Abstract
Environments with strong gradients in physical conditions, such as rocky intertidal, induce animal morphological strategies to face them. The gastropod Trophon geversianus inhabit within the intertidal and subtidal habitats of Patagonian rocky shores. Although there is a wide knowledge of the phenotypic differences of this species regarding habitats (i.e., intertidal/subtidal), little is known about the interaction between habitat and latitude. Here, we studied form variation (size and shape) by using 3D geometric morphometric of T. geversianus shells from alive gastropods and analyzed the phenotypic effect from micro-scale (habitat), macro-scale (latitude), and the interaction habitat-latitude (site). Lastly, we tested the classification accuracy of the shape variable for each predictor variable and a synthetic variable (from a cluster analysis). We found that habitats and sites had the greatest influence on shape variation. Moreover, we found that the largest shell sizes were more likely to be located in subtidal habitats. Also, the size differences between sites were not negligible. Finally, habitat demonstrated the highest classification accuracy for shape, even higher than genetically determined (sex) and synthetic variables. We found that the gastropods from the intertidal habitat presented a globular morph with shorter spire and larger relative size of the shell aperture, while subtidal gastropod showed an elongated morph, with smaller aperture and longer spire. We present evidence of the complexity of size and shape variation in T. geversianus, highlighting that site-dependence on shape variation must be considered in geometric morphometrics studies at a latitudinal scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the biological and physical factors which influence patterns of phenotypic variation has long fascinated evolutionary ecologists (Piersma and Van Gils 2011). Evolutionists have long maintained that plasticity is central in the origin of phenotypic differences between species (Jablonka and Lamb 1995), because of its implication in evolution’s most fundamental events: the origin of novel, complex traits and the origin of new species (Pfenning et al. 2010). The environment is well known to alter phenotypic traits at different geographical scales (Rice 2012). Therefore, rigid structures of marine organisms contain considerable information about their history, including the changing conditions of mineralization and environmental stress or disease (Okoshi 1996). Still, the influence of micro scales, such as habitats (intertidal-subtidal) or intertidal levels (low, middle, high tide levels; see Raffaelli and Hawkins 2012), and macro-scales, such as latitudinal clines, continues to be poorly understood.
In general, species with large latitudinal distributions exhibit morphological variation that could be a consequence of local adaptation (Johannesson 1986; 1996; Partridge and Coyne 1997; Márquez et al. 2015) or phenotypic plasticity (Bourdeau et al. 2015) to different physical conditions. Mollusks present intra- and interspecific phenotypic variation that correlates with environmental gradients of space or time, and it is considered to be a response to these gradients (Vermeij 1972; Conde-Padín et al. 2007). A seminal study on intertidal environments in mollusks previously described a relationship between size gradients and physical stressors or predation and other biotic interactions (Vermeij 1972). For example, a positive correlation between sea temperature and shell strength was described along the distribution of the gastropod Nucella lapillus (Vermeij and Currey 1980). In Acanthina monodon, morphometric phenotypes were related to wave exposure and hydrology along with the species range of distribution (Sánchez et al. 2011). A more recent study of shell shape variation in Lottia subrugosa suggested that factors affecting limpet morphology at large spatial scales may act at smaller scales as well (Vieira and Bueno 2019). Studies of changes in shell shape at different environmental conditions carried out in the gastropod Littorina saxatilis are interesting examples of evolutionary works (Grahame et al. 2006): two different ecotypes (genetically determined phenotypes associated with certain ecological conditions) were described as living in micro-scales, separated only by a few meters. The “crab ecotype” presented a thick and elongated shell opened by a small aperture, while the “wave ecotype” was characterized by a shell with a more compressed spire, larger aperture, and smaller size (Johannesson 1986; Conde-Padín et al. 2007). Moreover, Grahame (2006) found those ecotypes in different areas of the UK, Spain, and along the Swedish coast. On a small scale, work carried out on Siphonaria lessonii described two ecomorphotypes in the high and middle intertidal levels of the same rocky intertidal in Atlantic Patagonia (Livore et al. 2018) related to environmental stress caused by exposure to air, wave action and variation in temperature in the micro-scale. Also, other marine gastropod species reported shell form variation in response to biological and physical factors (Crothers 1975; Irie 2005 Supplementary data in Márquez et al. 2015). Marine gastropods on rocky intertidal shores exhibit substantial morphological variation that is often correlated with strong environmental gradients, even on very small spatial scales (Trussell and Etter 2001).
Environments with strong gradients in physical conditions, such as rocky intertidal, require morphological strategies to face them. Rocky intertidal are unstable environments characterized by a wide range of ultra-violet (UV) radiation, temperature, and wind (Denny and Wethey 2001; Raffaelli and Hawkins 2012). In North Patagonia, desiccation is an order of magnitude higher than in other intertidal areas studied in different parts of the world, due to low local rainfall (see Table 1 in Bertness et al. 2006) and persistent dry west winds, which flow relatively unobstructed (Crespi-Abril et al. 2018), with high persistence and intensity (Paruelo et al. 1998). The muricid gastropod Trophon geversianus (Pallas 1776) inhabits both the intertidal and subtidal habitats of northern Patagonian rocky shores. This gastropod shows a continuous distribution along the South-Western Atlantic Ocean from Buenos Aires (38°00’ S; 57° 32’ W) to the Burdwood Bank (54° 30′ S; 60° 30′ W) (Pastorino 1994, 2005). T. geversianus reproduces through egg capsules, where the embryos develop until hatching as crawling juveniles (Cumplido et al. 2010) and exhibits different phenotypic traits related to particular environmental factors along with its distribution. Márquez et al. (2015) described two ecomorphotypes without genetic differences between intertidal and subtidal environments for this gastropod. However, subsequent works that studied the same species at a latitudinal scale did not consider this phenotypic variation at the micro-scale level, and reported two additional ecomorphotypes corresponding to Magellan and Patagonian biogeographic provinces, a positive correlation between size and seawater pH, and rejected the possibility of ecogeographic rules (Malvé et al. 2016, 2018). Nevertheless, the ecogeographic rules, such as Bergmann's and Allen's, which express the relationship between body size and environmental temperature, demands a great number of morphological analyses before they can be rejected or approved (Bergmann 1848; Mayr 1956).
