The effect of modifying 1,1,3-trihydroperfluoro-1-propanol on the properties of oriented polyester yarns was investigated. We demonstrate a stabilizing effect of the modifier, which contributes improved strength properties of polyethylene terephthalate and an increase in its resistance to thermal oxidation and hydrolytic aging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Improving the properties of polyesters, polyethylene terephthalate (PET) in particular, is important in view of the widespread use of the products of this polymer [1, 2]. One of the most important directions in the study of thermal and hydrolytic stability of polymers is establishing the relationship between the kinetics of degradation processes and the polymer structure.
A noticeable change in the stability of PET is achieved upon modification of the structure of its macromolecules by introducing functional groups of organoelemental comonomers containing moieties of stabilizers, and by changing the supramolecular structure (density of the amorphous phase, the degree of crystallinity, stereochemical features) by varying the sample preparation conditions [1–3]. Methods for surface modification of polymers also can improve the thermal and hydrolytic stability of polyesters [1].
The use of poly- and perfluorinated compounds is of great interest because it allows one to significantly improve a number of properties (thermal, optical, wear resistance, hydrolytic stability) of heterochain polymers (polydieneurethanes, polythiourethanes, polysulfides, and polyamide 6) even at low concentrations (10–3 – 5 wt. %) of the modifier [4–10].
The purpose of this work is to study the modifying effect of 1,1,3-trihydro perfluoro-1-propanol on thermal and hydrolytic stability of oriented polyester yarns surface-modified with it.
For the study we used oriented polyester yarns with a linear density of 170 tex (TU 6-13- 53578992-87-2007, CJSC “Gazpromkhimvolokno”, Volzhsky, Russia). To modify the polyester we used 1,1,3-trihydroperfluoro-1-propanol HCF2CF2CH2OH (polyfluorinated alcohol – PFA, TU 2412-001-23184793-99, JSC “HaloPolymer”, Perm, Russia).
Surface modification of 5 wt. % PET with the PFA was performed by the solution method using n-hexane at 40 °C for 2 hours. The modified strands were dried at 40 °C under vacuum.
Polyester yarns were tested in an environmental chamber for temperature and humidity recycling “Binder MKF 240” according to the regime shown in Fig. 1. The number of cycles was 30. The relative humidity of air in the chamber was automatically maintained at 95 ± 2%.
Mechanical testing of the polymer samples was performed in H5K-S machines from the company “Tinius Olsen” by the standard methods.
Structural and morphological features of the modified PET were studied by IR Fourier transform spectroscopy (using the “Nicolet-6700” spectrometer), powder reflection X-ray diffraction in the Bragg – Brentano geometry (automated diffractometer “DRON-3”, CuKα radiation (λ = 1.5418 Å, Ni-filter), and scanning electron microscopy combined with microprobe analysis (“Versa 3D DualBeam” microscope).
The ability of partially crystalline polymers to crystallize in the course of technological procedures largely determines their properties and fields of application. Thus, modification of polyesters with functional additives (polyhydric phenols, fluoroalcohols) promotes the formation of stable intermolecular complexes, resulting in irreversible binding of low molecular weight polymer compounds [1, 11–15]. The prospect of the use of compounds containing the group H(CF2CF2)nCH2 is associated with the possibility of multifunctional interaction with macromolecules of PET (Appendix, diagram 1), which has been previously determined by pulsed solid-state nuclear magnetic resonance 1H [1, 12].
At the same time, when selecting a surface modification process one should be guided by the fact that, for example, a number of solvents (acetone, nitromethane, fluorobenzene) cause crystallization of PET even at room temperature, hindering the diffusion of the modifier into the polymer [1, 2]. Choosing n-hexane as a solvent is dictated by the fact that it has practically no effect on the crystallization of PET in the temperature range 20-80 °C and reduces the proportion of polyassociates (Appendix, diagram 2), thereby facilitating the penetration of alcohols into the polymer [1].
As follows from the data presented in Table 1, administration of PFA improves strength properties of polyester yarns subjected to thermo-oxidative and hydrolytic aging by an average of 1.2 times. This result is due to a compounding effect of molecules of the fluorinated modifier on restructuring of the supramolecular PET structure, resulting in an increase in the degree of polyester crystallinity from 50 to 58%, an increase in the relative integrated intensities of reflexes in all three crystallographic directions (010, 110 and 100) with a corresponding narrowing of the diffraction lines (the difference in full width at half maximum for the modified polyester compared to the initial sample is 2°), which contributes to a more uniform distribution of the external load on macromolecular chains and production of materials with improved properties (Table 2).
The increase in crystallinity of the modified polyester may be facilitated by a partial transition of glycol residue conformers from gauche- to trans- conformation, which leads to the formation of a more “rigid” structure due to the interaction of CH2 protons with the nearest and remote fluorine atoms of the CF2 groups, as well as opportunities for the association of the maximum number of polar atoms in each basic unit (Diagram 1). Formation of these intermolecular complexes apparently will complicate the diffusion of volatile decomposition products and enhance thermal stability of PET. Conformational transitions in PET are analyzed in detail in [1, 13, 15–17] with the aide of FT-Raman spectroscopy and FT-IR spectroscopy of multiple frustrated total internal reflection.
