Abstract
Using goldfish (Carassius auratus) as the model animal, the present study revealed the types of the DNA damage induced by monocrotophos, a highly toxic organophosphorus pesticide, and explored the mechanism underlying the DNA-damaging effect of this pesticide. Results of the alkaline comet assay showed that global DNA damage (including single- and double-strand breaks and alkali-labile sites) in peripheral erythrocytes of goldfish, measured as olive tail moment, was significantly increased by exposure to 0.01, 0.10, and 1.00 mg/L monocrotophos for 24, 48, 96, and 168 h. In particular, alkali-labile sites rather than single- or double-strand breaks, distinguished by the alkaline, pH 12.1, and neutral comet assays, were mainly induced by monocrotophos at 48 h. Oxidative damage in DNA bases and telomeric DNA was investigated by using the alkaline comet assay combined with endonuclease III or formamidopyrimidine DNA glycosylase and with fluorescence in situ hybridization, respectively. Further, glutathione peroxidase activity significantly decreased at 24 h but increased at 96 and 168 h, and malondialdehyde concentrations significantly increased at 48 h but gradually decreased at 96 and 168 h, which indicated an over-production of reactive oxygen species (ROS) at short exposure durations, but effective scavenging at long exposure durations in the peripheral blood tissues. Accordingly, our results suggest that DNA damage induced by monocrotophos in fish blood cells is possibly due to the inhibition of ROS scavenging and resulted accumulation of ROS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Monocrotophos is an organophosphorus pesticide used for pest control in agriculture and forestry. It has been banned in Western countries like the USA, but is still widely used in some African and Asian countries because of its good insecticidal efficacy and low price (Tariq et al. 2004; Kumari et al. 2007; Krause et al. 2013). Monocrotophos residues in brinjal, okra, cucurbits, crucifers, and green chili were found to be 0.023–1.140 mg/kg (Swarnam and Velmurugan 2013) on the Andaman Islands, India, and its concentration in the industrial wastewater near Lucknow City, India, was reported to be 8.32 ± 3.9 μg/L (Anjum and Malik 2013). Monocrotophos residues can enter the aquatic environment through surface runoff or be absorbed by organisms via the food chain and thereby alter the genetic materials of exposed organisms and induce DNA damage (Saleha Banu et al. 2001; Ali and Kumar 2008), which includes DNA single-strand breaks (SSB), double-strand breaks (DSB), alkali-labile sites (ALS), DNA base oxidation, DNA–protein cross-links, and DNA–DNA cross-links (Ribas-Maynou et al. 2014). Using the alkaline comet assay, Saleha Banu et al. (2001) first reported the DNA strand breaks caused by monocrotophos exposure in fish blood cells. Other studies also demonstrated that monocrotophos can induce DNA strand breaks in mouse peripheral blood leukocytes (Mahboob et al. 2002), in human lymphocytes (Jamil et al. 2004), and in the gill, kidney, and lymphocytes of Channa punctatus (Ali and Kumar 2008). However, to our knowledge, these studies have not clarified the specific types of DNA damage detected by Saleha Banu et al. (2001), which may include SSB, DSB, or ALS, and the specific pathway through which monocrotophos induces DNA strand breaks is still unknown.
It has been reported that organophosphate (OP) pesticides mainly induce DNA strand breaks and base damage by increasing reactive oxygen species (ROS) generation in tissues, which can attack DNA directly (Bagchi et al. 1995; Lu et al. 2012). Specifically, OP pesticides generate excessive ROS via two possible pathways: (a) In the oxidation–reduction cycle catalyzed by the cytochrome P450s (CYPs), the chemical bond –P=O, transformed from the –P=S or originally existing in OP pesticides, can easily obtain an electron and transfer it to the oxygen (O2) molecule to produce superoxide anion (\({\text{O}}_{ 2}^{ \cdot - }\)), and then the \({\text{O}}_{2}^{ \cdot - }\) can be further converted into other ROS like the hydroxyl radicals (\(^{ \cdot } {\text{OH}}\)) (Bondy and Naderi 1994; Kovacic 2003); (b) OP pesticides can also reduce the antioxidant ability of tissues by inhibiting the ROS scavenging enzyme activities, and thus excessive ROS accumulate. Yu et al. (2008) reported that chlorpyrifos increases the levels of DNA damage and lipid peroxidation and decreases activities of antioxidant enzymes superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px) in the retina of mice, suggesting that inhibition of antioxidant enzyme activities may increase the level of ROS and further lead to the damage of DNA. Recently, Kashyap et al. (2011) found that exposure of 10−5 M monocrotophos increased the level of ROS in PC12 cells by elevating the mRNA expressions of CYP1A1/1A2, 2B1/2B2, 2E1 enzymes; however, whether the DNA damage induced by this pesticide is associated with alterations in the antioxidant system and accumulation of ROS is still equivocal.
Therefore, to investigate the types of DNA damage induced by monocrotophos, different versions of the comet assay were applied in peripheral erythrocytes of goldfish (Carassius auratus) exposed to 0.01, 0.10, and 1.00 mg/L of this pesticide: The alkaline (pH > 13), pH 12.1, and neutral (pH 8.2–8.5) comet assays were used to identify the global DNA damage (includes DSB, SSB, and ALS), DSB and SSB, and DSB, respectively; and the alkaline comet assay combined with endonuclease III (Endo III) or formamidopyrimidine DNA glycosylase (FPG) and with fluorescence in situ hybridization (FISH) was used to detect oxidative damage in DNA bases and telomeric DNA, respectively. Further, the potential mechanism underlying the DNA-damaging effect of monocrotophos was explored with respect to the changes of the ROS scavenging ability in the peripheral blood tissues of goldfish, as evaluated by the SOD and GSH-Px activities; the level of lipid peroxidation, a relatively delayed manifestation that follows ROS accumulation (Dwivedi and Flora 2011), was assessed by the malondialdehyde (MDA) concentrations.
