Abstract
Despite their prevalence in nature, the evolution of sex-specific female ornaments is still not well understood. Although in some cases (often carotenoid-based ornaments) they appear to honestly signal quality, such as fecundity, it has been suggested that some female ornaments evolved to deceptively obtain matings. One such case is the long-tailed dance fly (Rhamphomyia longicauda) where females possess two sex-specific ornaments: pinnate scales on the hind femur and tibia and abdominal sacs that are inflated in female-biased “display” swarms. Because females rely on male nuptial food gifts to initiate and sustain egg development, female ornaments are thought to have evolved in the context of deceiving males to obtain gifts. For males, the costs of being deceived may be reduced if female ornaments on average provide valuable information about female quality such as fecundity to males. Here, we use static allometry (with body size as a proxy for condition) of both ornamental and non-ornamental traits in females (and homologous non-ornamental traits in males) in order to determine whether they indicate condition to males. Most male traits scaled isometrically with body size, however, as often expected for sexually selected traits, female ornaments (abdomen area and tibia scale length) showed significant positive allometry and had steep slopes relative to non-ornamental traits. In addition, male leg hairs (homologous with female scales) showed positive static allometry, probably because they are involved in nuptial-prey capture or in grasping mates. As larger females invest more in ornamentation relative to smaller females, their ornaments may exaggerate differences in female condition and thus inform male mating decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although males typically possess elaborate or showy traits used to increase mating success, there are an increasing number of species known where females possess sexually selected ornamental traits (Darwin 1871; Trivers 1972; Amundsen 2000; Tobias et al. 2012; Nordeide et al. 2013; Hare and Simmons 2018). Strong sexual selection and female-specific ornamentation are typically found in species where males make large investments in offspring such as nuptial feeding or paternal care and thus females are highly motivated to mate, often relying on these direct benefits for offspring development (Trivers 1972; Gwynne 1981, 1991, 1993; Gwynne and Simmons 1990; Jones et al. 2001). On the other hand, potential male mating rate is more limited in such paternally investing species, leading males to discriminate among potential mates, especially when females vary in quality (Trivers 1972; Gwynne 1991; Jones et al. 2001; Bondurianksy 2001). Thus, it is reasonable to predict that female ornaments evolved to advertise quality to choosy males (Andersson 1994).
Because male fitness is typically limited by the number of offspring sired, the highest quality females are those that provide the greatest opportunity for fertilization success (Fitzpatrick et al. 1995; Bondurianksy 2001; Herridge et al. 2016). For many animals, female quality is determined by fecundity (the number of eggs a female can produce) but can also include offspring quality or viability (reviewed in: Bonduriansky 2001; Noredeide et al. 2013). When investment in ornamentation is costly however, females that produce these elaborate structures may reduce the resources available for egg production, thus reducing fecundity (or egg size) relative to females that do not invest in these traits (Fitzpatrick et al. 1995). Such trade-offs are expected to constrain the evolution of ornamental traits in females because males would be unlikely to be attracted to traits that reduce fitness, even if they honestly signal fecundity (Fitzpatrick et al. 1995).
Chenoweth et al. (2006) suggest that female ornaments may be adaptive even with costs to fecundity, if they function as valuable signals when direct assessment of female quality is difficult (e.g. when body size does not scale predictably with fecundity, or if visual signaling occurs in poor light conditions). This may explain female ornamentation in empid dance flies, Rhamphomyia longicauda (Wheeler et al. 2012), where female sex-specific pinnate leg scales and inflatable abdominal sacs are displayed in lek-like mating swarms in poor light conditions at dusk and dawn. Prior to entering swarms, females inflate their abdominal sacs and pull their legs up alongside the abdomen, increasing their apparent size to males entering the swarm from below (Cumming 1994; Funk and Tallamy 2000). Males arrive carrying nutritious prey-items (usually small flies, mayflies, or caddisflies) that they hand over just before mating (Funk and Tallamy 2000). Because female empids do not hunt for prey, they rely on these mating gifts for egg development (Downes 1970; Funk and Tallamy 2000; Hunter and Bussière 2019) and thus mate frequently (Herridge 2016; Browne 2021).