As we mentioned above, the influence of geographic scales on the phenotype remains in debate. Most marine species have been assumed to be demographically open populations that are interconnected by high gene flow (Sanford and Kelly 2011). However, increasing evidence shows that marine populations are less connected to each other (Palumbi 2004; Levin 2006), highlighting the importance of studies on short-scale variations (Livore et al. 2018; Vieira and Bueno 2019). Some authors claim that local adaptation is more common than is supposed, whereas others state that phenotypic plasticity is ubiquitous (Sanford and Kelly 2011; Bourdeau et al. 2015). The incorporation of micro-scale information in latitudinal analyses could be the cornerstone to bring light to the underlying evolutionary processes.
Therefore, our aim was to analyze the shell shape variation in T. geversianus at two different geographic scales: across different tidal or habitat levels (micro-scale: less than 0.3 km apart), and latitudinally (macro-scale: more than 400 km apart). Specifically, we aimed to identify the most influential geographical scales (micro vs macro) on shell form variation of T. geversianus. We also aimed to assess whether intertidal and subtidal T. geversianus ecomorphotypes were represented in a latitudinal scale; and which physical condition was most influential in morphometric variation?
Material and methods
Study area
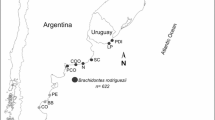
The study area covered three localities with environmental heterogeneity along a latitudinal gradient (410 km straight-line) of Patagonian rocky shores (Fig. 1): Golfo Nuevo (GN; 42° 47′ S-64° 57′ W), Bahía Camarones (CA; 44° 53′ S; − 65° 39′ W), and Comodoro Rivadavia (CO; 45° 57’S; 67° 32’W). The semi-enclosed GN is characterized by high salinity, higher average temperature, and weak vertical water stratification related to low water exchange (Rivas and Beier 1989; Rivas 1990). CA is a bay open to the sea but protected by a tombolo in the south (Schillizzi et al. 2014). The point located in CO is an open ocean shoreface, characterized by a high flow of water, intense wave energy, and strong winds (Labraga 1994). Each site has different environmental conditions of air desiccation and physical stress. Annual means of air temperature, surface irradiance, precipitation, winds, air CO2, organic carbon, sea salt, surface sea temperature and fetch were recorded to analyze the ambient heterogeneity (Supplementary information 1). All physical variables were acquired from the GES-DISC Interactive Online Visualization ANd aNalysis Infrastructure (GIOVANNI) available by NASA’s Goddard Earth Sciences (GES) Data and Information Services Center (DISC) (https://giovanni.gsfc.nasa.gov/giovanni/), except fetch, which was calculated following Burrows (2008) as a proxy of the wave action at coastal sites. Fetch was defined as the unobstructed length of water, in which wind from a certain direction can blow over. The higher the fetch incises from a certain direction; the more energy is imparted onto the surface of the water (Harborne et al. 2006). In this work, we considered 200 km as the “transition point”, i.e., a distance where the fetch is long enough to consider waves fully developed (Harborne et al. 2006; Burrows et al. 2008). Lastly, we present a descriptive analysis of physical variables across localities.
Sampling
During 2016, we randomly and manually collected 272 living adults, with a total length between 1.5 and 6 cm, (see the adult size in Cumplido and Bigatti 2020), 203 females, and 69 males of T. geversianus snails along the 3 localities, approximately the same number from shallow subtidal (SUB) and mid intertidal level (IN) habitats (see Fig. 1 for sampling details). The distance between habitats (IN and SUB) within each locality did not exceed 0.3 km, representing the spatial micro-scale. The linear distance between the farther locality sites was approximately 410 km, representing the latitudinal macro-scale. Snails of SUB habitat were sampled by freediving at 5–10 m depth, whereas snails of IN habitat were collected manually during low tides. We denoted each combination of locality and habitat as site, i.e., GN:SUB, GN:IN, CA:SUB, CA:IN, CO:SUB, CO:IN.
Geometric morphometrics
The shape of each snail was captured by the Cartesian coordinates of a three dimensional (3D) configuration of 17 anatomically homologous points using the same protocol as Márquez et al. (2015), with the inclusion of one type II landmark (Supplementary information 2). All specimens were measured by one observer (RANV) using a Microscribe G2X digitizer. Landmark configurations were superimposed by generalized Procrustes analysis (Gower 1975; Rohlf and Slice 1990). This procedure translates and rotates the landmark configurations to a common origin and scales them to unit centroid size, which is defined as the sum of squared distance of all landmarks from their centroid (Rohlf and Slice 1990; Slice et al. 1996). We used the centroid size (hereafter size) of each snail as a good proxy of shell size (Rohlf and Slice 1990; Zelditch et al. 2012). To improve the visualization of the shell shape changes in 3D data, we used a surface-based technique for warping (Klingenberg 2011). In Landmark Editor software (Wiley et al. 2005), we deform surfaces from 3D coordinates of the starting and target shapes exported from MorphoJ version 1.06c (Klingenberg 2008), using the thin-plate spline function.