Materials and methods
Chemicals and fish
Monocrotophos (3-hydroxyl-N-methyl-cis-crotonamide dimethyl phosphate, 40 % water-soluble preparation) was purchased from the Qingdao pesticide factory, People’s Republic of China. All other chemicals were of analytical grade and purchased from Sigma (St. Louis, MO, USA) or Sinopharm Chemical Reagent Co., Ltd. (Beijing, People’s Republic of China).
Goldfish (C. auratus) with average weight and length of 8.53 ± 0.83 cm and 24.05 ± 2.74 g were obtained from a local supplier in Qingdao, People’s Republic of China, and were acclimated under laboratory conditions for 2 weeks before exposure.
Exposure design and sample collection
Goldfish (C. auratus) were exposed to 0.01, 0.10, and 1.00 mg/L monocrotophos in a semi-static manner for 24, 48, 96, and 168 h. Control treatments were established for each exposure duration, and triplicate exposures were performed for each treatment and control. Test compound concentrations were maintained in a semi-static manner (half water renewal daily), and quantification of monocrotophos in exposure solutions was analyzed by the ultra-performance liquid chromatography–tandem quadrupole mass spectrometry (UPLC-MS/MS). Monocrotophos was undetected in the control tanks, while the analyzed concentrations (mean ± standard deviation; and % analyzed/nominal) in the test solutions were 0.001 ± 0.0001 (25 %), 0.009 ± 0.0004 (22.5 %), and 0.091 ± 0.0062 mg/L (22.75 %) (Zhang et al. 2013). The concentrations mentioned in the following referred to the nominal monocrotophos pesticide values. Throughout the experiment, the water temperature was maintained at 20 ± 2 °C, the dissolved oxygen remained at 7.0 ± 0.1 mg/L, the pH was 7.5 ± 0.5, and the photoperiod was 14-h light and 10-h dark. No food was provided for the fish during exposure.
After goldfish had been anesthetized in 75 mg/L MS-222 (Sigma, St. Louis, MO, USA), peripheral blood samples were obtained from the caudal vein using a heparinized syringe. Three fish were randomly sampled for each treatment and control group at each time point. For the comet assay, a fraction of each blood sample was diluted into a single-cell suspension in phosphate-buffered saline (PBS) (pH 7.4), with a cell density of 105 cells/mL and cell viability (evaluated by trypan blue exclusion assay) higher than 95 %. For the evaluation of SOD and GSH-Px activities and MDA concentrations, the hemolysate was collected. Heparinized blood (200 μL), prepared by adding heparin sodium to the remaining fractions of the samples to avoid blood coagulation, was diluted with 4 mL physiological saline (0.9 % NaCl) and centrifuged at 2,000g/min for 3 min. The precipitated erythrocytes were collected and resuspended with 0.8 mL pre-cooled ddH2O and 0.4 mL 95 % ethanol and then mixed for 30 s. Trichloromethane (0.4 mL) was added and mixed for 1 min, followed by centrifugation at 3,500g/min for 8 min. The supernatant was the hemolysate used for evaluation.
Comet assay
The comet assay was conducted under red light, according to the procedure described by Singh et al. (1988) with some modifications.
For the alkaline comet assay, 25-µL single-cell suspension was mixed with 75 µL low-melting-point agarose and pipetted onto a glass microscope slide pre-coated with a layer of 45 µL 0.6 % (w/v) normal melting point agarose. The slides were maintained at 4 °C for 10 min to allow solidification of the agarose and then immersed in lysis buffer (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris-base, pH 10, with 1 % Triton X-100 and 10 % dimethyl sulfoxide added immediately before use) to lyse the cells at 4 °C for 1.5 h. After being washed in 0.2 M phosphate buffer (pH 7.4), the slides were immersed in electrophoresis buffer (0.3 M NaOH and 1 mM Na2EDTA, pH > 13) for 20 min to allow the unwinding of DNA at 4 °C. Electrophoresis was performed for 20 min at 25 V (300 mA). The slides were then washed with pre-cooled neutralization buffer (0.4 M Tris–HCl, pH 7.5) three times for 5 min each, drained and stained with 30 µL ethidium bromide (20 μg/mL) for 15 min. Analysis of the slides was performed at 200× magnification using a fluorescence microscope (Olympus BX-51, Tokyo, Japan) equipped with a CCD video camera. For each fish in each treatment group, two slides were processed and 50 cells randomly selected from each slide were analyzed.
Procedures for the pH 12.1 and neutral comet assays were essentially the same as for the alkaline comet assay, except that for the pH 12.1 comet assay, the pH of the electrophoresis buffer was adjusted to 12.1, and for the neutral comet assay, lysis of cells and electrophoresis were all performed at room temperature and electrophoresis was conducted in TBE buffer (90 mM Tris, 90 mM boric acid, 2 mM EDTA, pH 8.2–8.5).