Experimental manipulations of female trait size in display swarms in the field (using plastic models) have shown that males are most attracted to females with large ornaments (Funk and Tallamy 2000), with abdomen area being the most important (Murray et al. 2018). However, when comparing mated and unmated females, Wheeler et al. (2012) found that female ornaments are under stabilizing sexual selection which suggests that males may avoid mating with the most elaborately ornamented females. This finding supports the prediction of Chenoweth et al. (2006) that stabilizing sexual selection will result from stochastic overinvestment in ornamentation that reduces fecundity, and thus the attractiveness of the most ornamented females. However, such stabilizing selection is also expected if males avoid the most attractive, frequently-mated females because of the increased risk and intensity of sperm competition (Funk and Tallamy 2000; Wheeler et al. 2012; Herridge et al. 2016; Murray et al. 2018).
The potential for female ornaments in R. longicauda to function as honest indicators of quality however, was first questioned by Funk and Tallamy (2000), because one of the female ornaments, inflated abdomen size, was found to explain a low proportion of variance in egg size (r2 = 0.23) relative to a non-inflatable congeneric, Rhamphomyia sociabilis (r2 = 0.72) (Funk and Tallamy 2000). This study suggested that female inflatable abdomens in R. longicauda may have evolved to deceive males by masking the degree of egg development in order to avoid rejection. Funk and Tallamy (2000) noted that egg development (size) may be particularly important to males in species with last male sperm precedence (common in insects; Simmons 2001) because mature eggs, and thus impending oviposition, means a reduced likelihood of female remating that would compromise paternity. Further studies of a different population of R. longicauda have found slightly higher relationships between inflated abdomen area and egg size (r2 = 0.334; Bussière et al. 2008, r2 = 0.49; Wheeler 2008), although these are still low compared to R. sociabilis (Funk and Tallamy 2000). Regardless, the relationship between female quality and ornament size in R. longicauda is consistently positive and significant (Funk and Tallamy 2000; Bussière et al. 2008; Wheeler et al. 2012) and importantly, is similar to other species where female traits are considered honest signals of quality (e.g. r2 = 0.35 in barn swallows; Møller 1993, r2 = 0.32–0.40 in Inca terns; Velando et al. 2001, r2 = 0.44 in scissor tail fly catchers; Regosin and Pruett-Jones 2001, r2 = 0.11 in penguins; Massaro et al. 2003, r2 = 0.34–0.35 in dance fly Rhamphomyia tarsata; LeBas et al. 2003, and r2 = 0.68 in mantids; Barry 2015).
Females in ornamented dance fly species, however, may necessarily deceive their first mate because they have no mature eggs and require nuptial gifts to initiate egg development: compared to mated Empis aestiva females, a species with leg scale ornamentation similar to R. longicauda, eggs of unmated individuals did not develop (become larger) with age. This trend was not found in an unornamented species (Rhamphomyia crassirostris) where females developed eggs regardless of their mating status (Hunter and Bussière 2019). Further, the fact that the number of developed eggs depends on future matings would make it nearly impossible for males to accurately assess female quality. The limited ability of males to detect deception (unless males can assess weight post-pairing; Murray et al. 2018) makes the evolution of deceptive traits more likely (Mokkonen and Lindstedt 2016) in dance flies.