Design and statistical analyses
We applied a linear model using Generalized Least Squares (GLS) to analyze the effect of the macro (locality) and micro-scale (habitat)on size. Four effects were tested: locality, habitat, sex, and the interaction between locality and habitat (hereafter site effect). As well, we modeled the heteroscedasticity by adding the sites as a constant variance function structure (Zar 1999), and then we compared variation between sites. Furthermore, we performed post hoc pairwise comparisons (Tukey’s test) between parallel levels of the site effect (i.e., between localities within habitats, and between habitats within localities). We studied the association between size and environmental factors with the correlation coefficient applied on each habitat separately because environmental factors were measured for each locality (Nakagawa and Schielzeth 2013).
To study the association between shape (Procrustes coordinates) and size we conducted a multivariate linear regression (Bookstein 1991; Klingenberg 2011) and analyze the allometric effect. Then, the shape analysis was divided into two analytical procedures: differentiation and classification. First, we performed a Procrustes Analysis of Variance (ANOVA) to analyze the difference among sites (Goodall 1991; Collyer et al. 2015). The linear predictor had the same four factors mentioned above with the addition of size because the allometry was relevant (Adams et al. 2013). Also, we analyzed the shape variance of each site (estimated as Procrustes variance). We then performed a post hoc pairwise comparison of Procrustes distance and variance between parallel levels of the site effect (Collyer et al. 2015). In addition, the effect sizes, and confidence interval of Procrustes distance comparisons were calculated by residual randomization in permutation procedure using two alternatives null models: the model without the site effect (site-reduced model), and the model without site and habitat effect (site-habitat-reduced model). This procedure allowed us to characterize the complex phenotypic variation of the Trophon model. Lastly, the association between shape variation and physical variables was studied using the two-block partial-least-squares (2B-PLS) analysis between localities within each habitat (Rohlf and Corti 2000).
Second, we performed a Linear Discriminant Analysis for each of the previously known effects (i.e., locality, habitat, sex, and site) to analyze the best shape classification. Jackknife cross-validation values were used to quantify and validate the results (Efron and Gong 1983). We also, performed a model-based clustering with the first axes of the Principal Component Analysis that explained at least 80% of the total variation (Fraley and Raftery 2002). This procedure aims to find an “objective” conformational clustering and whether these are attributable to any of the sources of variation analyzed. Additionally, we used Bayesian Information Criteria to determine the model and the number of clusters for the clustering analysis (Scrucca et al. 2016). Lastly, to evaluate the performance of the objective clustering, we ran a cross-validated Linear Discriminant Analysis and compared the average of classification success of this method with the classification based on the previously known factors.
Statistical analyses and fetch were performed in R statistical software (R Core Team 2020, version 4.0.3). The packages nlme (Pinheiro et al. 2020), emmeans (Lenth et al. 2020), car (Fox and Weisberg 2019), and DHARMa (Harting 2020) were used to perform GLS and Tukey’s test; geomorph (Adams et al. 2020) was used for morphometric analysis; and mclust (Scrucca et al. 2016) was used for cluster analysis.
Results
Physical variables
The descriptive analysis of physical variables showed that air temperature, surface irradiance, wind, air carbon dioxide, organic carbon and sea surface temperature decrease with the latitude (Supplementary information 1). In contrast, fetch follows the opposite pattern, and the other physical variables did not show a clear pattern. The locality CO is notable for the extreme conditions, with the minimum and maximum records. In GN, organic carbon (3.02E-6) was higher, with values three times greater than the other localities.
Size analyses
Principal results of size and shape analyses are reported in Table 1. The GLS analysis showed differences in the interaction factor with no appreciable sex differences (Table 1a). Post hoc comparison showed that snails of SUB habitat were larger than snails of IN habitat within localities (Fig. 2a). The difference between localities within habitat showed a complex pattern. For IN habitat, snails of CA and GN were the smallest, while CO the largest. Concerning SUB habitat, snails of GN were the largest, while snails of CO showed an opposite pattern with slight differences with snails of CA. In absolute numbers, snails within habitat (IN- SUB) had a greater range of sizes than snails within localities (GN, CA, CO; see Table 2).
Pairwise comparison of means between sites. (a) Distances between size mean values and confidence intervals (95%) of the difference between sites. There are clear differences between size sites when zero is not included in the confidence interval (b) Distance between shape means (black line inside each box) and confidence intervals under site-reduced null model (95%, grey box), and site-habitat-reduced null model (95%, dotted line box). Lines outside the boxes denote clear differences. Each comparison is pointed out on the left. Abbreviations: Golfo Nuevo (GN), Bahía Camarones (CA), Comodoro Rivadavia (CO), intertidal (IN) and subtidal (SUB)
In general, the variation was more noticeable in SUB than IN habitat for all localities (Fig. 3a, Table 2). Concerning IN habitat, CA samples presented the higher variability with clear differences from the other localities. On the other hand, GN was the locality with the minimum variation. For SUB habitat, GN was the locality with remarkably least variation. The other comparisons were statistically undetermined. Curiously, we found different latitudinal patterns between intertidal and subtidal mean size. Intertidal mean size increases with latitude, while the opposite was true for subtidal mean size, reaching a peak in GN. Concerning physical variables, variation between IN localities was mainly influenced by salinity (R2 = 0.60), fetch (R2 = 0.48), and air temperature (R2 = 0.40), whereas we did not find any physical variables that influence SUB variation between localities (Table 3).