For the alkaline comet assay with the use of Endo III or FPG enzyme, additional procedures were adopted after lysis of cells but before electrophoresis (Thorne et al. 2009). Slides were washed in enzyme reaction buffer (10 mM Bis–Tris propane HCl, 10 mM MgCl2, 1 mM DTT, pH 7.0, 0.1 mg/mL FBS) two times for 8 min each and air-dried. Of the six slides prepared for each sample, two were treated with 100 mU Endo III per slide (New England Biolabs, Hitchin, UK) to recognize and convert oxidative pyrimidines into strand breaks, another two with 100 mU FPG per slide (New England Biolabs, Hitchin, UK) to recognize and convert oxidative purines, and the remaining two with enzyme reaction buffer as control slides. The slides were then incubated for 45 min at 37 °C and subsequently immersed in electrophoresis buffer. The subsequent steps were the same as for the alkaline comet assay.
Digital images of the slides were acquired using Image-Pro Express software (Media Cybernetics Inc., Silver Spring, MD, USA) and analyzed with the CASP software (Końca et al. 2003). DNA damage was expressed as olive tail moment (OTM, arbitrary units), defined as the distance between the center of mass of the tail and the center of mass of the head, in micrometers, multiplied by the percentage of DNA in the tail (Tice et al. 2000).
Comet-FISH
An oligonucleotide probe, which can bind specifically to the targeted telomeric DNA sequences, was labeled with tetramethyl rhodamine (TAMRA) at the 5′ end of the sequence and was synthesized by Shanghai Sangon Biotechnology Co. Ltd. (Shanghai, People’s Republic of China). The probe sequence was 5′-CCCTAACCCTAACCCTAA-3′.
The whole comet-FISH procedure was performed under red light following the protocol described by Arutyunyan et al. (2004, 2005) and Schlörmann and Glei (2009). After electrophoresis, the slides prepared as described above were dehydrated in an ethanol series (75, 85, and 100 %, 5 min each), rehydrated in ddH2O for 10 min, denatured in 0.5 M NaOH for 30 min, and neutralized in 0.01 M PBS (pH 7.4) for 1 min. The slides were then dehydrated in an ethanol series (75, 85, and 100 %, 5 min each) again and air-dried. Diluted probes (5 μL) were mixed with 7 μL hybridization solution, denatured at 75 °C for 5 min, maintained at 4 °C for 2 min, and then pipetted onto the air-dried slides. Slides were then sealed with sealing films and placed in a humidified chamber at 37 °C overnight. The next day the slides were placed at room temperature for 5 min before removing the sealing films and then were washed in 2× saline sodium citrate (SSC) solution at 65 °C for 5 min, 1× SSC solution at 65 °C for 5 min, and 1× phosphate-buffered detergent solution at room temperature for 5 min. Finally, 30 μL of 1:2,000 diluted SYBR-Green I (Shanghai Sangon Biotechnology Co. Ltd., Shanghai, People’s Republic of China) was pipetted onto each slide for counterstaining.
The slides were then analyzed with an Olympus BX-51 fluorescence microscope equipped with a blue filter for the view of comet cells and a green filter for hybridization signals. Fifty randomly selected comet cells with successful hybridization were analyzed for each slide. Telomeres without DNA damage displayed hybridization signals in the heads of comet cells, whereas those with DNA damage also displayed hybridization signals in the tails. Levels of DNA damage in telomeres were calculated by the ratio of the number of comet cells with hybridization signals in tails to the number of comet cells successfully hybridized.
Measurement of SOD and GSH-Px activities and MDA concentration
SOD and GSH-Px activities, MDA concentrations, and hemoglobin (Hb) contents in the peripheral blood tissues of goldfish were determined using corresponding assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, People’s Republic of China) according to the manufacturer’s instructions. The activity of SOD was expressed as units per gram of Hb, where one unit of enzyme was defined as the amount of enzyme inhibiting the rate of reduction reaction of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyltetrazolium chloride by 50 % in 1 mL reaction solution. The activity of GSH-Px was expressed as units per gram of Hb, where one unit of enzyme was defined as the micromoles of GSH oxidized per minute, deducting non-enzymatic reaction. The MDA concentration was defined as the moles of MDA per gram of Hb.
Statistical analysis
Results of the comet assay, SOD and GSH-Px activities, and MDA concentrations were expressed as the mean ± standard deviation. Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple comparisons. For the results of comet-FISH, χ 2 analysis was used to evaluate the significance of differences in the percentage of comet cells with hybridization signals in the tails between the control group and each treatment. Values were considered significant when P < 0.05.
Results
Global DNA damage
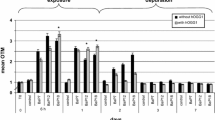
DNA in the control group maintained integrity, as almost no tails were observed in comet cells (Fig. 1a). However, comet cells in the 0.01 mg/L treatment group displayed obvious tails (Fig. 1b), and those in the 0.10 and 1.00 mg/L treatment groups showed gradual increases in the intensity and length of comet tails (Fig. 1c, d). Results at exposure durations of 24, 48, 96, and 168 h showed that OTM values in peripheral erythrocytes were significantly increased by 0.01, 0.10, and 1.00 mg/L monocrotophos (P < 0.05). At the same exposure concentration, the OTM value significantly increased at 24 h and reached a peak value at 48 h, followed by gradual decreases at 96 h and 168 h, although the levels remained significantly higher than the corresponding control (P < 0.05) (Fig. 2).