The evolution of deception is even more likely in systems where traits provide some valuable information on average, as this lowers the costs of being deceived (West-Eberhard 1979; Mokkonen and Lindstedt 2016; Johnstone and Grafen 1993). Although ornamental traits in dance flies mask female quality (number of developed eggs), differences in larval acquisition of resources prior to eclosion may allow higher condition females to invest more in ornamentation relative to those in lower condition (Andersson 1994; King et al. 2011; Somjee 2020). Although condition does not provide direct information about ovarian development, we suggest that females in high condition have an overall greater chance of producing more developed eggs: high condition females may be better at deceiving males (can produce large ornaments) and are probably able to swarm for longer and/or more frequently (Somjee 2020), thus increasing their chances of obtaining the matings necessary to begin and sustain egg development. Moreover, higher condition females may even have a better chance of surviving until oviposition (Gwynne et al. 2015), further increasing the fitness benefits of mating with a higher condition female. Thus, despite masking the degree of egg development, we suggest female ornaments in R. longicauda may reduce the costs of deception by exaggerating, and thus reliably signalling, differences in female condition. One way to examine whether female ornaments in R. longicauda are related to condition, is to measure the investment in traits relative to body size. While body size is not a direct measure of condition, it is closely related and often used as a proxy (e.g. Emlen 1997; Johnstone et al. 2009; Emlen et al. 2012). Not only do larger individuals often store more energy per gram of tissue, but they also have lower resting metabolic rates relative to smaller individuals of the same species (Thommen et al. 2019; Somjee 2020).
In this study, we measure static allometry of inflated abdomen area and pinnate-scale ornaments as well as several non-ornamental female traits to determine how smaller females invest in ornamental traits relative to larger ones. If female ornaments have a signalling value to males, we expect ornamental traits to be more closely related to female condition and thus exhibit steeper allometric slopes relative to non-ornamental traits. If female ornaments show positive static allometry, larger females invest relatively more in ornamentation than smaller individuals, suggesting these traits exaggerate differences among females and thus reliably indicate condition. On the other hand, if female ornaments show negative allometry, this suggests they minimize differences in female condition (and thus are probably not reliable cues) because smaller females invest disproportionately in these traits.
We can gain further insight into the evolution of female ornaments by comparing their investment in traits relative to males. In R. longicauda, males do not possess the abdominal and leg-scale ornaments but have the likely ancestral state of the traits: non-inflatable abdomens and leg hairs instead of scales on the tibiae and femora. A previous study (Bussière et al. 2008) compared the nature of sexual selection on males and females in R. longicauda, but this did not include any ornamental traits. Here, we measure allometric patterns on female ornaments and homologous male traits with the prediction that allometric slopes will be steeper for females, as is often observed when sexual selection has led to an exaggerated trait in one sex (Petrie 1988; Green 1992).
Methods
Dance fly biology
Empid dance flies include many species where males provide their mates with nuptial gifts (Cumming 1994). In our study species, R. longicauda, females gather in large swarms during dusk or dawn and males enter the swarm with nutritious prey-items which they exchange with females for mating (Funk and Tallamy 2000). It is thought that females’ reliance on mating for nutrition (Downes 1970) drives sexual competition among females and has led to the evolution of the two female-specific ornaments used to attract males. When in the lek-like mating swarms, females inflate their abdominal sacs and pull up their scaly legs alongside the abdomen, which increases their apparent size (Funk and Tallamy 2000).
Specimen collection and measurement
We collected 224 female and 113 male R. longicauda from mating swarms in the Credit river valley, near Glen Williams, Ontario, Canada (43.6865660, − 79.9260960) from mid-June to early July of 2017 and 2018. Males were caught individually and transferred to vials where they were frozen and then stored in > 70% ethanol. Females were collected using a sweep net and flash frozen with liquid nitrogen in order to preserve the inflated abdominal sacs. Once frozen, females were stored in ethanol. We took images of male and females, using a camera fitted to a dissecting microscope and measured male and female traits using ImageJ. Measurements included thorax scutum length as an estimate of body size (as in Wheeler 2008; and Herridge 2016), ornamental traits: inflated abdomen area (as an estimate of pleural sac size), and the length of the longest scale (hairs in males) on the femur and tibia (Fig. 1) as well as non-ornamental traits: wing length, hind femur length, and hind tibia length. We did not include measures of tibial or femoral scale area (as in LeBas et al. 2003; Herridge 2016; Wheeler et al. 2012), as these traits were highly correlated with the length of the leg segment in both sexes (tibia length: females R = 0.92, males R = 0.80; femur length: females R = 0.84, males R = 0.85), thus likely are not independent measurements.