Pairwise comparison of variance between sites. (a) Distances between size variance means values and confidence intervals (95%) of the ratio between sites. There are clear differences between size sites when one is not included in the confidence interval (b) Distance between shape variances (black line inside each box) and confidence intervals (95%, grey box). Lines outside the boxes denote clear differences. Each comparison is pointed out on the left. Abbreviations: Golfo Nuevo (GN), Bahía Camarones (CA), Comodoro Rivadavia (CO), intertidal (IN) and subtidal (SUB)
Shape analyses
Besides incorporating size into the general model, we performed a multivariate regression for allometry visualization through the two factors (Supplementary information 3). The relationship between shape and size was allometric (permutation test with 10 000 random permutations, P = 0.0001). Comparing between habitats the variation explained by allometry was 6.78% (Supplementary information 3a), between localities was 7.89% (Supplementary information 3b) and pooling between sites explain 3.7% of shell shape variation (Supplementary information 3c). The shell shape variation related to bigger snails was associated with a fusiform shape, the smaller relative size of the shell aperture, lateral compression of the last whorl, and anterior movement of the apertural maximum height.
Procrustes ANOVA of shape showed a strong effect of the site factor on shape, where the effect of the localities is slightly higher than the effect of the habitat (Table 1b). Concerning post hoc comparison of sites under the null site-reduced model, CO locality showed the greatest differences of shape between habitats, whereas the habitat-differences of the other localities were not noticeable (Fig. 2b). For IN habitat, the northern localities (GN and CA) showed the smallest differences, while the remaining two comparisons showed clear differences. In contrast, the main difference in SUB habitat was between CA and GN, and the other comparisons did not show noticeable differences. Under the null site-habitat-reduced model the habitat comparisons within localities become statistically clearest: in addition to the shape changes between habitats described for CO, shape was also modeled by the habitat regardless of the locality; i.e., there is one shape pattern for the intertidal habitat and another shape pattern exclusively for the subtidal habitat (Supplementary information 4). As expected, the remaining comparison did not change under this null model.
Analyzing the variance, there was a higher shape variation in localities of IN than SUB habitat (Table 2), except for CO where this difference was not detectable. Following the same pattern as the size, CA showed the greatest level of variation in IN habitat (Fig. 3b). Pairwise differences between SUB localities were less conspicuous: GN was the locality with the lowest variation, while CA and CO had a similar variation. The physical variables showed a strong correlation (IN-R2 = 0.859, SUB-R2 = 0.771 for the first dimension; see Table 3) and a similar pattern of association in both IN and SUB habitat: the fetch (loadings or singular vectors: IN: −0. 99, SUB: −0. 97) was the variable with the strongest effect on shape variation. The irradiance showed certain influence but modeled shape oppositely (IN: 0.13, SUB: 0.21).
The principal results of classificatory analyses are reported in Fig. 4. Except for sex, all previously known factors classified snails with high accuracy: the overall classification accuracy was 73.16% for sex, 89.34% for locality, 88.6% for site, and 95.59% for habitat. Only CO: IN in site classification was correctly classified to one level of a category (Supplementary information 5). On the other hand, objective analysis inferred that shape is classified into three clusters. In this sense, one cluster was composed of 98% of CO: IN, another cluster was composed of 81% of GN: SUB and two snails from CA: IN and GN: IN, and the remaining snails belonged to the last cluster. The third cluster was represented by rounded shells with wider apertures, while the second cluster was represented by shorter apertures and slender shells. Analyzing habitat shell shape differences, we found that the intertidal snails showed a globular morph with shorter spire and larger relative size of the shell aperture, while subtidal organisms showed an elongated morph, with a smaller aperture and a longer spire. Finally, discriminant analysis using this cluster as a classificatory variable showed an overall classificatory accuracy of 94.85%. In this case, one category was correctly classified by the discriminant analysis (the first commented).
Shell shape represented by the first two principal components (PC1 and PC2), which together explain 29.88% of the total phenotypic variation. Representation of different snails (circles, triangles and squares) come from model-based clustering analysis showing the better classification. The wireframes plots (inset right) and the computer tomography rendered shells (left) represent the consensus shape of each group from the cluster analysis. Abbreviations: Golfo Nuevo (GN), Bahía Camarones (CA), Comodoro Rivadavia (CO), intertidal (IN) and subtidal (SUB)
Discussion
The 3D geometric morphometric methods and computer tomography allowed us to describe, with high resolution, the shell size and shape variations in Trophon geversianus from different environments along central Atlantic Patagonia. In this sense, the site-dependence on shape variation makes any generalization difficult; we found higher shape differences between habitats and site. On the other hand, we found a clear pattern of size variation; in each locality, the bigger sizes were located in the subtidal. Finally, sex was the less influential factor for shape and size variation.
Gastropods present a wide phenotypic variation under a diverse spatial scale. For example, Johannesson (1986) found intraspecific differences in the shell thickness and apertural amplitude at a micro-environments level, while Vieira and Bueno (2019) found shell shape variation in an intermediate spatial scale. Ecomorphotypes in gastropods related to thermal tolerance, dehydration, and wave action were described even in smaller geographic scales. For instance, in north Patagonia rocky shore intertidal, a spatial segregation related to differences in desiccation tolerance was found in populations of the false limpet Siphonaria lesonii (Livore et al. 2018). Likewise, two ecomorphotypes of Trophon geversianus related to dehydration, wave pressure, and predation were described in the same area: one for the intertidal level and the other for the subtidal level (Márquez et al. 2015). In the present work, the habitat (IN-SUB) was the most determining classificatory variable for shape, which resulted in the best average classification accuracy and a clear differentiation, regardless of locality. In other words, habitat acts as a modeler of shape in the same way for each locality. Additionally, CO snails showed a locality-dependence on shape differences between habitats. In summary, the assumption of only two ecomorphotypes along a latitudinal range underestimates the real complex patterns of T. geversianus form.
