Abstract
Rensch's rule is an allometric rule on sexual size dimorphism. It states that in small-sized species, females are larger than males, whereas in larger species, males are relatively larger than females. Several studies have explored this pattern, and its inverse in lizard species. China has a unique and high diversity of species, with a variety of ecological systems which shape diversity of phenotypes. In this study, sexual size dimorphism and Rensch’s rule were determined using a dataset of Chinese lizard species. The findings show that Chinese lizards generally exhibit female-biased sexual size dimorphism. In addition, clutch size was positively correlated with sexual size dimorphism. Agamidae species were the only taxa that followed Rensch’s rule (slope of males against females was steeper than 1). Clutch size was correlated with sexual size dimorphism in groups that do not follow Rensch's rule. This finding implies that strong fecundity selection limits application of Rensch's rule in these groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most animals exhibit anatomical differences in body size based on sex. These differences define sexual size dimorphism (SSD). Nature of SSD changes are consistent with changes in body size of species that exhibit an allometric trend to Rensch’s rule (Rensch 1950, 1959; Fairbairn 1997). Rensch’s rule states that in small-sized species, females are larger than males, whereas in large-sized species, males are larger than females (Rensch 1950, 1959). Over evolutionary time, male body sizes show greater variation compared with female body sizes, regardless of which sex is larger (Fairbairn 1997). Although Rensch’s rule may be less prevalent (e.g. snakes: Burbrink and Futterman 2019; lizards: Valdecantos, et al. 2019; salamanders: Peñalver-Alcázar et al. 2019), it defines most taxa, including insects (Blanckenhorn et al. 2007a,b), mammals (Lindenfors et al. 2007), and reptiles (Cox et al. 2007).
Several hypotheses are used to explain patterns that are consistent with Rensch’s rule, isometry (monomorphism from small-bodied to large-bodied species) and its inverse (De Lisle and Rowe 2013; Burbrink and Futterman 2019). One of these hypotheses is based on sexual selection. For example, mating success in males is enhanced by larger body sizes that are advantageous when encountering rivals and in defending territory (Trivers 1972; Cox et al. 2003; Wikelski and Romero 2003). On the contrary, sexual selection favors smaller male body sizes to increase mobility or agility (Zamudio 1998; Szekely et al. 2004; Grossi et al. 2016). Differential plasticity hypothesis proposes sex-specific plastic responses to environmental factors (Fairbairn 2005). In this hypothesis, SSD follows a pattern that is consistent with Rensch's rule when males exhibit greater phenotypic plasticity compared with females, especially in reptiles. For example, male geckos exhibit larger temperature-induced phenotypic plasticity in final body sizes compared with females, therefore, they follow Rensch’s rule (Starostova et al. 2010). Fecundity selection on the other hand favors larger females due to their reproductive characteristics (Cox et al. 2003) resulting in isometry or a pattern which is reverse to Rensch’s rule.
Rensch's rule describes co-evolution within defined female and male body sizes. It proposes that adult female and male body sizes are congenitally established (Teder and Tammaru 2005). However, previous studies report that some related subpopulations exhibit a positive allometric pattern in body size (Pyron et al. 2007; Lengkeek et al. 2008). The overall pattern of Rensch’s rule is not established, especially in lineages of taxa where both male- and female-biased SSD can be observed at the same time (mixed SSD). Furthermore, Webb and Freckleton (2007) report that within taxa that display mixed SSD species, female-biased SSD are smaller compared with male-biased SSD. Moreover, studies report that Rensch’s rule may not apply in lineages where females are the larger sex (De Lisle and Rowe 2013; Burbrink and Futterman 2019). These contradicting findings imply that there is need for further studies to validate ability of Rensch’s rule to distinguish between lineages with female- and male-biased SSD. In addition, more studies should be carried out to provide information on application of Rensch’s rule (Webb and Freckleton 2007; Colleoni et al. 2014).
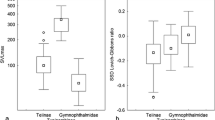
Lizards exhibit a wide array of SSD (Cox et al. 2003, 2007). SSD studies on lizard groups report contrasting results (Stuart-Fox 2009; Frýdlová and Frynta 2015). SSD can vary geographically (e.g. more female-biased in cold area geographically, Tarr et al. 2019), however, in most lizard species, males are predominantly larger in size compared with females (Cox et al. 2003). Some lizard lineages such as Gymnophthalmidae, Teiidae (Frýdlová and Frynta 2015), Eublepharidae (Gekkota; Kratochvíl and Frynta 2002), Varanidae (monitor lizards, Starostova et al., 2010) and Phrynosomatidae (Sceloporus lizards, Jiménez-Arcos et al. 2017) follow Rensch’s rule. However, Rensch’s rule can be expressed differently within some families of different lizard groups. For example, Liolaemus and Phymaturus both belong to Liolaemidae, however, the former genus follows Rensch’s rule (slope > 1, Pincheira-Donoso and Tregenza 2011), whereas Phymaturus genus does not follow Rensch’s rule (slope = 1, Valdecantos et al. 2019).