Statistical analysis
We square root-transformed abdomen area to ensure that all measurements were in the same units (mm) and confirmed that all traits were normally distributed using a Shapiro–Wilk test of normality. We then calculated the average trait size (± SD) for both males and females and used a Student’s T-test to test for significant differences. Next, we determined the allometric relationship using model II standard major axis regression (SMA; see Green 2000; Simmons and Tomkins 1996; Kelly 2014) of the log transformed traits (wing length, femur length, tibia length, \(\sqrt{\mathrm{abdomen}}\mathrm{ area}\), femur scale length, and tibia scale length) on log thorax length for both males and females. We determined whether traits deviated significantly from isometry using the 95% confidence intervals of the SMA slope.
Results
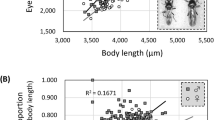
While females had significantly larger abdomens, legs, and leg scales (hairs in males) than males, there was no significant difference (with Bonferroni correction) in male and female thorax or wing size (Table 1). We found evidence of positive allometry for several male and female traits as slopes were significantly higher than one. In females, both abdominal area and tibia scale length scaled positively with body size with slopes significantly greater than those for non-ornamental traits (Table 2). Although femur scale length did not differ significantly from isometry, this trait also had a steep (but not significantly greater) slope relative to non-ornamental traits. Female femur and tibia length did not differ significantly from isometry, while wing length showed significant negative allometry. In males, three traits including wing length, femur length, and tibia length all scaled negatively with body size. Interestingly, male leg hairs on both the tibia and femur scaled positively with body size. Although the allometric intercept was higher in females across both these traits, males showed a steeper allometric slope for hairs compared to female scales (Table 2; Figs. 2, 3, 4).
Discussion
In the dance fly R. longicauda, we found evidence of positive allometry on two female ornaments: abdomen area and tibia scale length. Both these traits had allometric slopes greater than one and were steep relative to non-ornamental traits, including tibia and femur length. The relationship between body size and femur scale length did not differ significantly from isometry, however as predicted, the slope still tended to be steeper than that of non-ornamental traits. This finding suggests that ornaments may exaggerate differences among females and serve as reliable cues of condition, as larger (presumably higher condition) females invest more in ornamentation relative to smaller ones.
Although female ornaments likely evolved in the context of deceiving males to obtain important nutrition (Funk and Tallamy 2000; Hunter and Bussière 2019; Murray et al. 2018), they appear to provide useful information to selective males. Indeed, a male showing a preference for a highly ornamented female will be deceived if she is unmated and thus with no developed eggs. However, she is more likely to mate and develop eggs because she is in high condition; high condition, and thus highly ornamented females are expected to attract multiple mates (Funk and Tallamy 2000; Murray et al. 2018) and thus obtain plenty of protein for egg development. High condition R. longicauda females also may be better able to bear the costs of displaying in mating swarms (Somjee 2020) and thus are probably able to swarm for longer and/or more often. Finally, there is evidence that higher condition females may have a better chance of surviving until oviposition; larger (higher condition) females are better able to escape from spider webs that are often present near mating swarms (Gwynne et al. 2015).