Analyzing all the shell shape information, we found more variation and differences between localities in intertidal samples than subtidal. Some works pointed out that clustering analysis of shape captured intrinsic variation corresponding to genetic factors (such as sex or genus) (Vaux et al. 2017; Vrdoljak et al. 2019). Here, the complexity of the shell shape information required three clusters. Hence, the most conspicuous classification criterion was habitat, even more important than intrinsic factors such as sex or the artificial factor from the cluster analysis. Our results indicate that in T. geversianus, extrinsic factors are more decisive in modeling the shape variation than intrinsic ones. Considering that stressful environments can facilitate a developmental expression of cryptic genetic variation (Badyaev 2005), the fluctuating conditions of the intertidal environment could be the trigger of the great variation found in the shape (Stearns 2000; Pöhlmann et al. 2011). Nevertheless, this type of interpretation is still under debate, since changing environments might result in morphological stasis due to the energetic costs associated with stress tolerance (Parsons 1994). Our results suggest that the extreme environmental conditions that snails are exposed to in the Patagonian intertidal promote shape variation.
As we reported, the environmental variables, their interactions, and their intensity along the latitudinal gradient modulated size and shape variation in gastropods (Guerra-Varela et al. 2009; Hollander and Butlin 2010). We found that size variation is influenced by physical variables just in intertidal samples, particularly by salinity, air temperature, and fetch. In marine gastropods, Melatunan et al. (2013) found that an increase in temperature advantage the selection of smaller sizes, which can be more thermoregulatory effective. Our results agree: we found the lower size in GN intertidal, the place with the higher air and sea temperature. Moreover, we found the bigger intertidal snails in the locality with the highest fetch: CO. As a possible explanation of this phenomenon, Vieira and Bueno (2019) described that fetch stimulates the development of higher apertural sizes and, consequently, the total shell size. Finally, we found the predicted Bergmann's rule tendency in the intertidal. Malvé et al. (2016) pointed out an inverse correlation between size and pH along latitudinal localities on T. geversianus. In contrast, we found evidence that the pH model size variation only for intertidal samples, mainly associated with salinity, dissolved carbon, and, to a lesser extent, sea temperature. In this sense, the low magnitude in which those physical variables change along the latitudinal gradient could be potentiated by the increased concentration in the intertidal due to the desiccation, which might explain why those variables become more relevant in intertidal than subtidal habitats for size variation. Also, some authors claimed that wave force is one of the main factors determining intertidal organisms’ size (Vieira and Bueno 2019) and, in our study, size increased with fetch. Nevertheless, there is no such trend in subtidal samples, where the higher sizes were recorded in the northern locality GN, with lesser fetch. Overall, we highlighted the relevance of both macro and micro-scale environments, and the different dependence on size variation from physical variables. In this sense, we strongly recommend future studies to determine the micro-scale origin of the snails collected at each site.
The physical variables studied contribute in different ways to the shell shape variation in T. geversianus, where the most influential was the fetch. Johannesson et al. (1986) found that wave action generates a dislodgment effect on the intertidal and, in consequence, a bigger muscular foot in the gastropod Littorina saxatilis. Besides, in a recent study performed by Vieira and Bueno (2019), the authors described that fetch caused wide aperture and more conical shells that may be an effective strategy to the dislodgement effects in Lottia subrugosa. We found wider apertures in the intertidal shells as in previous studies (Márquez et al. 2015), particularly in the intertidal with the higher fetch, CA and CO, as a possible response to the dislodgement effect. Our results suggest that these physical variables mainly influence the shapes of snails from intertidal habitats.
Phenotypic variation among populations distributed along different environments could produce fixed ecotypes or even species (West-Eberhard 2003). In the present study, we found clear differences in shape between intertidal of CO with the other two intertidal localities. Nevertheless, the same comparisons did not show appreciable differences in subtidal habitat. The remaining comparison expresses an inverse pattern: the shell shapes of northern localities (GN-CA) were different in subtidal but this difference was not appreciable in intertidal habitat. Once again, we emphasize the complex pattern of the shell shape variation that is not generalizable to the effect of simple variables, such as latitude and habitat. Moreover, some authors explained this complexity as follows: in intertidal habitats, invertebrates are exposed to a wide range of physical factors during the tides (Dayton 1971; Denny and Wethey 2001), while the subtidal present more similar physical conditions but harder predation forces (Bertness et al. 2006; Rechimont et al. 2013). In the light of the evidence presented here, in future latitudinal works, we strongly encourage including ambiental complexity, such as micro-scale, to study the shape variation.
Previous studies carried out on T. geversianus in the same zone differ in the conclusions with the current study: They described large shape patterns, one belonging to the Argentinean and another to the Magellan biogeographic provinces, (Malvé et al. 2018), and rejected ecogeographic rules using habitat-pooled samples (Malvé et al. 2016). In contrast, we did not identify a latitudinal pattern in the shell shape of both intertidal and subtidal samples and disagree with not considering the sample’s habitat-origin. Furthermore, we highlighted the site-dependence on the shape as a key factor, neither latitude nor habitat separately.