The geographical area of China provides a good environment for studies on evolution of lizard species in relationship to SSD patterns within a phylogenetic comparative framework. China has a large geographical region contiguous with other geographical regions, and with diverse species (212 species belong to 10 families, Zhou et al. 2019; Wang et al. 2020a) and a variety of ecological strategies, therefore it can shape the diversity of phenotypes. However, detailed comparative phylogenetic studies on lizard SSD and Rensch's rule using bigger sample sizes have not been previously carried out in China. Previous studies only report on Rensch’s rule at intraspecific level (Liang and Shi, 2017 and Zhao et al. 2016). In this study, Rensch’s rule was used to determine relative effects of sexual selection and fecundity on macro-evolution of SSD.
Materials and methods
Data collection
Biometric data were compiled by measuring snout-vent length (SVL) of Chinese lizard specimens obtained from Xinjiang Agricultural University (13 species, Appendix S1). Measurements were performed using a Vernier caliper calibrated to 0.01 mm (see Appendix S1). SVL was used to measure body sizes in reptiles as a proxy for overall structural size (Meiri 2010). In addition, data on SVL and clutch size (CS) for different lizard species were retrieved from literature (published and unpublished) (Appendix S1).
Notably, SVL has limitations when used in large-scale comparative studies (Meiri 2010). For example, in lizards, SVL does not accurately reflect variations in body shape (Pincheira-Donoso et al. 2011). Body mass (BM) is, therefore, more reliable for comparative analyses. Although it is not as popular as SVL, mass can be directly compared across all clades in macroecological studies (Meiri 2010; Feldman et al. 2016; Regis and Meik 2017). In this study, SVL measurements were converted to BM using 9 family-specific allometric equations (Feldman et al. 2016; Meiri, 2018). Therefore, SVL results are similar to BM results in this study (see Appendix S2 for SVL results).
Clutch size (CS) was used as a measure of fecundity per reproductive episode (transient fecundity) (Pincheira-Donoso and Hunt 2017). This trait is mainly used when referring to fecundity selection (Cox et al. 2003; Pincheira-Donoso and Tregenza 2011), which is available for most Chinese lizard species.
A dataset of 176 Chinese lizard species (approximately 83% of extant Chinese lizard species (Wang et al. 2020a), belonging to 9 families (Agamidae, Anguidae, Dibamidae, Eublepharidae, Gekkonidae, Lacertidae, Scincidae, Shinisauridae, and Sphaerodactylidae) were classified into four major groups: Agamidae, Lacertidae, Scincidae, and Gekkota (including Eublepharidae, Gekkonidae, Sphaerodactylidae) was included in this study (Appendix S1). Anguidae, Dibamidae and Shinisauridae species had small sample sizes (n ≤ 2), therefore, they were excluded in analyses. The study was carried out following taxonomy of the checklists of Chinese reptiles (Wang et al. 2020a) and August, 2019 Reptile database (http://www.reptile-database.org/, Uetz et al. 2019).
Sexual dimorphism index was used to characterize SSD using the formula presented below (Lovich and Gibbons 1992; Smith 1999):
Dimorphism is arbitrarily defined as positive when females are the larger sex and negative when males are the larger sex. This index has been used in previous reptile studies (Cox et al. 2003, 2007). Data for individuals within species were not available, therefore, we arbitrarily regarded differences of less than 10% (−0.1 < SSD < 0.1) for each species as monomorphism (Table 1).
Phylogenetic relationships
A considerable portion of sexual dimorphism can be introduced by phylogenetic constraints and inertia. Phylogenetic history should, therefore, be taken into account in sexual dimorphism analyses (Cheverud et al. 1986; Smith and Cheverud 2002). Evolutionary relationships among studied species were determined as reported by Tonini et al. (2016). Species which had no morphological data were removed from the tree (Fig. 1). A total of 147 species were used to account for phylogenetic constraints on sexual dimorphism (Tonini et al. 2016). Maximum likelihood values of λ, which represents strength of the phylogenetic signal (Revell 2012) were used to account for phylogenetic non-independence.
Statistical analysis
T-tests were performed for each of the four major groups to test differences between SSD and zero, for a given clade. Cases which showed no difference between SSD and zero, were regarded as monomorphism.