Males are of course, still subject to sperm competition, which is expected to increase in intensity when females mate frequently (Parker 1970; Simmons 2001). The outcome of sperm competition is important for understanding the evolution of female ornaments because it is the final determinant of male fitness and is expected to affect mating preferences. If there is strong last male sperm precedence in R. longicauda, female condition may still inform male mate choice, but the cost of deception is probably higher because a low-quality female will need to re-mate to fully develop her eggs, potentially compromising this male’s paternity. On the other hand, if paternity is less biased (such as when sperm mixes within the spermatheca; Simmons 2001), the costs of deception will be lower, as the male is likely to gain some paternity regardless of a female’s ovarian development at the time of mating. Although there is evidence that multiple males sire offspring (2–6 sires; using the conservative method of allele counting; Browne 2021), the degree of last male sperm precedence is not known with confidence. While sclerotized, non-stretchable spermathecae of R. longicauda females makes sperm displacement and thus biased paternity in favour of the last male more likely (Simmons 2001), previous work shows that last males do not father more offspring than a female’s other mates (Browne 2021). On the other hand, we cannot rule out that this finding was influenced by our sampling methods, which may have disrupted the last mating male’s copulation prior to insemination (Browne 2021) and further, Herridge’s (2016) study shows that R. longicauda sperm stores are typically dominated by a single male (mating order unknown). Regardless of the outcome of sperm competition, paternity confidence is expected to decrease when females mate more frequently (Simmons 2001) so males may still be expected to avoid the most attractive (highly ornamented) individuals (Funk and Tallamy 2000; Wheeler et al. 2012; Herridge et al. 2016; Murray et al. 2018). Again, this could help to explain the finding of stabilizing sexual selection on inflated abdomen size (Wheeler et al. 2012), as males balance the costs of mating with a deceptive female lacking ovarian development and sperm competition intensity.
Surprisingly, we also found that two male traits—tibia hair length and femur hair length- scaled positively with body size (thorax length), while male hind tibia and femur length showed negative allometric slopes. Although females had higher allometric intercepts for both tibia and femur scales, consistent with female-biased sexual dimorphism, males actually showed steeper allometric slopes for (almost certainly non-ornamental) leg hairs that are homologous with female leg scales. Leg hairs may be sexually selected for grasping flying females. Alternatively, because males are the only sex that hunt, leg hairs are likely subject to natural (or sexual, given prey are used in obtaining mates) selection for hunting efficiency (Svensson and Petersson 1987; Svensson 1997). In other dance fly species, it has been suggested that leg adaptations, including fore-femur length and leg hairs (Empis boralis; Svensson and Petersson 1987, R. marginata; Svensson 1997) are related to prey capture and thus hairs may serve a similar function in R. longicauda. Although positive allometry is typically associated with strong sexual selection (Petrie 1988; Green 1992), natural selection can also produce positive allometric slopes when the benefit of expressing the trait is greater for larger males relative to small ones (Bonduriansky and Day 2003; van Lieshout et al. 2013).
Based on the finding of positive static allometry on secondary sexual traits, we suggest that female ornaments in R. longicauda may serve as valuable signals of condition despite the traits masking the degree of egg development (quality) from males. Although female ornaments are not expected to evolve in the context of signalling condition alone, we suggest this may have added importance in systems where females rely on gifts (matings) for egg development and thus cannot honestly signal quality.
Availability of data and material
Data are available to be posted to Dryad.
Code availability
R script available to be posted to Dryad.