Some authors explained the latitudinal trend in size by developmental plasticity (Van Voorhies 1996) or as an adaptive result (Partridge and Coyne 1997). However, we found a complex latitudinal pattern that caused size changes. An adaptationist hypothesis of the size latitudinal-trend would expect the same changes in both habitats since there are no genetic differences between intertidal and subtidal snails (Márquez et al. 2015). Also, the shape differences between habitats without genetic differences support the plasticity hypothesis (West Eberhard 2003). However, this is the second work in T. geversianus where the relationship between habitat and morphological traits is explored. In this sense, a sharp conclusion about the evolutionary processes requires more evidence about genetic pools in each site as well as including more sample sites. Future studies have to focus on covering biotic pressures to continue unraveling the causes of phenotypic variation (Takada and Rolán-Alvarez 2000; Templeton 1981).
Our main question about the most influential scale in the shell shape variation of T. geversianus has an ambiguous answer: we found that the principal shape classification was the habitat (even higher than an artificial cluster variable based on the shape) and differences between habitats were independent of the locality. The importance of the habitat as a modeler of the shape is enhanced considering the closeness between the habitats (~ 0.3 km) and the remoteness of the localities (~ 400 km). However, the site and locality strongly influenced the shell shape variation. In this sense, we could not say that habitat ecomorphotypes are more distinguishable than site ecomorphotypes, highlighting the site-dependence on the shell shape variation in T. geversianus. In conclusion, we emphasize the importance of having prior information on the sample’s origin. If analyses are carried out leaving out important (and accessible) information, then a spurious classification may be obtained.
Code availability
R script for model construction is available at https://doi.org/10.6084/m9.figshare.14474241.v1
Data availability
The data underlying this work are available at https://doi.org/10.6084/m9.figshare.14474241.v1
References
Adams DC, Collyer ML, Kaliontzopoulou A, Sherratt E (2020) Geomorph: Software for geometric morphometric analyses. R package version 3.0.5. https://cran.r-project.org/package=geomorph. Accessed 20 July 2020
Adams DC, Rohlf FJ, Slice DE (2013) A field comes of age: geometric morphometrics in the 21st century. Hystrix 24(1):7
Badyaev AV (2005) Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc Royal Soc B Biol Sci 272:877–886
Bergmann C (1848) Über die verhältnisse der wärmeökonomie der thiere zu ihrer grösse. Göttingen Studien
Bertness MD, Crain CM, Silliman BR, Bazterrica MC, Reyna MV, Hidalgo F, Farina J (2006) The community structure of western Atlantic patagonian rocky shores. Ecol Monogr 76:439–460
Bookstein FL (1991) Morphometric tools for landmark data. Cambridge/New York/Port Chester. Melbourne, Sydney, Cambridge University Press
Bookstein F, Schafer K, Prossinger H, Seidler H, Fieder M, Stringer C, Weber GW, Arsuaga JL, Slice DE, Rohlf FG, Recheis W, Mariam AJ, Marcus LF (1999) Comparing frontal cranial profiles in archaic and modern homo by morphometric analysis. Ana Rec (new Anat) 257:217–224
Bourdeau PE, Butlin RK, Brönmark C, Edgell TC, Hoverman JT, Hollander J (2015) What can aquatic gastropods tell us about phenotypic plasticity? A review and meta-analysis. Heredity 115:312–321
Burrows MT, Harvey R, Robb L (2008) Wave exposure indices from digital coastlines and the prediction of rocky shore community structure. Mar Ecol-Prog Ser 353:1–12
Collyer ML, Sekora DJ, Adams DC (2015) A method for analysis of phenotypic change for phenotypes described by high-dimensional data. Heredity 115:357–365
Conde-Padín P, Grahame JW, Rolán-Alvarez E (2007) Detecting shape differences in species of the Littorina saxatilis complex by morphometric analysis. J Mollus Stud 73:147–154
Crespi-Abril AC, Soria G, De Cian A, López-Moreno C (2018) Roaring forties: an analysis of a decadal series of data of dust in Northern Patagonia. Atmos Environ 177:111–119
Crothers JH (1975) On variation in Nucella lapillus (L.): shell shape in populations from the south coast of England. J Mollus Stud 41:489–498
Cumplido M, Averbuj A, Bigatti G (2010) Reproductive seasonality and oviposition induction in Trophon geversianus (Gastropoda: Muricidae) from Golfo Nuevo, Argentina. J Shellfish Res 29:423–428
Cumplido M, Bigatti G (2020) Maturity assessment for the Implementation of the first fishery regulation in Patagonian marine gastropods. Malacologia 63(1):139–147
Dayton PK (1971) Competition, disturbance, and community organization: the provision and subsequent utilization of space in a rocky intertidal community. Ecol Monogr 41:351–389
Denny MW, Wethey DS (2001) Physical processes that generate patterns in marine communities. Marine Community Ecology. Sinauer Associates Inc., Sunderland, pp 3–37
Efron B, Gong G (1983) A leisurely look at the bootstrap, the jackknife, and cross-validation. Amer Statist 37:36–48
Fox J, Weisberg S (2019) An R companion to applied regression (Third). R package version 1.4.6. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. Accessed 20 July 2020