Effect of clutch size on sexual size dimorphism (SSD) was determined by modelling SSD as the response variable whereas clutch size and female body mass were modelled as predictor variables using phylogenetic generalized least square (PGLS) models (Orme et al. 2012). PGLS models were run separately for the following groups; i. all lizard species, ii. male-biased dimorphic species, and iii. female-biased dimorphic species.
Log-10 male masses versus log-10 female masses were plotted for all the species and, separately for the four groups (the four groups accounted for 98% of the lizards in our dataset, see Table 1). Rensch’s rule is manifested when the slope of regressions was greater than 1. When regression slopes are significantly less than 1, SSD is consistent with the inverse of Rensch's rule. When regression slopes are not significantly different from 1, SSD evolves in overall isometry with body size.
Reduced major axis (RMA) regression slope is the ratio of size variances (Price and Phillimore 2007; Smith 2009). This slope is appropriate for testing Rensch’s rule compared with ordinary least square (OLS) regression slope (Abouheif and Fairbairn 1997), as there is variance in both female and male sizes. However, RMA is unable to fix this kind of measurement error (see Smith 2009; Kilmer and Rodríguez 2017). Therefore, the method correct for testing of Rensch’s rule between OLS or RMA is unknown. Meiri and Liang (2021) reports that OLS and RMA regression should be used when testing Rensch’s rule, because RMA is less conservative.
In this study, Rensch’s rule was also tested by phylogenetic-informed analyses using phylogenetic reduced major axis (PRMA) regression and phylogenetic generalized least square (PGLS) models (Orme et al. 2012). Both RMA and OLS, referred in this study as non-phylogenetical analyses, were used to test Rensch’s rule. Therefore, they enabled incorporation of the 29 species missing from the phylogenetic tree. However, OLS slope is always shallower than RMA slope (Ludbrook 2010, and this study), and results of OLS are similar to RMA results in this study, therefore, we only discuss OLS regression results (for RMA results, see Appendix S2).
Non-phylogenetic (RMA, OLS) and phylogenetic-informed (PRMA, PGLS) analyses using only male-biased (SSD < −0.10) and only female-biased (SSD > 0.10) sexual dimorphic species were repeated to test Rensch’s rule in lizards. Small sample size was a limitation during analysis using only male- or female-biased sexual dimorphic species in the four major groups.
Statistical analyses were performed using R (3.6.1, R Core Team 2017). Graphs in this study were generated using “ggtree” (version 2.4.1, Yu et al. 2017) and “treeio” (version 1.14.3, Yu 2019) packages. RMA was performed using smart package (sma function) (version 3.4-8, Warton et al. 2012). PRMA was performed using phytools package (phyl.RMA function, version 0.7-20, Revell 2012). PGLS was performed using caper package (version 1.0.1, Orme et al. 2012).
Results
SSD
Sexual size dimorphism data for 176 species were included in this study. Approximately 25–63 species were recorded for the four groups. Sexual dimorphism indices varied significantly, from a strong male bias of − 1.93 in Pseudocalotes brevipes to a female bias of 1.42 in Goniurosaurus hainanensis (Figs. 1, 2). 30% of the 176 species showed male-biased dimorphism (16–42% in the four groups) whereas female-biased dimorphism was observed in 38% (29–55% in the four groups; Table 1). A total of 56 species (32%) exhibited monomorphism. All four major clades exhibited broad ranges of sexual size dimorphism. Female-biased dimorphism was observed in Gekkota and Scincidae species (43% and 56% respectively), whereas Laterata was monomorphic (48%, Table 1 and Fig. 2). Chinese lizards exhibited monomorphism (Lizards as whole, mean: 0.01 ± 0.03, t = 0.27, p = 0.788). Among the four major clades, three clades showed monomorphism (Agamidae, mean: −0.09 ± 0.06, t = -1.49, p = 0.138; Gekkota, mean: 0.04 ± 0.05, t = 0.88, p = 0.383; Lacertidae, mean: 0.05 ± 0.04, t = 1.26, p = 0.222), whereas Scincidae showed female-biased dimorphism (mean: 0.15 ± 0.06, t = 2.68, p = 0.012).
Distribution of sexual size dimorphism for 176 species (4 major groups). Positive values represent female-biased dimorphism whereas negative values represent male-biased dimorphism. The black dashed line represents zero sexual dimorphism. SDI values between − 0.10 and 0.10 in body mass are indicated by straight lines. Phylogenetic relationships are based on Tonini et al. (2016). Four major groups represented 98% of the sample size in this study. Four representative species were selected randomly within each group; Gekkota: Mediodactylus russowii, Scincomorpha: Plestiodon chinensi; Lacertidae: Eremias arguta, Acrodontia: Phrynocephalus mystaceus, respectively (Photos: Tao Liang)
Fecundity selection
Clutch size was significantly correlated with SSD (p < 0.05, but marginally correlated in male-biased group: p = 0.059). SSD increased with increase in CS in lizards after controlling for female body size consistent with the hypothesis of fecundity selection. Therefore, our phylogenetically-informed analyses imply that processes resulting in evolutionary variations in female body size is linked to evolutionary variations in litter size (Table 2).