Change history
23 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10682-022-10163-y
References
Amundsen T (2000) Why are female bird ornamented? Trends Ecol Evol 15:149–155
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Barry KL (2015) Sexual deception in a cannibalistic mating system? Testing the femme fatale hypothesis. Proc R Soc B 282:20141428
Bonduriansky R (2001) The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol Rev 76:305–339
Bonduriansky R, Day T (2003) The evolution of statis allometry in sexually selected traits. Evolution 57:2450–2458
Browne JH (2021) Understanding paternity and female ornaments in systems where females compete for nuptial gifts: a tale of long tails and short tails. Dissertation, University of Toronto, Canada
Bussière LF, Gwynne DT, Brooks R (2008) Contrasting sexual selectin on males and females in a role-reversed swarming dance fly, Rhamphomyia longicauda. J Evol Biol 21:1683–1691
Chenoweth SF, Doughty P, Kokko H (2006) Can non-directional male mating preferences facilitate honest female ornamentation? Ecol Lett 9:179–184
Cumming JM (1994) Sexual selection and the evolution of dance fly mating systems (Diptera: Empididae: Empidinae). Can Entomol 126:907–920
Darwin C (1871) The decent of man and selection in relation to sex. John Murray, London
Downes JA (1970) The feeding and mating behaviour of the specialized Empidinae (Diptera: Empididae: Empidinae). Can Entomol 126:907–920
Emlen DJ (1997) Diet alters male horn allometry in the beetle onthophagys acuminatus (Coleoptera: Scarabaeidae). Proc R Soc Londn B 264:567–574
Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC (2012) A mechanism of extreme growth and reliable signalling in sexually selected ornaments and weapons. Science 337:860–864
Fitzpatrick S, Berglund A, Rosenqvist G (1995) Ornaments or offspring: costs to reproductive success restrict sexual selection processes. Biol J Lin Soc 55:251–260
Funk DH, Tallamy DW (2000) Courtship role reversal and deceptive signals in the long-tailed dance fly, Rhamphomyia longicauda. Anim Behav 59:411–421
Green AJ (1992) Positive allometry is likely with mate choice, competitive display and other functions. Anim Behav 43:170–172
Green AJ (2000) The scaling and selection of sexually dimorphic characters: an example using the marbled teal. J Avian Biol 31:345–350
Gwynne DT (1981) Sexual difference theory: Mormon Crickets show role reversal in mate choice. Science 213:779–780
Gwynne DT (1991) Sexual competition among females: What causes courtship-role reversal? Trends Ecol Evol 6:118–121
Gwynne DT (1993) Food quality controls sexual selection in Mormon crickets by altering male mating investment. Ecology 74:1406–1413
Gwynne DT, Simmons LW (1990) Experimental reversal of courtship roles in an insect. Nature 346:172–174
Gwynne DT, Punzalan D, Hunt J (2015) Viability selection on female fly finery in the wild. Biol J Lin Soc 116:530–540
Hare RM, Simmons LW (2018) Sexual selection and its evolutionary consequences in female animals. Biol Rev 94:000–000
Herridge EJ (2016) Polyandry rates and reproductive success in a nuptial gift giving dance fly Rhamphomyia longicauda. Dissertation, University of Stirling
Herridge EJ, Murray RL, Gwynne DT, Bussière LF (2016) Diversity in mating and parental sex roles. In: Kliman RM (ed) Encyclopedia of evolutionary biology. Elsevier, Oxford, pp 453–458
Hunter FDL, Bussière LF (2019) Comparative evidence supports a role for reproductive allocation in the evolution of female ornament diversity. Ecol Entomol 44:324–332
Johnstone RA, Grafen A (1993) Dishonesty and the handicap principle. Anim Behav 46:759–764
Johnstone RA, Rands SA, Evans MR (2009) Sexual selection and condition-dependence. J Evol Biol 22:2387–2394
Jones AG, Walker D, Avise JC (2001) Genetic evidence for extreme polyandry and extraordinary sex-role reversal in a pipefish. Proc R Soc Lond B 268:2531–2535
Kelly CD (2014) Sexual selection, phenotypic variation, and allometry in genitalic and non-genitalic traits in the sexually size-dimorphic stick insect Micrarchus hystriculeus. Biol J Linn Soc 113:471–484
King EG, Roff DA, Fairbairn DJ (2011) Trade-off acquisition and allocation in Gryllus firmus: a test of the Y model. J Evol Biol 24:256–264