ALS, SSB, and DSB
DNA in the peripheral erythrocytes of goldfish showed the most serious damage at 48 h after exposure to monocrotophos, as indicated by results of Fig. 2, and so this exposure duration was selected for the following studies. For the alkaline and pH 12.1 comet assays, OTM values in all treatment groups increased significantly compared with corresponding controls (P < 0.05); for the neutral comet assay, OTM values in the 0.01 and 1.00 mg/L treatment groups increased significantly (P < 0.05), but no significant changes were observed in the 0.10 mg/L treatment group (Fig. 3). At the same monocrotophos exposure concentration, OTM values assessed by the three versions of comet assay were significantly different from each other (P < 0.05), with those of the alkaline 5.1–15.3 times higher than the pH 12.1 and those of the pH 12.1 4.9–31.5 times higher than the neutral (Fig. 3).
Olive tail moment (OTM) values of DNA in peripheral erythrocytes of goldfish assessed by comet assay under different pH conditions. Goldfish were exposed to 0.01, 0.10, and 1.00 mg/L monocrotophos for 48 h. *Significantly different from the control group under the same pH conditions; P < 0.05. At the same exposure concentration, different letters represent significant differences between the OTM values under different pH conditions (P < 0.05)
Oxidative DNA damage
Figure 4 shows the levels of oxidative DNA damage in peripheral erythrocytes of goldfish assessed by alkaline comet assay with the use of Endo III and FPG. After incubation with Endo III and FPG, OTM values in all treatment groups increased significantly compared with their corresponding negative controls (P < 0.05). In the case of incubation with Endo III, the OTM values in the treatment groups were 1.3–2.2 times higher than the corresponding negative controls, and in the case of incubation of FPG, those were 1.6–2.4 times higher.
Olive tail moment (OTM) values of DNA in peripheral erythrocytes of goldfish assessed by alkaline comet assay with the use of formamidopyrimidine DNA glycosylase (FPG) or endolase III (Endo III). Goldfish were exposed to 0.01, 0.10, and 1.00 mg/L monocrotophos for 48 h. *Significantly different from corresponding negative controls; P < 0.05
DNA damage in telomeres
Fluorescently labeled DNA probes were observed in the heads or tails of comet cells under a fluorescence microscope (Fig. 5). Comet cells without the addition of DNA probes served as the negative control, in which no hybridization signals were detected in the heads or in the tails. Comet cells in control groups with no pesticide exposure served as controls, in which hybridization signals were only detected in the heads, indicating less or no DNA damage in telomeres. Conversely, hybridization signals in the tails of comet cells were detected in all pesticide treatment groups. Compared with the control, the percentage of comet cells with hybridization signals in the tails was significantly increased by 0.01, 0.10, and 1.00 mg/L monocrotophos exposure for 48 h (P < 0.05, Table 1).
Representative comet-FISH images in peripheral erythrocytes of goldfish exposed to monocrotophos. Goldfish were exposed to 0.01, 0.10, and 1.00 mg/L monocrotophos for 48 h. a Group with no probes added (negative control); b group with no pesticide exposure (control); c 0.01 mg/L; d 0.10 mg/L; e 1.00 mg/L. 1, comet cells under the fluorescence microscope with blue filter; 2, comet cells under the fluorescence microscope with green filter; 3, combined images of 1 and 2. The arrows show the location of the small hybridization signals in the heads or tails of the comets
Activities of SOD and GSH-Px and concentrations of MDA
Activities of SOD
No significant differences in the SOD activities in peripheral blood tissues of goldfish were observed between treatment groups and their corresponding controls after monocrotophos exposure for 24, 48, 96, and 168 h (Fig. 6).
Activities of GSH-Px
Figure 7 shows the changes of GSH-Px activities in peripheral blood tissues of goldfish exposed to monocrotophos. The GSH-Px activities in all treatment groups decreased significantly compared with the control at 24 h (P < 0.05), followed by nonsignificant differences at 48 h. At 96 h, the GSH-Px activities in treatment groups increased significantly (P < 0.05) compared with the control, whereas activities at 168 h exhibited a decreasing trend compared with those at 96 h, but were still significantly higher than the control (P < 0.05).
Concentrations of MDA
MDA concentrations in the treatment groups decreased significantly at 24 h and 168 h compared with the corresponding controls (P < 0.05), but increased significantly and reached a peak value for each treatment group at 48 h (P < 0.05). No significant differences were observed between treatment groups and the control at 96 h (Fig. 8).
Discussion
By applying the pH > 13 comet assay, waterborne or oral exposure to monocrotophos has been found able to induce DNA strand breaks on different nontarget animals (Saleha Banu et al. 2001; Mahboob et al. 2002; Jamil et al. 2004; Ali and Kumar 2008). The results of the pH > 13 comet assay in the present study showed that waterborne exposure of 0.01, 0.10, and 1.00 mg/L monocrotophos for 24–168 h was able to induce DNA damage in peripheral erythrocytes of goldfish, which was consistent with the reports of Saleha Banu et al. (2001) and Ali and Kumar (2008) using fish as the model animal. However, it was just the global DNA damage confirmed in these previous studies utilizing the pH > 13 comet assay (Tice et al. 2000), without clear evidence on the specific types of DNA strand breaks and the pathway through which monocrotophos caused DNA strand breaks. Therefore, the pH 12.1 and pH 8.2–8.5 comet assays were performed in this study. The significantly increased OTM values in these three versions of comet assay, compared with their corresponding controls, specifically suggested induction of ALS, DSB, and SSB in peripheral erythrocytes of goldfish exposed to 0.01, 0.10, and 1.00 mg/L monocrotophos for 48 h. Wen et al. (2011) demonstrated that DSB and SSB but not ALS can be detected by the pH 12.1 comet assay; Horváthová et al. (1998) and Tice et al. (2000) reported that by keeping the same molarity of the ingredients in unwinding and electrophoresis procedures, the presence of increased migration of DNA fragments at pH > 13 compared with that at pH 12.1 indicates the induction of ALS. Since the OTM values at pH > 13 were 5.1–15.3 times higher than those at pH 12.1, we deduce that compared with the DNA strand breaks, ALS was the main type of DNA damage produced by monocrotophos exposure. As the neutral comet assay was reported to be more specific for the detection of DSB (Shahidi et al. 2007; Cortés-Gutiérrez et al. 2012; Ribas-Maynou et al. 2012) and the OTM values at pH 12.1 were 4.9–31.5 times higher than those at pH 8.2–8.5, it was predominantly SSB with much fewer DSB detected by the pH 12.1 comet assay in this study. Consistently, Tice et al. (2000) suggested that almost all genotoxic agents induce SSB orders of magnitude more than DSB.