Rensch’s rule
PGLS regression analysis showed that male size against female size regression slope was significantly steeper than 1 (PGLS: slope = 1.05 ± 0.02, P(slope=1) < 0.05). This implies that the variance in male body sizes was larger compared with the variance of female body sizes. This reflects allometric relationship between the sexes that is in accordance with Rensch’s rule. Agamidae species was the only species among the four major groups that exhibited Rensch’s rule (PGLS: slope = 1.10 ± 0.05, p(slope=1) > 0.05). Gekkota (PGLS: slope = 0.98 ± 0.04), Lacertidae (PGLS: slope = 1.02 ± 0.04) and Scincidae (PGLS: slope = 1.01 ± 0.05) contradicted Rensch’s rule (P (slope=1) > 0.05 in all cases, Fig. 3 and Appendix S2). Moreover, Rensch’s rule was not applicable in either male- or female biased dimorphic taxa (P (slope=1) > 0.05) (Appendix S2). Non-phylogenetic and phylogenetic analyses (phylogenetic signals were weak) revealed similar results (See Fig. 3 and Appendix S2).
Phylogenetic major axis and ordinary least square regressions of males on females for all lizards (a) and four major taxa (b Agamidae, c Gekkota, d Lacertidae, e Scincidae). Grey dotted lines represent isometric relationships with a zero intercept. Blue lines represent ordinary least square (OLS) slopes
Discussion
Studies report that lizards mainly exhibit male-biased sexual size dimorphism (Cox et al. 2003, 2007). However, our results show that Chinese lizards exhibits a monomorphic and marginally female-biased SSD. Chinese lizards generally follow Rensch's rule as a whole, implying a great variability in male and female body sizes. However, the patterns were inconsistent within the four major groups. Agamidae species was the only species that was consistent with Rensch’s rule.
SSD in Chinese lizards is female-biased
Sexual selection and fecundity selection are the most important hypotheses in explaining sexual dimorphism (Abouheif and Fairbairn 1997; Aleksic et al. 2009; Cox et al. 2003; Liang et al. 2015; Liang and Shi 2017; Wan et al. 2018). In lizards, sexual selection plays a key role in shaping male-biased dimorphism (Cox et al. 2003, 2007). In this study, we observed that Agamidae species were male-biased. In Agamidae species, for example Ctenophorus ornatus and Trapelus sanguinolentus male-male combat is common in the wild environment (LeBas and Marshall 2000; personal observation). This may because larger body sizes are positively correlated with a successful outcome following aggressive encounters (Frýdlová and Frynta 2015; Lailvaux and Irschick 2007; Liang et al. 2018; Lovich and Gibbons 1992; Schuett 1997; Stuart-Fox 2009; Zucker and Murray 1996).
Fecundity selection stimulates increased body sizes in females so that they can hold more or larger eggs (Blanckenhorn 2005; Darwin 1871; Scharf and Meiri 2013), resulting in female-biased SSD. SSD results in this study were consistent with fecundity selection hypothesis, whereby female sizes were larger compared with male sizes in general (SDI = 0.01). This finding implies that fecundity selection contributes to female-biased dimorphism that exhibits a positive relationship between clutch (or litter) size and female size in lizards (Shine 1988; Cox et al. 2003; Pincheira-Donoso and Tregenza 2011). Several studies report that some lizard lineages do not exhibit a relationship between fecundity selection and female-biased SSD (Pincheira-Donoso and Tregenza 2011; Pincheira-Donoso and Hunt 2017). For example, previous studies report that CS and SSD in lineages are not significantly correlated with male-biased SSD (Cox et al. 2003; Pincheira-Donoso and Tregenza 2011; Pincheira-Donoso and Hunt 2017). This observation can be attributed to the fact that SSD varies based on geographical region (Tarr et al. 2019; Tsuji and Fukami 2020). However, the effect of geography on SSD across Chinese lizards, should be explored further.