LeBas NR, Hockham LR, Ritchie MG (2003) Nonlinear and correlational sexual selection on ‘honest’ female ornamentation. Proc R Soc Lond B 270:2159–2165
Massaro M, Davis LM, Darby JT (2003) Carotenoid-derived ornaments reflect parental quality in male and female yellow-eyed penguins (Megadyptes antipodes). Behav Ecol Sociobiol 55:169–175
Mokkonen M, Lindstedt C (2016) The evolutionary ecology of deception. Biol Rev 91:1020–1035
Møller AP (1993) Sexual selection in the barn swallow Hirundo rustica III. Female Tail Ornam Evolut 47:417–431
Murray RL, Wheeler J, Gwynne DT, Bussière LF (2018) Sexual selection on multiple female ornaments in dance flies. Proc R Soc B 285:20181525
Nordeide JT, Kekäläinen J, Janhunen M, Kortet R (2013) Female ornaments revisited—are they correlated with offspring quality? J Anim Ecol 82:26–38
Parker GA (1970) Sperm competition and its evolutionary consequences in the insects. Biol Rev 45:525–567
Petrie M (1988) Intraspecific variation in structures that display competitive ability: large animals invest relatively more. Anim Behav 36:1174–1179
Regosin JV, Pruett-Jones S (2001) Sexual selection and tail-length dimorphism in scissor-tailed flycatchers. Auk 118:167–175
Simmons LW (2001) Sperm competition and its evolutionary consequences in the insects. Princeton University Press, New Jersey
Simmons LW, Tomkins JL (1996) Sexual selection and the allometry of earwig forceps. Evol Ecol 10:97–104
Somjee U (2020) Positive allometry of sexually selected traits: Do metabolic maintenance costs play an important role? BioEssays 43:e2000183
Svensson BG (1997) Swarming behavior, sexual dimorphism, and female reproductive status in the sex role-reversed dance fly species Rhamphomyia marginata. J Insect Behav 10:783–804
Svensson BG, Petersson E (1987) Sex-role reversed courtship behaviour, sexual dimorphism and nuptial gifts in the dance fly, Empis borealis (L.). Ann Zool Fenn 24:323–334
Thommen A, Werner S, Frank O, Philipp J, Knittelfelder O, Quek Y, Fahmy K, Shevchenko A, Friedrich BM, Jülicher F, Rink JC (2019) Body size-dependent energy storage causes Kleiber’s law scaling of the metabolic rate in planarians. Life 8:e38187
Tobias J, Montgomerie R, Lyon BE (2012) The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philos Trans R Soc B 367:2274–2293
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the decent of man. Aldine, Chicago
van Lieshout E, Svensson PA, Wong BBM (2013) Consequences of paternal care on pectoral fin allometry in a desert-dwelling fish. Behav Ecol Sociobiol 67:513–518
Velando A, Lessells CM, Márquez JC (2001) The function of females and male ornaments in the Inca tern: evidence for links between ornament expression and both adult condition and reproductive performance. J Avian Biol 32:311–318
West-Eberhard MJ (1979) Sexual selection, social competition, and evolution. Proc Am Philos Soc 123:222–234
Wheeler J (2008) Sexual selection and female ornamentation in a role-reversed dance fly. Dissertation, University of Toronto, Canada
Wheeler J, Gwynne DT, Bussière LF (2012) Stabilizing sexual selection for female ornaments in a dance fly. J Evol Biol 25:1233–1242
Acknowledgements
Thanks to: Samreen Munim and Erik Etzler for assistance in the field, Kavya Manikonda for specimen imaging, and to Rosalind Murray, Marc Johnson, and Doug Currie for comments on the research (funded by a NSERC Discovery Grant to DTG). An additional thanks to the reviewers for their detailed and insightful comments on the manuscript.
Funding
Funded by a NSERC Discovery grant to DTG.
Author information
Authors and Affiliations
Contributions
JHB—concept and design, specimen/data collection, data analysis, primary author, DTG—contributions to concept and design, specimen collection, substantial editing and revisions.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflicts of interest.
Consent for publication
All authors included on the submission contributed to the manuscript and have given consent to submit.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Browne, J.H., Gwynne, D.T. Deceived, but not betrayed: static allometry suggests female ornaments in the long-tailed dance fly (Rhamphomyia longicauda) exaggerate condition to males. Evol Ecol 36, 631–641 (2022). https://doi.org/10.1007/s10682-021-10148-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-021-10148-3