: Siluriformes): germinative cysts, follicle formation and oocyte development. J Morphol 272:1290–1306. doi:10.1002/jmor.10981
Quagio-Grassiotto I, Wildner DD, Guimarães-Bassoli ACD (2014) A cytochemical approach to describe oocyte development in the freshwater ostariophysan, Serrasalmus maculatus (Characiformes). Micron 60:18–28. doi:10.1016/j.micron.2014.01.001
Rocha MJ, Rocha E (2006) Morphofunctional aspects of reproduction from synchronous to asynchronous fishes—an overview. In: Reinecke M, Zaccone G, Kappor BG (eds) Fish endocrinology. Science Publishers, Enfield, pp 570–624
Saito A, Kano Y, Suzuki M, Tomura H, Takeda J, Tanaka S (2002) Sequence analysis and expressional regulation of messenger RNAs encoding beta subunits of follicle-stimulating hormone and luteinizing hormone in the red-bellied newt, Cynops pyrrhogaster. Biol Reprod 66:1299–1309. doi:10.1095/biolreprod66.5.1299
Sink TD, Lockmann RT, Fecteau KA (2008) Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu, and golden shiners. Fish Physiol Biochem 34:95–101. doi:10.1007/s10695-007-9150-9
So WK, Kwok HF, Ge W (2005) Zebrafish gonadotropins and their receptors: II. Cloning and characterization of zebrafish follicle stimulating hormone and luteinizing hormone subunits—their spatial–temporal expression patterns and receptor specificity. Biol Reprod 72:1382–1396. doi:10.1095/biolreprod.104.038216
Sreenivasulu G, Senthilkumaran B (2009) New evidences for the involvement of 20β-hydroxysteroid dehydrogenase in final oocyte maturation of air-breathing catfish. Gen Comp Endocrinol 163:259–269. doi:10.1016/j.ygcen.2009.04.022
Swanson P, Dickey JT, Campbell B (2003) Biochemistry and physiology of fish gonadotropins. Fish Physiol Biochem 28:53–59. doi:10.1023/B:FISH.0000030476.73360.07
Tam JKV, Lee LTO, Cheng CHK, Chow BKC (2011) Discovery of a new reproductive hormone in teleost: pituitary adenylate cyclase-activating polypeptide-related peptide (PRP). Gen Comp Endocrinol 173:405–410. doi:10.1016/j.ygcen.2011.06.003
Tanaka M, Nakajin S, Kobayashi D, Fukada S, Guan G, Todo T, Senthilkumaran B, Nagahama Y (2002) Teleost ovarian carbonyl reductase-like 20β-hydroxysteroid dehydrogenase: potential role in the production of maturation-inducing hormone during final oocyte maturation. Biol Reprod 66:1498–1504. doi:10.1095/biolreprod66.5.1498
Tymchuk W, Sakhrani D, Devlin R (2009) Domestication causes large-scale effects on gene expression in rainbow trout: analysis of muscle, liver and brain transcriptomes. Gen Comp Endocrinol 164:175–183. doi:10.1016/j.ygcen.2009.05.015
Varsamos S, Xuereb B, Commes T, Flik G, Spanings-Pierrot C (2006) Pituitary hormone mRNA expression in European sea bass Dicentrarchus labrax in seawater and following acclimation to fresh water. J Endocrinol 191:473–480. doi:10.1677/joe.1.06847
Vazzoler AEAM (1996) Biologia da reprodução de peixes teleósteos: teoria e prática. EDUEM, Maringá
Vischer HF, Teves ACC, Ackermans JCM, van Dijk W, Schulz RW, Bogerd J (2003) Cloning and spatiotemporal expression of the follicle-stimulating hormone β subunit complementary DNA in the African catfish (Clarias gariepinus). Biol Reprod 68:1324–1332. doi:10.1095/biolreprod.102.009985
von Ihering R (1937) A method for inducing spawning in fish. Prog Fish Cult 34:15–16
Yoshiura Y, Kobayashi M, Kato Y, Aida K (1997) Molecular cloning of the cDNAs encoding two gonadotropin beta subunits (GTH-I beta and -II beta) from the goldfish, Carassius auratus. Gen Comp Endocrinol 105:379–389. doi:10.1006/gcen.1996.6839
Young G, Kusakabe M, Nakamura I, Lokman PM (2005) Gonadal steroidogenesis in teleost fish. In: Melamed P, Sherwood N (eds) Hormones and their receptors in fish reproduction. Molecular aspects of fish and marine biology, vol 4. World Scientific Publishing, Hackensack, pp 155–223
Zhou Y, Niu Y, Tão M, Deng X, Liu S, Liu Y, Li J (2010) Molecular cloning characterization and expression of FSH and LH beta subunits from grass carp (Ctenopharyngodon idella). Fish Physiol Biochem 36:213–221. doi:10.1007/s10695-008-9223-4
Zohar Y, Mylonas CC (2001) Endocrine manipulations of spawning in culture fish: from hormones to genes. Aquaculture 197:99–136. doi:10.1016/S0044-8486(01)00584-1
Acknowledgments
This work was supported by a research Grant from FAPESP (01/10483-1). The authors would also like to thank the fishermen who collected the wild females and the Ponte Nova Fish Farm for providing the facilities for maintaining the captive animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreira, R.G., Honji, R.M., Melo, R.G. et al. The involvement of gonadotropins and gonadal steroids in the ovulatory dysfunction of the potamodromous Salminus hilarii (Teleostei: Characidae) in captivity. Fish Physiol Biochem 41, 1435–1447 (2015). https://doi.org/10.1007/s10695-015-0097-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0097-y