Both sexual and fecundity selection involve different larger body parts rather than overall size alone. Head and axilla-groin length are the main determinants of body size, regardless of neck length. This phenomenon of different approaches to ‘being large’ in females (larger axilla-groin but smaller head to support more eggs/offspring, Darwin 1871; Scharf and Meiri 2013) and males (larger or wider head but smaller axilla-groin for increased bite force, Jerry et al. 2006; Verwaijen et al. 2002; Wang et al. 2020b) is common in lizards (Scharf and Meiri 2013). Studies report that males have larger heads in Chinese lizard populations whereas females have larger abdomens (Chen et al. 2015; Liang et al. 2018; Liao et al. 2013; Lin 2004; Wan et al. 2018). Therefore, both sexual and fecundity selection play a role in determining SSD in Chinese lizards. However, fecundity selection has a significant role compared with sexual selection.
Chinese lizards are infrequently consistent with Rensch’s rule
Rensch's rule is only observed in Agamids where males are always larger compared with females in large-bodied species (e.g. genus Laudakia and Calotes), whereas females are larger compared with males in small-bodied species (genus Phrynocephalus; Wang 2011; Wu et al. 2005). Sexual selection favors large-bodied males, especially in territorial species (Trivers 1972; Cox et al. 2003; Wikelski and Romero 2003). In small-bodied species, females are always larger than males (Rensch 1959; Webb and Freckleton 2007). This enhances production of relatively larger eggs in small females (negative egg size allometry, Kratochvíl and Frynta 2006; Meiri et al. 2015).
Rensch’s rule is not as prevalent as it is sometimes portrayed, especially in female-biased dimorphic species (Shine et al. 1998; Valdecantos et al. 2019; Burbrink and Futterman 2019). In lineages where females are larger than males, female sizes exhibit more variations compared with males. This is probably due to fecundity selection that promotes large female abdominal volumes (Darwin 1871; Scharf and Meiri 2013) that can hold a larger clutch size (or relatively large eggs). This explains why Rensch’s rule was not applicable in three of the four Chinese lizard clades included in this study. Inconsistency between geckos and Rensch’s rule as reported in this study has been reported previously by Cox et al. (2007). However, Kratochvíl and Frynta (2002) reported that geckos were consistent with Rensch’s rule. These differences are attributed to the fact that the lizard group in Kratochvíl and Frynta (2002) belongs to eublepharid geckos (Eublepharidae) that are different from those studied by Cox et al. (2007) and this study.
Male- and female-biased dimorphic species exhibited a size based isometric relationship, and a positive relationship between CS and SSD. This may because fecundity selection favors larger females to lay more eggs resulting in inconsistency with Rensch’s rule. Alternatively, this phenomenon can be explained by selection promoting larger sizes in separate male- and female-biased groups. When both male and female sizes are subjected to uniform selection pressures across species, they exhibit similar size variation. Therefore, Rensch’s rule does not need to be tested in separate male- and female-biased groups, because mixed-biased allometry (both male- and female-biased SSD exist) are important in SSD evolutionary studies because they provide the necessary data for comparative analysis of whether SSD is an adaptive trait or not (Abouheif and Fairbairn 1997; Fairbairn, 1997; Colwell 2000).
Phylogenetically-informed analyses are useful for clarifying potential influence of phylogeny on SSD of related species. In this study, results of phylogenetically-informed analyses showed low phylogenetic signal (λ) in both male- and female-biased dimorphic species. This implies that phylogenetic constraints and inertia do not influence SSD allometry of Chinese lizards.
Conclusions
Fecundity selection plays a significant role compared with sexual selection in determining SSD of Chinese lizards. Agamidae species exhibits Rensch’s rule. This is attributed to the strong sexual selection in males (in larger species) and a lower limit to egg size (in smaller species), resulting in more constrained female body sizes. Male- and female-biased dimorphic species exhibit a size based isometric relationship, because both male and female sizes are subjected to a uniform selection pressure across species, therefore, they exhibit a similar variation, and because fecundity selection favors larger females to lay more eggs resulting in inconsistency with Rensch’s rule. Rensch’s rule is less prevalent in Chinese lizards because fecundity selection evolves in larger female sizes, and females are larger compared with males in most species.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