Fraley C, Raftery AE (2002) Model-based clustering, discriminant analysis, and density estimation. J Ame Stat Assoc 97:611–631
Goodall C (1991) Procrustes methods in the statistical analysis of shape. J R Stat Soc Ser B-Stat Methodol 53:285–321
Gower JC (1975) Generalized procrustes analysis. Psychometrika 40:33–51
Grahame JW, Wilding CS, Butlin RK (2006) Adaptation to a steep environmental gradient and an associated barrier to gene exchange in Littorina saxatilis. Evolution 60:268–278
Guerra-Varela J, Colson I, Backeljau T, Breugelmans K, Hughes RN, Rolán-Alvarez E (2009) The evolutionary mechanism maintaining shell shape and molecular differentiation between two ecotypes of the dogwhelk Nucella lapillus. Evol Ecol 23:261–280
Harborne AR, Mumby PJ, ZŻychaluk K, Hedley JD, Blackwell PG, (2006) Modeling the beta diversity of coral reefs. Ecology 87:2871–2881
Harting F (2020) DHARMa: residual diagnostics for hierarchical (Multi-Level / Mixed) regression models. R package version 0.3.1. https://CRAN.R-project.org/package=DHARMa. Accessed 20 July 2020
Hollander J, Butlin RK (2010) The adaptive value of phenotypic plasticity in two ecotypes of a marine gastropod. BMC Evol Biol 10:333
Irie T (2005) Geographical variation of shell morphology in Cypraea annulus (Gastropoda: Cypraeidae). J Mollus Stud 72:31–38
Jablonka E, Lamb M (1995) Epigenetic inheritance and evolution: the Lamarckian dimension. Oxford University Press, Oxford New York
Johannesson B (1986) Shell morphology of Littorina saxatilis Olivi: the relative importance of physical factors and predation. J Exp Mar Biol Ecol 102:183–195
Johannesson B (1996) Population differences in behaviour and morphology in the snail Littorina saxatilis: phenotypic plasticity or genetic differentiation? J Zool 240:475–493
Klingenberg CP (2008) MorphoJ. University of Manchester, UK, Faculty of Life Sciences
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Res 11:353–357
Labraga JC (1994) Extreme winds in the Pampa del Castillo Plateau, Patagonia, Argentina, with reference to wind farm settlement. J Appl Meteorol 33:85–95
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2020) Estimated marginal means, aka least-squares means. R package version 1.4.6. https://CRAN.R-project.org/package=emmeans. Accessed 20 July 2020
Levin LA (2006) Recent progress in understanding larval dispersal: new directions and digressions. Integr Comp Biol 46:282–297
Livore JP, Mendez MM, Bigatti G, Márquez F (2018) Habitat-modulated shell shape and spatial segregation in a Patagonian false limpet (Siphonaria lessonii). Mar Ecol Prog Ser 606:55–63
Malvé ME, Gordillo S, Rivadeneira MM (2016) Connecting pH with body size in the marine gastropod Trophon geversianus in a latitudinal gradient along the south-western Atlantic coast. J Mar Biolog Assoc UK 98:449–456
Malvé ME, Rivadeneira MM, Gordillo S (2018) Biogeographic shell shape variation in Trophon geversianus (gastropoda: Muricidae) along the southwestern Atlantic coast. Palaios 33:498–507
Márquez F, Nieto Vilela RA, Lozada M, Bigatti G (2015) Morphological and behavioral differences in the gastropod Trophon geversianus associated to distinct environmental conditions, as revealed by a multidisciplinary approach. J Sea Res 95:239–247
Mayr E (1956) Geographical character gradients and climatic adaptation. Evolution 10:105–108
Melatunan S, Calosi P, Rundle SD, Widdicombe S, Moody AJ (2013) Effects of ocean acidification and elevated temperature on shell plasticity and its energetic basis in an intertidal gastropod. Mar Ecol Prog Ser 472:155–168
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Okoshi K (1996) Biomineralization in molluscan aquaculture growth and disease. Bull Ins Océanogra 14:151–169
Palumbi SR (2004) Marine reserves and ocean neighborhoods: the spatial scale of marine populations and their management. Annu Rev Environ Resour 29:31–68
Pallas PS (1776) Specilegia Zoologica, quibus novae imprimis et obscurae animalium species iconibus, descriptionibus atque commentariis illustrantur, Berolini, pp 15
Parsons PA (1994) Morphological stasis: an energetic and ecological perspective incorporating stress. J Theor Biol 171:409–414
Partridge L, Coyne JA (1997) Bergmann’s rule in ectotherms: is it adaptive? Evolution 51:632–635
Paruelo JM, Beltrán A, Jobbagy E, Sala OE, Golluscio RA (1998) The climate of Patagonia: general patterns and controls on biotic processes. Ecol Austral 8:85–101
Pastorino G (1994) Moluscos costeros recientes de Puerto Pirámide, Chubut, Argentina. Academia Nacional de Ciencias, Córdoba
Pastorino G (2005) A revision of the genus Trophon montfort, 1810 (Gastropoda: Muricidae) from southern south America. Nautilus 119:55–82
Pfenning DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP (2010) Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol 25:459–467
Piersma T, Van Gils JA (2011) The flexible phenotype: a body-centred integration of ecology, physiology and behaviour. Oxford University Press, New York
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core T (2020) Linear and nonlinear mixed effects models. R package version 3.1–147. https://CRAN.R-project.org/package=nlme. Accessed 20 July 2020
Pöhlmann K, Koenigstein S, Alter K, Abele D, Held C (2011) Heat-shock response and antioxidant defense during air exposure in Patagonian shallow-water limpets from different climatic habitats. Cell Stress Chaper 16:621–632