By utilizing the pH > 13, pH 12.1, and pH 8.2–8.5 comet assays, this is the first study to reveal that ALS are the main type of DNA damage induced by monocrotophos exposure in fish blood cells. ALS in DNA mainly include apurinic or apyrimidinic (AP) sites (Cortés-Gutiérrez et al. 2014), and due to the loss of hydrogen bonding and other non-covalent interactions, the stability of duplex DNA is destroyed at AP sites (Greenberg 2014). Thus, AP sites increased by monocrotophos exposure may contribute to the presence of SSB when the AP endonuclease in cells nicks the damaged DNA backbone (Kim et al. 1998). Moreover, an unrepaired SSB may be converted to a further DSB in DNA destabilized by AP sites (Ma et al. 2011). Accordingly, induction of ALS (AP sites) may be one of the main pathways through which monocrotophos induces DNA strand breaks (including SSB and DSB) in goldfish peripheral erythrocytes.
As AP sites in cells can be formed in the base excision repair of oxidative DNA damage initiated by DNA glycosylases (Jin et al. 2014), the abnormally increased ALS in peripheral erythrocytes of goldfish exposed to monocrotophos might be, at least partly, due to the generation of oxidized DNA bases. Results of the alkaline comet assay showed that comet cells in the treatment groups, after incubation with Endo III or FPG enzyme, all exhibited higher OTM values compared with the controls, directly suggesting oxidation of DNA bases upon monocrotophos exposure. In addition to DNA bases in non-telomeric regions, guanine-rich telomeric DNA is particularly vulnerable to attack from ROS, since guanines are prone to be oxidized and further converted to SSB, which are less efficiently repaired in telomeres than the rest of the genome (von Zglinicki et al. 2005; Wang et al. 2010). Results of the comet-FISH showed that compared with the control, exposure of 0.01, 0.10, and 1.00 mg/L monocrotophos significantly increased the percentage of comet cells with hybridization signals in the tails, the value of which reached maximum in the 0.10 mg/L treatment group but reduced slightly in the 1.00 mg/L group. Arutyunyan et al. (2004) found that ex vivo incubation with bleomycin increased telomeric DNA damage in the human peripheral blood cells in a dose-dependent manner, and verified that the dose–response relationship of DNA damage was not dramatically altered by the hybridization procedure of the comet-FISH. Thus, the results of comet-FISH in this study demonstrated DNA strand breaks in telomeres and indirectly validated the oxidative DNA damage caused by monocrotophos exposure; however, compared with bleomycin, telomeric DNA damage induced by monocrotophos exhibited a dose-independent increase. Opresko et al. (2005) reported that AP sites generated in the base excision repair pathway of 8-oxo-7,8-dihydroguanine, oxidative products of guanines, decrease the binding of telomeric proteins TRF1 and TRF2 to the telomere sequence, leading to the disruption of telomere length, maintenance and function; damage in the structure and function of telomere may further result in the formation of micronuclei, nucleoplasmic bridges, nuclear buds, and chromosome fragmentation (Gisselsson et al. 2001; Pampalona et al. 2010). The present study confirmed the telomeric DNA damage induced by monocrotophos exposure in peripheral erythrocytes of goldfish, and this is in accordance with our findings that this pesticide produced nuclear abnormalities in peripheral erythrocytes and structural chromosomal aberration in kidney cells of Monopterus albus (unpublished data). Collectively, results of the alkaline comet assay with the use of Endo III or FPG enzyme and comet-FISH indicated the oxidation of DNA bases caused by monocrotophos exposure.