References
Abouheif E, Fairbairn DJ (1997) A comparative analysis of allometry for sexual size dimorphism: assessing Rensch’s rule. Am Nat 149:540–562
Aleksic I, Ivanovic A, Crnobrnja-isailovic J et al (2009) Sex size and shape differences in the lacertid community (Podarcis spp. and Archaeolacerta sp.) from the Lake Skadar region (Montenegro). Ital J Zool 76:43–52
Blanckenhorn WU (2005) Behavioral causes and consequences of sexual size dimorphism. Ethology 111:977–1916
Blanckenhorn WU, Dixon AFG, Fairbairn DJ et al (2007a) Proximate causes of Rensch’s rule: does sexual size dimorphism in arthropods result from sex differences in development time? Am Nat 169:245–257
Blanckenhorn WU, Meier R, Teder T (2007b) Rensch’s rule in insects: patterns among and within species. In: Fairbairn DJ, Blanckenhorn WU, Szekely T (eds) Evolutionary studies of sexual size dimorphism. Sex, size and gender roles. Oxford University Press, Oxford
Burbrink FT, Futterman I (2019) Female-biased gape and body-size dimorphism in the New World watersnakes (tribe: Thamnophiini) oppose predictions from Rensch’s rule. Ecol Evol 9:9624–9633
Chen SY, Bi JH, He ZC et al (2015) Sexual dimorphism and reproductive output of Eremias argus from Ordos, China. Chin J Zool 50:214–220
Cheverud JM, Dow MM, Leutenegger W (1986) A phylogenetic autocorrelation analysis of sexual dimorphism in primates. Am Anthropol 88:916–922
Colleoni E, DenëL M, Padoa-Schioppa E et al. (2014) Rensch's rule and sexual dimorphism in salamanders: patterns and potential processes. J Zool 293:143–151
Colwell RK (2000) Rensch’s rule crosses the line: convergent allometry of sexual size dimorphism in hummingbirds and flower mites. Am Nat 156:495–510
Cox RM, Butler M, John-Alder HB (2007) The evolution of sexual size dimorphism in reptiles. Oxford University Press, Oxford
Cox RM, Skelly SL, John-Alder HB (2003) A comparative test of adaptive hypotheses for sexual size dimorphism in lizards. Evolution 57:1653–1669
Darwin C (1871) The descent of man and selection in relation to sex. John Murray, London
De Lisle SP, Rowe L (2013) Correlated evolution of allometry and sexual dimorphism across higher taxa. Am Nat 182(5):630–639
Fairbairn DJ (1997) Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annu Rev Ecol Syst 28:659–687
Fairbairn DJ (2005) Allometry for sexual size dimorphism: testing two hypotheses for Rensch’s rule in the water strider aquarius remigis. Am Nat 166:S69–S84
Feldman A, Sabath N, Pyron RA et al (2016) Body sizes and diversification rates of lizards, snakes, amphisbaenians and the tuatara. Glob Ecol Biogeogr 25:187–197
Frýdlová P, Frynta D (2015) Strong support for Rensch’s rule in an American clade of lizards (Teiidae and Gymnophtalmidae) and a paradox of the largest tejus. Sci Nat 102:1–11
Grossi B, Solis R, Veloso C et al (2016) Consequences of sexual size dimorphism on energetics and locomotor performance of Grammostola rosea (Araneae; Teraphosidae). Physiol Entomol 41:281–288
Jerry F, Husak A, Lappin K et al (2006) Bite-Force performance predicts dominance in male venerable collared lizards (Crotaphytus antiquus). Copeia 2006(2):301–306
Jiménez-Arcos VH, Sanabria-Urbán S, Castillo RC (2017) The interplay between natural and sexual selection in the evolution of sexual size dimorphism in Sceloporus lizards (Squamata: Phrynosomatidae). Ecol Evol 7:905–917
Kilmer JT, Rodríguez RL (2017) Ordinary least squares regression is indicated for studies of allometry. J Evol Biol 30:4–12
Kratochvíl L, Frynta D (2002) Body size, male combat and the evolution of sexual dimorphism in eublepharid lizards (Squamata: Eublepharidae). Biol J Lin Soc 76:303–314
Kratochvíl L, Frynta D (2006) Body size effect on egg size in eublepharid geckos (Squamata: Eublepharidae), lizards with invariant clutch size: negative allometry for egg size in ectotherms is not universal. Biol J Lin Soc 88:527–532
Lailvaux SP, Irschick DJ (2007) The evolution of performance-based male fighting ability in Caribbean Anolis lizards. Am Nat 170:573–586
LeBas NR, Marshall NJ (2000) The role of colour in signaling and male choice in the agamid lizard Ctenophorus ornatus. Proc R Soc Lond B 267(1442):445–452
Lengkeek W, Didderen K, Cote IM et al (2008) Plasticity in sexual size dimorphism and Renschs rule in Mediterranean blennies (Blenniidae). Can J Zool 86(10):1173–1178