Raffaelli D, Hawkins SJ (2012) Intertidal ecology. Springer Science & Business Media, London
Rechimont ME, Galván D, Sueiro MC, Casas G, Piriz ML, Diez ME, Primost M, Zabala MS, Márquez F, Brogger M, Fernández Alfaya JE, Bigatti G (2013) Benthic diversity and community structure of a north patagonian rocky shore. J Mar Biolog Assoc UK 93:2049–2058
Rice SH (2012) The place of development in mathematical evolutionary theory. J Exp Zool Part B 318:480–488
Rivas AL, Beier EJ (1989) Temperature and salinity fields in the north patagonian gulfs. Oceanol Acta 13:15–20
Rivas AL (1990) Heat-balance and annual variation of mean temperature in the north-patagonian gulfs. Oceanol Acta 13:265–272
Rohlf FJ, Corti M (2000) Use of two-block partial least-squares to study covariation in shape. Syst Biol 49:740–753
Rohlf FJ, Slice DE (1990) Extensions of the Procrustes Method for the optimal superimposition of landmarks. Syst Biol 39:40–59
Sánchez R, Sepúlveda R, Brante A, Cárdenas L (2011) Spatial pattern of genetic and morphological diversity in the direct developer Acanthina monodon (Gastropoda: Mollusca). Mar Ecol Prog Ser 434:121–131
Sanford E, Kelly MW (2011) Local adaptation in marine invertebrates. Annu Rev Mar Sci 3:509–535
Scrucca L, Fop M, Murphy TB, Raftery AE (2016) mclust 5: clustering, classification and density estimation using Gaussian finite mixture models. R j 8:289
Schillizzi R, Spagnuolo J, Luna L (2014) Morfología de la costa atlántica entre Punta Ninfas y Cabo Dos Bahías, Chubut, Argentina. Revista Mus La Plata 14:1–15
Slice D, Bookstein F, Marcus L, Rohlf F (1996) Advances in morphometrics.In: Marcus LF, Corti M, Loy A, Naylor GJP, Slice D (Eds) A glossary for geometric morphometries, vol 284. pp 531–551
Stearns SC (2000) Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87:476–486
Takada Y, Rolán-Alvarez E (2000) Assortative mating between phenotypes of the intertidal snail Littorina brevicula: a putative case of incipient speciation? Ophelia 52:1–8
Team RC (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/
Templeton AR (1981) Mechanisms of speciation-a population genetic approach. Annu Rev Ecol Syst 12:23–48
Trussell GC, Etter RJ (2001) Integrating genetic and environmental forces that shape the evolution of geographic variation in a marine snail Microevolution rate, pattern, process. Springer, New York, pp 321–337
Van Voorhies WA (1996) Bergmann size clines: a simple explanation for their occurrence in ectotherms. Evolution 50:1259–1264
Vaux F, Crampton JS, Marshall BA, Trewick SA, Morgan-Richards M (2017) Geometric morphometric analysis reveals that the shells of male and female siphon whelks Penion chathamensis are the same size and shape. Molluscan Res 37:194–201
Vermeij GJ (1972) Intraspecific shore-level size gradients in intertidal molluscs. Ecology 53:693–700
Vermeij GJ, Currey JD (1980) Geographical variation in the strength of thaidid snail shells. Biol Bull 158:383–389
Vieira EA, Bueno M (2019) Small spatial scale effects of wave action exposure on morphological traits of the limpet Lottia subrugosa. J Mar Biolog Assoc UK 99:1309–1315
Vrdoljak J, Padró J, De Panis D, Soto IM, Carreira VP (2019) Protein–alkaloid interaction in larval diet affects fitness in cactophilic Drosophila (Diptera: Drosophilidae). Biol J Linnean Soc 127:44–55
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, New York
Wiley DF, Amenta N, Alcantara DA, Ghosh D, Kil YJ, Delson E, Harcourt-Smith W, Rohlf FJ, St John K, Hamann B (2005) Evolutionary morphing VIS 05 IEEE visualization. IEEE, pp 431–438
Zar JH (1999) Biostatistical analysis. Pearson Education, India
Zelditch ML, Swiderski DL, Sheets HD (2012) Geometric morphometrics for biologists: a primer. Academic press, Cambridge
Acknowledgements
We thank Julio Rúa, Nestor Ortiz and Ricardo Vera for field logistics. We also thank Pablo Spagnolatti for helping in 3D visualization. We are very grateful to Lic. Julia Ratowiecki and MSc. Sebastian Srugo for English revisions, which help us to improve the manuscript. This work was part of the doctoral thesis of RANV at the Universidad Nacional de la Patagonia San Juan Bosco. This is the publication 156 of the Laboratorio de Reproducción y Biología Integrativa de Invertebrados Marinos (LARBIM). We specially thank two anonymous reviewers for their valuable suggestions, which enriched the manuscript.
Funding
This study was partially funded by ANPCyT-FONCyT (PICT 2018–0969 to GB, PICT 2017–1750 to SG and PICT 2015–3696 and-2018–03197 to FM). RANV is a postdoctoral fellow, JV a doctoral fellow of CONICET; FM, GB and SG are members of the Research Career (CONICET).
Author information
Authors and Affiliations
Contributions
RANV, JV, FM, SG, and GB contributed to the study conception and design. Data collection was performed by RANV, FM and GB, and data analysis was performed by RANV and JV. The first draft of the manuscript was written by RANV and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nieto-Vilela, R.A., Vrdoljak, J., Giulianelli, S. et al. Geometric morphometrics reveal complex shape variation patterns at different geographic scales in the patagonian gastropod Trophon geversianus. Evol Ecol 35, 705–721 (2021). https://doi.org/10.1007/s10682-021-10125-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-021-10125-w