Thermo-oxidative stability of polyester yarns may be increased by the possible generation of electrophilic polyfluorinated radicals from PFA [1]
which are different from the non-fluorinated analogues in that the tetrahedral configuration is retained, and they react with macroradicals that form during thermal decomposition of macromolecular chains.
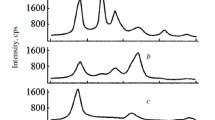
Analysis of FT-IR spectra of PET in the 4000-3000 cm–1 wavenumber region also points to an inhibitory effect of PFA to thermal degradation of polyester macromolecules, leading to oxidative functionalization of the polymer by means of formation of hydroxyl and carboxyl groups (Fig. 2). As can be seen from electron micrographs, modified polyester yarns are characterized by a significantly smaller fraction of microprojections, cracks, and grooves resulting from polymer degradation (Fig. 3).
Microprobe analysis showed that after exposing the polyester yarn in the environmental chamber the concentration of PFA in the analyzed layer (~1 micron) decreases slightly, from 1.5-1.7 to 1.4-1.5 wt. %, resulting in hydrophobization of PET and enhancement of its hydrolytic stability.
References
S. V. Kudashev, V. F. Zheltobryukhov, and T. I. Danilenko, Polyethylene Terephthalate: Features, Modifications, Structure, and Recycling Trends [in Russian], Volgograd State Technical University, Volgograd, 148 p. (2014).
B. V. Petukhov, Polyester Fibers [in Russian], Khimiya, Moscow, 272 p. (1976).
S. V. Kudashev and V. F. Zheltobryukhov, Utilization and Recycling of Carbon and Heterochain Polymers, Volgograd State Technical University, Volgograd, 43 p. (2013).
S. V. Kudashev, Ya. V. Zubavichus, et al., Trenie i Iznos, 34, No. 5, 524-529 (2013).
I. A. Novakov, N. A. Rakhimova, et al., Khim. Promst. Segodnya, No. 3, 31-44 (2012).
I. A. Novakov, N. A. Rakhimova, et al., Khim. Promst. Segodnya, No. 7, 28-38 (2012).
I. A. Novakov, N. A. Rakhimova, et al., Trenie i Iznos, 32, No. 4, 344-354 (2011).
I. A. Novakov, N. A. Rakhimova, et al., Trenie i Iznos, 32, No. 5, 476-488 (2011).
A. P. Krasnov, N. A. Rakhimova, et al., Trenie i Smazka v Mashinakh i Mekhanismakh, No. 2, 29-35 (2011).
I. A. Novakov, N. A. Rakhimova, et al., Zh. Prikl. Khim., 84, No. 6, 995-1003 (2011).
S. V. Kudashev, U. R. Urmantsev, et al., Zh. Obshch. Khim., 84, No. 2, 326-328 (2014).
S. V. Kudashev, U. R. Urmantsev, et al., Proceedings of the Volgograd State Technical University, Series: Chemistry and technology of elemental organic monomers and polymeric materials, Volgograd State Technical University, Volgograd, 4, No. 107, 130-139 (2013).
S. V. Kudashev, V. N. Arisova, et al., Khim. Tekhnol., 15, No. 3, 155-158 (2014).
S. V. Kudashev, V. N. Arisova, et al., Proceedings of the Volgograd State Technical University, Series: Chemistry and technology of elemental organic monomers and polymeric materials, Volgograd State Technical University, Volgograd, 7, No. 134, 101-106 (2014).
S. V. Kudashev, U. R. Urmantsev, et al., Zh. Prikl. Khim., 86, No. 6, 972-978 (2013).
S. V. Kudashev, V. N. Arisova, et al., Proceedings of the Volgograd State Technical University, Series: Chemistry and technology of elemental organic monomers and polymeric materials, Volgograd State Technical University, Volgograd, 19, No. 122, 81-85 (2013).
S. V. Kudashev, V. N. Arisova, et al., Proceedings of the Volgograd State Technical University, Series: Chemistry and technology of elemental organic monomers and polymeric materials, Volgograd State Technical University, Volgograd, 19, No. 122, 86-90 (2013).
The authors are grateful to V. N. Arisova, Ph.D. (Volgograd State Technical University) for the help in carrying out X-ray diffraction studies.
The work was supported by the economic contract for the Science & Research Work No. 20-53 / 676-14 (01.01.2014-31.12.2016) and the Federal Target Program grant “Research and scientific-pedagogical personnel of innovative Russia” for 2009-2013 (agreement No. 14.V37.21.1201).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimicheskie Volokna, No. 1, pp. 18-22, January – February, 2016.
Appendix
Appendix

Diagram 1

Diagram 2
Rights and permissions
About this article
Cite this article
Kudashev, S.V., Nistratov, A.V., Danilenko, T.I. et al. Modifying Effect of 1,1,3-Trihydroperfluoro-1-Propanol on the Properties of Oriented Polyester Yarns. Fibre Chem 48, 16–20 (2016). https://doi.org/10.1007/s10692-016-9731-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10692-016-9731-4