Liang T, Li Y, Zheng P et al (2018) Sexual dimorphism and female reproduction of Eremias arguta. Arid Zone Res 35:1160–1166
Liang T, Lv SP, Wu KL et al (2015) Sexual dimorphism and female reproduction of Phrynocephalus helioscopus (Agamidae). Chin J Ecol 34:1602–1606
Liang T, Shi L (2017) Sexual dimorphism and morphological variation of three populations of Phrynocephalus helioscopus: test of Bergmann’s rule, Allen’s rules and Rensch’s rule. Sichuan J Zool 36:249–257
Liao HH, Xu F, Yang WK (2013) The Sexual Size Dimorphism of Teratoscincus przewalskii. Sichuan J Zool 32:808–813
Lin ZH (2004) Sexual dimorphism in head and body size and the growth during reproductive period in the lizard, Japalura splendida. Sichuan J Zool 23:277–280
Lindenfors P, Gittleman JL, Jones KE (2007) Sexual dimorphism in mammals. Oxford University Press, Oxford
Lovich JE, Gibbons JW (1992) A review of techniques for quantifying sexual size dimorphism. Growth Dev Aging Gda 56:269–281
Ludbrook J (2010) Linear regression analysis for comparing two measurers or methods of measurement: But which regression? Clin Exp Pharmacol Physiol 37(7):692–699
Meiri S (2010) Length–weight allometries in lizards. J Zool 281:218–226
Meiri S (2018) Traits of lizards of the world: Variation around a successful evolutionary design. Glob Ecol Biogeogr 27:1168–1172
Meiri S, Feldman A, Kratochvíl L (2015) Squamate hatchling size and the evolutionary causes of negative offspring size allometry. J Evol Biol 28:438–446
Meiri S, Liang T (2021) Rensch’s rule – definitions and statistics. Glob Ecol Biogeogr. https://doi.org/10.1111/geb.13255
Orme CDL, Freckleton RP, Thomas GH et al (2012) Caper: comparative analysis of phylogenetics and evolution in R. Methods Ecol Evol 3:145–151
Peñalver-Alcázar M, Galán P, Aragón P (2019) Assessing Rensch’s rule in a newt: roles of primary productivity and conspecific density in interpopulation variation of sexual size dimorphism. J Biogeogr 46:2558–2569
Pincheira-Donoso D, Tregenza T (2011) Fecundity selection and the evolution of reproductive output and sex–specific body size in the Liolaemus lizard adaptive radiation. Evol Biol 38:197–207
Pincheira-Donoso D, Hunt J (2017) Fecundity selection theory: concepts and evidence. Biol Rev 92:341–356
Pincheira-Donoso D, Fox SF, Scolaro JA et al (2011) Body size dimensions in lizard ecological and evolutionary research: exploring the predictive power of mass estimation equations in two liolaemidae radiations. Herpetol J 21:35–42
Price TD, Phillimore AB (2007) Reduced major axis regression and the island rule. J Biogeogr 34:1998–1999
Pyron M, Fincel M, Dang M (2007) Sexual size dimorphism and ecomorphology of spotfin Shiner (Cyprinella spiloptera) from the Wabash River watershed. J Freshw Ecol 22(4):687–696
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Regis KW, Meik JM (2017) Allometry of sexual size dimorphism in turtles: a comparison of mass and length data. PeerJ 5:e2914
Rensch B (1950) Die Abhängigkeit der relativen Sexual differenz von der Körpergrösse. Bonner Zoologische Beitrage 1:58–69
Rensch B (1959) Evolution above the species level. Columbia University Press, New York
Revell LJ (2012) Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Scharf I, Meiri S (2013) Sexual dimorphism of heads and abdomens: Different approaches to ‘being large’ in female and male lizards Biol J Linnean Soc 110: 665–673
Schuett GW (1997) Body size and agonistic experience affect dominance and mating success in male copperheads. Anim Behav 54:213–224
Shine R (1988) The evolution of large body size in females: a critique of Darwin’s “fecundity advantage” model. Am Nat 131:124–131
Shine R, Harlow PS, Keogh JS et al (1998) The allometry of life–history traits: insights from a study of giant snakes (Python reticulatus). J Zool 244:405–414
Smith RJ (1999) Statistics of sexual size dimorphism. J Hum Evol 36:423–459
Smith RJ (2009) Use and misuse of the reduced major axis for line–fitting. Am J Phys Anthropol 140:476–486
Smith RJ, Cheverud JM (2002) Scaling of sexual dimorphism in body mass: a phylogenetic analysis of Rensch’s rule in Primates. Int J Primatol 23:1095–1135
Starostova Z, Kubica L, Kratochvil L (2010) Macroevolutionary pattern of sexual size dimorphism in geckos corresponds to intraspecific temperature–induced variation. J Evol Biol 23:670–677
Stuart-Fox D (2009) A test of Rensch’s rule in dwarf chameleons (Bradypodion spp.), a group with female–biased sexual size dimorphism. Evol Ecol 23:425–433
Szekely T, Freckleton RP, Reynolds JD (2004) Sexual selection explains Rensch’s rule of size dimorphism in shorebirds. Proc Natl Acad Sci USA 101:12224–12227