Oxidative damage in DNA bases is directly related to the production of ROS, especially \(^{ \cdot } {\text{OH}}\) (Cooke et al. 2003). Results of Kashyap et al. (2011) indicated that induced expression of CYPs might have up-regulated the production of ROS in cells after monocrotophos exposure. The antioxidant enzymes, on the other hand, play an important role in scavenging the over-produced ROS in tissues, but whether monocrotophos can interfere with the scavenging of ROS is still unknown. After exposed to 0.01, 0.10, and 1.00 mg/L monocrotophos for 24, 48, 96, and 168 h, no significant differences were observed in the activities of SOD, the enzyme responsible for dismutation of \({\text{O}}_{2}^{\cdot - }\) to O2 and hydrogen peroxide (H2O2), in the peripheral blood tissues of goldfish. However, at 24 h, GSH-Px activities were significantly inhibited, which may interfere with the decomposition of H2O2 and result in over-production of \(^{ \cdot } {\text{OH}}\). The highly reactive \(^{ \cdot } {\text{OH}}\) reacts with DNA by addition to double bonds of DNA bases and by abstraction of an H atom from the methyl group and 2′-deoxyribose of thymine (Cooke et al. 2003), consequently leading to the significantly increased level of DNA damage at 24 h. However, the excessive ROS-induced lipid peroxidation at 24 h might be balanced to some extent by stimulating effects of monocrotophos on non-enzyme antioxidants such as vitamin E and vitamin C, which exist in the membrane or in plasma and protect lipoproteins from oxidation (Machlin and Bendich 1987; Betancor et al. 2012). Thus, MDA concentrations did not exhibit a trend to increase even though the GSH-Px activities decreased. At 48 h, no significant alterations were observed for the GSH-Px activities, but the level of lipid peroxidation and DNA damage all reached peak values, which can be interpreted as the results of ROS accumulation in peripheral erythrocytes. At 96–168 h, the GSH-Px activities increased significantly in compensation for the increased ROS, and MDA concentrations decreased gradually; both the changes of GSH-Px activities and MDA concentrations suggested the scavenging of excessive ROS, and so the levels of DNA damage decreased gradually due to DNA repair. Briefly, it is supposed that interfering with the scavenging process and leading to the accumulation of ROS are possibly involved in the mechanisms underlying the DNA-damaging effect of monocrotophos in fish blood cells.
Saleha Banu et al. (2001) reported that DNA damage (mean comet tail length) in the nucleated erythrocytes of Tilapia mossambica, orally exposed to 0.313–4.375 mg/kg monocrotophos (98 % pure), reached maximum at 24 h and decreased thereafter to control levels at 96 h, exhibiting a higher DNA repair ability than that in goldfish used in the present study. Such difference may be due to the different exposure dose of monocrotophos or the distinct life span of erythrocytes, which varies among species over a range of 80–500 days with an erythrocyte mutation period of 17–23 days (Dinnen et al. 1988; Avery et al. 1992; Fischer et al. 1998; Çavaş and Könen 2007). Ali and Kumar (2008) reported that DNA damage (the percentage of DNA in comet tails) in the gill, kidney, and lymphocytes of C. punctatus exposed to 1.59–4.78 mg/L monocrotophos pesticide (36 % effective concentration) decreased gradually after reaching peak values at 96 h. Thus, compared with C. punctatus, goldfish with the most serious DNA damage occurring at 48 h exhibited a higher DNA repair ability. In addition, the level of DNA damage in the study of Ali and Kumar (2008) was 4–5 times higher than the control in the gill, and 3–4 times higher in the kidney and lymphocytes of C. punctatus; however, that in peripheral erythrocytes of goldfish reached as much as 42.0–44.3 times higher than control levels, indicating a more sensitive response to monocrotophos exposure in the peripheral erythrocytes of goldfish.
In conclusion, the present study shows that monocrotophos produces DNA strand breaks in peripheral erythrocytes of goldfish by inducing ALS, SSB, and DSB, and induction of ALS (AP sites) is one of the main pathways through which monocrotophos further produces SSB and DSB. And such ALS increases in DNA are, at least partly, resulted from the monocrotophos-induced oxidative DNA damage, as indicated by the altered activities of antioxidant enzymes which disturbed the scavenging of ROS. It is worth to note that abnormally increased DNA damage is always potent inducers of cell death and many tumors (Vamvakas et al. 1997; Khanna and Jackson 2001; Jackson 2002), which can further influence the growth, development, reproduction, and population dynamics of organisms (Poletta et al. 2013; Santos et al. 2013; Einaudi et al. 2014; Ribas-Maynou et al. 2014). Considering that liposoluble monocrotophos, remaining in food and the aquatic environment, may accumulate in tissues of organisms (Arifin et al. 1997), appropriate discharge standards should be established in practical use to reduce the ecological risk of this pesticide.