Tarr S, Meiri S, Hicks JJ et al (2019) A biogeographic reversal in sexual size dimorphism along a continental temperature gradient. Ecography 42:706–716
Teder T, Tammaru T (2005) Sexual size dimorphism within species increases with body size in insects. Oikos 108(2):321–334
Tonini JFR, Beard KH, Ferreira RB et al (2016) Fully–sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biol Cons 204:23–31
Trivers R (1972) Parental investment and sexual selection. Aldine Publishing Company, Chicago
Tsuji K, Fukami T (2020) Sexual dimorphism and species diversity: from clades to sites. Trends Ecol Evol 35:105–114
Uetz P, Freed P, Hošek J (2019) The reptile database. http://www.reptile–database.org. Accessed 14 Aug 2019
Valdecantos S, Lobo F, Perotti MG et al (2019) Sexual size dimorphism, allometry and fecundity in a lineage of South American viviparous lizards (Liolaemidae: Phymaturus). Zoologischer Anzeiger 279:152–163
Verwaijen D, van Damme R, Herrel A (2002) Relationships between head size, bite force, prey handling efficiency and diet in two sympatric lacertid lizards. Funct Ecol 16:842–850
Wan LX, Zhang HJ, Li HJ (2018) Sexual dimorphism of Eremias multiocellata from three populations in Gansu region. Sichuan J Zool 37:139–148
Wang K, Ren JL, Chen HM et al (2020) The updated checklists of amphibians and reptiles of China. Biodiversity Science 28:189–218 ([in Chinese])
Wang XQ, Liang T, An J et al (2020) Comparison of bite force performance and head shape between males and females of Trapelus sanguinolenta. Chin J Zool 55:29–36 ([in Chinese])
Wang Z (2011) Adapting to extreme climate: the evolution of viviparity in Phrynocephalus lizards. Nanjing Normal University, Nanjing
Warton DI, Duursma RA, Falster DS et al (2012) Smatr 3—an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259
Webb TJ, Freckleton RP (2007) Only half right: species with female–biased sexual size dimorphism consistently break Rensch’s rule. PLoS ONE 2:e897
Wikelski M, Romero LM (2003) Body size, performance and fitness in Galapagos marine iguanas. Integr Comput Biol 43:376–386
Wu PF, Wang ZY, Guo HY et al (2005) The growth and growth differences between female and male of Phrynocephalus vlangalii. J Sichuan Univ 42:1252–1257
Yu GC (2019) Treeio: base classes and functions for phylogenetic tree input and output. R package version 1.8.2. https://guangchuangyu.github.io/software/treeio.
Yu GC, Smith D, Zhu HC et al (2017) Ggtree: an R package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 8:28–36
Zamudio KR (1998) The evolution of female–biased sexual size dimorphism: a population–level comparative study in horned lizards (Phrynosoma). Evolution 52:1821–1833
Zhao L, Chen Y, Lou SL et al (2016) Reciprocal sexual size dimorphism and Rensch’s rule in toad–headed lizards, Phrynocephalus vlangalii. Salamandra 52:261–268
Zhou RB, Peng XP, Hou M et al (2019) A new species of genus Goniurosaurus—G. Sinensis J Shihezi Univ (Nat Sci) 37:549–556
Zucker N, Murray L (1996) Determinants of dominance in the tree lizard Urosaurus ornatus: the relative importance of mass, previous experience and coloration. Ethology 102:812–825
Acknowledgements
We thank all the researchers whose work contributed to our dataset. We are also thankful to Dr Lu Zhou, Dr Emma Sherratt, and the two anonymous referees for commenting on an earlier draft of the manuscript. We appreciate Ping-ping Gao for helping with collecting morphological traits; We sincerely thank Prof. Shai Meiri for helping with understanding the concept of Rensch’s rule, organizing the statistics, and stimulating discussion. Finally, we acknowledge Qian Han for assisting with developing the species pictures in Fig. 2. We thank freescience for English editing.
Funding
A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
TL, LS and CHL conceived the ideas and designed the methodology; TL and LS collected the data; TL analysed the data, TL, LS, GB and CHL led the writing of the manuscript. All authors contributed critically to drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the supplementary information.
Appendix S1
. Body size (mm), mass (Log-10 transformed, g), clutch sizes, and references of Chinese lizards. (DOCX 69 kb)
Appendix S2
. Results of reduced major axis (RMA) and ordinary least square (OLS) regression of male size on female size (length and mass). (DOCX 43 kb)
Rights and permissions
About this article
Cite this article
Liang, T., Shi, L., Bempah, G. et al. Sexual size dimorphism and its allometry in Chinese lizards. Evol Ecol 35, 323–335 (2021). https://doi.org/10.1007/s10682-021-10104-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-021-10104-1