Abbreviations
- ROS:
-
Reactive oxygen species
- SSB:
-
Single-strand breaks
- DSB:
-
Double-strand breaks
- ALS:
-
Alkali-labile sites
- OP:
-
Organophosphate
- CYPs:
-
Cytochrome P450s
- O2 :
-
Oxygen
- \({\text{O}}_{ 2}^{\cdot - }\) :
-
Superoxide anion
- \(^{ \cdot } {\text{OH}}\) :
-
Hydroxyl radicals
- SOD:
-
Superoxide dismutase
- GSH-Px:
-
Glutathione peroxidase
- Endo III:
-
Endonuclease III
- FPG:
-
Formamidopyrimidine DNA glycosylase
- FISH:
-
Fluorescence in situ hybridization
- MDA:
-
Malondialdehyde
- PBS:
-
Phosphate-buffered saline
- OTM:
-
Olive tail moment
- SSC:
-
Saline sodium citrate
- Hb:
-
Hemoglobin
- AP sites:
-
Apurinic or apyrimidinic sites
- H2O2 :
-
Hydrogen peroxide
References
Ali D, Kumar S (2008) Long-term genotoxic effect of monocrotophos in different tissues of freshwater fish Channa punctatus (Bloch) using alkaline single cell gel electrophoresis. Sci Total Environ 405:345–350
Anjum R, Malik A (2013) Evaluation of mutagenicity of wastewater in the vicinity of pesticide industry. Environ Toxicol Pharmacol 35:284–291
Arifin M, Roxas NP, Tejada AW, Penalba FF, Sevilla CC, Sajise PE (1997) Repeated oral dose of monocrotophos in goats: its bioaccumulation. Philippine Agriculturist (Philippines)
Arutyunyan R, Gebhart E, Hovhannisyan G, Greulich KO, Rapp A (2004) Comet-FISH using peptide nucleic acid probes detects telomeric repeats in DNA damaged by bleomycin and mitomycin C proportional to general DNA damage. Mutagenesis 19:403–408
Arutyunyan R, Rapp A, Greulich KO, Hovhannisyan G, Haroutiunian S, Gebhart E (2005) Fragility of telomeres after bleomycin and cisplatin combined treatment measured in human leukocytes with the Comet-FISH technique. Exp Oncol 27:38–42
Avery EH, Lee BL, Freedland RA, Cornelius CE (1992) Bile pigments in gallbladder and freshly-secreted hepatic duct bile from fed and fasted rainbow trout, Oncorhynchus mykiss. Comp Biochem Physiol Comp Physiol 101:857–861
Bagchi D, Bagchi M, Hassoun EA, Stohs SJ (1995) In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology 104:129–140
Betancor MB, Caballero MJ, Terova G, Corà S, Saleh R, Benítez-Santana T, Bell JG, Hernández-Cruz CM, Izquierdo M (2012) Vitamin C enhances vitamin E status and reduces oxidative stress indicators in sea bass larvae fed high DHA microdiets. Lipids 47:1193–1207
Bondy SC, Naderi S (1994) Contribution of hepatic cytochrome P450 systems to the generation of reactive oxygen species. Biochem Pharmacol 48:155–159
Çavaş T, Könen S (2007) Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay. Mutagenesis 22:263–268
Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214
Cortés-Gutiérrez EI, Hernández-Garza F, García-Pérez JO, Dávila-Rodríguez MI, Aguado-Barrera ME, Cerda-Flores RM (2012) Evaluation of DNA single and double strand breaks in women with cervical neoplasia based on alkaline and neutral comet assay techniques. Biomed Res Int. doi:10.1155/2012/385245
Cortés-Gutiérrez EI, Dávila-Rodríguez MI, López-Fernández C, Fernández JL, Crespo F, Gosálvez J (2014) Localization of alkali-labile sites in donkey (Equus asinus) and stallion (Equus caballus) spermatozoa. Theriogenology 81:321–325
Dinnen RD, Tomlinson SM, Hart D, Chopra C, Heddle JA (1988) Application of a micronucleus assay to the peripheral blood cells of rainbow trout, Salmo gairdneri. Can Tech Rep Fish Aquat Sci 1607:69–78
Dwivedi N, Flora SJ (2011) Concomitant exposure to arsenic and organophosphates on tissue oxidative stress in rats. Food Chem Toxicol 49:1152–1159
Einaudi L, Courbiere B, Tassistro V, Prevot C, Sari-Minodier I, Orsiere T, Perrin J (2014) In vivo exposure to benzo (a) pyrene induces significant DNA damage in mouse oocytes and cumulus cells. Hum Reprod 29:548–554
Fischer U, Ototake M, Nakanishi T (1998) Life span of circulating blood cells in ginbuna crucian carp (Carassius auratus langsdorfii). Fish Shellfish Immun 8:339–349
Gisselsson D, Jonson T, Petersén A, Strömbeck B, Dal Cin P, Höglund M, Mitelman F, Mertens F, Mandahl N (2001) Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc Natl Acad Sci USA 98:12683–12688
Greenberg MM (2014) Abasic and oxidized abasic site reactivity in DNA: enzyme inhibition, cross-linking, and nucleosome catalyzed reactions. Acc Chem Res 47:646–655
Horváthová E, Slameňová D, Hlinčíková L, Mandal TK, Gábelová A, Collins AR (1998) The nature and origin of DNA single-strand breaks determined with the comet assay. Mutat Res DNA Repair 409:163–171
Jackson SP (2002) Sensing and repairing DNA double-strand breaks. Carcinogenesis 23:687–696
Jamil K, Shaik AP, Mahboob M, Krishna D (2004) Effect of organophosphorus and organochlorine pesticides (monochrotophos, chlorpyriphos, dimethoate, and endosulfan) on human lymphocytes in-vitro. Drug Chem Toxicol 27:133–144
Jin J, Hwang BJ, Chang PW, Toth EA, Lu AL (2014) Interaction of apurinic/apyrimidinic endonuclease 2 (Apn2) with Myh1 DNA glycosylase in fission yeast. DNA Repair (Amst) 15:1–10
Kashyap MP, Singh AK, Kumar V, Tripathi VK, Srivastava RK, Agrawal M, Khanna VK, Yadav S, Jain SK, Pant AB (2011) Monocrotophos induced apoptosis in PC12 cells: role of xenobiotic metabolizing cytochrome P450s. PLoS ONE 6:e17757
Khanna KK, Jackson SP (2001) DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 27:247–254
Kim K, Biade S, Matsumoto Y (1998) Involvement of flap endonuclease 1 in base excision DNA repair. J Biol Chem 273:8842–8848
Końca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Góźdź S, Koza Z, Wojcik A (2003) A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res 534:15–20
Kovacic P (2003) Mechanism of organophosphates (nerve gases and pesticides) and antidotes: electron transfer and oxidative stress. Curr Med Chem 10:2705–2709
Krause KH, van Thriel C, De Sousa PA, Leist M, Hengstler JG (2013) Monocrotophos in Gandaman village: India school lunch deaths and need for improved toxicity testing. Arch Toxicol 87:1877–1881
Kumari B, Madan VK, Kathpal TS (2007) Pesticide residues in rain water from Hisar, India. Environ Monit Assess 133:467–471
Lu XT, Ma Y, Wang C, Zhang XF, Jin DQ, Huang CJ (2012) Cytotoxicity and DNA damage of five organophosphorus pesticides mediated by oxidative stress in PC12 cells and protection by vitamin E. J Environ Sci Health B 47:445–454
Ma W, Westmoreland JW, Gordenin DA, Resnick MA (2011) Alkylation base damage is converted into repairable double-strand breaks and complex intermediates in G2 cells lacking AP endonuclease. PLoS Genet. doi:10.1371/journal.pgen.1002059
Machlin LJ, Bendich A (1987) Free radical tissue damage: protective role of antioxidant nutrients. FASEB J 1:441–445
Mahboob M, Rahman MF, Danadevi K, Banu BS, Grover P (2002) Detection of DNA damage in mouse peripheral blood leukocytes by the comet assay after oral administration of monocrotophos. Drug Chem Toxicol 25:65–74
Opresko PL, Fan J, Danzy S, Wilson DM III, Bohr VA (2005) Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res 33:1230–1239
Pampalona J, Soler D, Genescà A, Tusell L (2010) Telomere dysfunction and chromosome structure modulate the contribution of individual chromosomes in abnormal nuclear morphologies. Mutat Res 683:16–22
Poletta GL, Gigena F, Loteste A, Parma MJ, Kleinsorge EC, Simoniello MF (2013) Comet assay in gill cells of Prochilodus lineatus exposed in vivo to cypermethrin. Pestic Biochem Physiol 107:385–390
Ribas-Maynou J, Garcia-Peiro A, Abad C, Amengual MJ, Navarro J, Benet J (2012) Alkaline and neutral Comet assay profiles of sperm DNA damage in clinical groups. Hum Reprod 27(3):652–658
Ribas-Maynou J, Fernández-Encinas A, García-Peiró A, Prada E, Abad C, Amengual MJ, Navarro J, Benet J (2014) Human semen cryopreservation: a sperm DNA fragmentation study with alkaline and neutral Comet assay. Andrology 2:83–87
Saleha Banu B, Danadevi K, Rahman MF, Ahuja YR, Kaiser J (2001) Genotoxic effect of monocrotophos to sentinel species using comet assay. Food Chem Toxicol 39:361–366
Santos R, Palos-Ladeiro M, Besnard A, Reggio J, Vulliet E, Porcher JM, Bony S, Sanchez W, Devaux A (2013) Parental exposure to methyl methane sulfonate of three-spined stickleback: contribution of DNA damage in male and female germ cells to further development impairment in progeny. Ecotoxicology 22:815–824
Schlörmann W, Glei M (2009) Comet fluorescence in situ hybridization (Comet-FISH): detection of DNA damage. Cold Spring Harb Protoc. doi:10.1101/pdb.prot5220
Shahidi M, Mozdarani H, Bryant PE (2007) Radiation sensitivity of leukocytes from healthy individuals and breast cancer patients as measured by the alkaline and neutral comet assay. Cancer Lett 257:263–273
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Swarnam TP, Velmurugan A (2013) Pesticide residues in vegetable samples from the Andaman Islands, India. Environ Monit Assess 185:6119–6127
Tariq MI, Afzal S, Hussain I (2004) Pesticides in shallow groundwater of bahawalnagar, Muzafargarh, DG Khan and Rajan Pur districts of Punjab, Pakistan. Environ Int 30:471–479
Thorne D, Wilson J, Kumaravel TS, Massey ED, McEwan M (2009) Measurement of oxidative DNA damage induced by mainstream cigarette smoke in cultured NCI-H292 human pulmonary carcinoma cells. Mutat Res 673:3–8
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Vamvakas S, Vock EH, Lutz WK (1997) On the role of DNA double-strand breaks in toxicity and carcinogenesis. CRC Crit Rev Toxicol 27:155–174
von Zglinicki T, Martin-Ruiz CM, Saretzki G (2005) Telomeres, cell senescence and human ageing. Signal Transduct 5:103–114
Wang Z, Rhee DB, Lu J, Bohr CT, Zhou F, Vallabhaneni H, de Souza-Pinto NC, Liu Y (2010) Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genet 6:e1000951
Wen Y, Zhang PP, An J, Yu YX, Wu MH, Sheng GY, Fu JM, Zhang XY (2011) Diepoxybutane induces the formation of DNA–DNA rather than DNA–protein cross-links, and single-strand breaks and alkali-labile sites in human hepatocyte L02 cells. Mutat Res Fund Mol M 716:84–91
Yu F, Wang Z, Ju B, Wang Y, Wang J, Bai D (2008) Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Exp Toxicol Pathol 59:415–423
Zhang X, Tian H, Wang W, Ru S (2013) Exposure to monocrotophos pesticide causes disruption of the hypothalamic-pituitary-thyroid axis in adult male goldfish (Carassius auratus). Gen Comp Endocrinol 193:158–166
Acknowledgments
This work was supported by the National Natural Science Foundation of China [30671618].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, F., Wang, B., Zhang, X. et al. Induction of DNA base damage and strand breaks in peripheral erythrocytes and the underlying mechanism in goldfish (Carassius auratus) exposed to monocrotophos. Fish Physiol Biochem 41, 613–624 (2015). https://doi.org/10.1007/s10695-015-0032-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0032-2