Abstract
Ontogenetic habitat shifts are predicted to increase the fitness and survival of individuals by allowing effective utilization of spatially distributed resources. Evidence supports nutritional requirements and predation pressure as drivers of habitat shifts. Likewise, intraspecific interactions are thought to lead to ontogenetic habitat shifts, however, empirical evidence is lacking. Here, we test if intraspecific male–male interactions are responsible for ontogenetic habitat shifts in Xanthagrion erythroneurum, a damselfly that undergoes developmental colour change. The juvenile males are yellow and change colour to red with sexual maturity. Field observations showed that the proportion of juvenile males is higher in adjacent woods than in primary mating arenas by ponds. We measured male–male interactions by the pond and in the woods, predicting the habitat switch would reduce male antagonistic interactions such as male aggression and male–male mating attempts. We showed that juvenile males receive less aggression in woods than at the pond mating arena. We conclude that lower population density and lower male encounter rates in the woods reduce the cost of male aggression for juvenile males. Our study provides evidence that stage-dependent habitat choice resulting from intrasexual antagonistic interactions may drive ontogenetic habitat shifts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Different developmental stages often require specific habitats (Moran 1994), and habitat shifts at different developmental stages can generate stage-structured populations (Shine et al. 2003). Habitat shifts are thought to increase fitness and survival at different life stages by optimizing the utilization of spatially distributed resources (Miller and Rudolf 2011). Some evidence exists supporting physiological limitations, differential nutritional requirements, and predator avoidance as selective agents of ontogenetic habitat shifts (Moran 1994; Grof-Tisza et al. 2015). Antagonistic interactions within and between sympatric species are predicted to be potential drivers of ontogenetic habitat shifts (Morris 2003; Martin et al. 2013; Delaney and Warner 2017), but are poorly supported by experimental evidence.
Antagonistic interactions between juveniles and adults are common in many vertebrate and invertebrate taxa, including damselflies (Corbet 1999; Martin et al. 2013; Delaney and Warner 2017). Damselflies shift from aquatic to terrestrial habitats after emerging from their larval stage (Corbet 1999). The adults, however, return to aquatic habitats for breeding as the females oviposit in the water or on submerged plants (Corbet 1999). Adult males assemble in mating arenas (lakes, ponds, or streams) and engage in scramble competition where they aggressively attack conspecifics to defend or access breeding grounds (Khan and Herberstein 2019, 2020b). In high-density assemblages, males often attempt to mate with conspecific males (Miller 1987; Beatty et al. 2015). Adult male aggression and male mating attempts are costly for juvenile males (Gering 2017). We predict that these costly interactions can be mitigated if juveniles relocate away from the aquatic breeding ground, thereby driving ontogenetic habitat shifts.
In this study, we aim to determine the patterns of ontogenetic habitat shift in X. erythroneurum damselflies, which exhibit ontogenetic colour change whereby the juvenile males change colour from dull yellow to conspicuous red upon sexual maturity (Fig. 1a, b; Khan and Herberstein 2020a). Juvenile dull colouration signals subordinance in animals, and can reduce adult aggression (Hawkins et al. 2012). In this study species, dull juvenile colouration, however, does not reduce adult aggression. Rather, yellow males incur higher conspecific aggression within breeding areas (Khan and Herberstein 2020b). We therefore hypothesized that such male–male aggressions could drive ontogenetic habitat shift. We calculated the proportion of juvenile and adult males active at the pond breeding territory and in the adjacent woods, away from the breeding area, to quantify the developmental habitat shift. We predicted, juvenile males would occupy woods over pond habitat if it reduces adult male aggression. Next, we aim to determine whether this ontogenetic habitat shift reduces costly male–male interactions incurred by yellow males. We performed a behavioural experiment to determine male aggression and mating attempts with juvenile males at the pond and wood habitats predicting that males at the wood habitat will receive fewer aggression and mating attempt than males at the pond habitat. In accordance with our prediction, we found that juvenile males at the wood receive male–male interactions than in pond habitats.
Ontogenetic colour variation of male. a sexually immature yellow male b mature red male. Male proportions and population density in pond and woods. c proportions of newly emerged males (n = 8), d proportions of yellow males (n = 16), e proportions of red males at the pond and the woods (n = 16), f population density (number of captured individuals/min) at the pond (n = 11) and the woods (n = 9). Bold lines indicate medians. Boxes enclose 25th to 75th percentiles. Error bars enclose the data range, excluding outliers. Dots are data points of each study day; dots that are vertically outside the error bars are outliers, > 1.5 times the interquartile range; * indicates p < 0.05
Materials and methods
Study species and field site
X. erythroneurum is an Australian damselfly commonly found in ponds, lakes, and marshes (Theischinger and Hawking 2016; Khan and Herberstein 2020a). Adult males of this species are distinguished from other sympatric species by their conspicuous red colouration and blue abdominal bands on terminal abdominal segments (Khan and Herberstein 2019). X. erythroneurum exhibits ontogenetic colour changes: the juvenile males are yellow and attain a conspicuous red colour 6–7 days after emergence (Fig. 1a, b; Khan and Herberstein 2020a). This yellow to red colour shift signals sexual maturity in males as only red males mate (Khan and Herberstein 2020a). Yellow males never mate, even in the absence of competitors (Khan and Herberstein 2020a). Adult females, like adult males, are red in colour, whereas juvenile (age 1–7 days) and subadult females (7–14 days) exhibit yellow body colour like juvenile males (Khan and Herberstein, unpublished data).
We conducted field studies at a pond situated on the North Ryde campus of Macquarie University, Sydney, Australia (33.772 S, 151.114 E). In the Sydney region, X. erythroneurum start emerging in September. The juveniles are seen in flight until December, whereas the adults remain active until June (Khan and Herberstein 2019). We did not require permits for this study as it was conducted outside national parks and protected areas, and X. erythroneurum is not protected in Australia.
Habitat selection
To determine whether red and yellow males prefer different habitats, we calculated damselfly frequencies by a pond (vegetation surrounding the pond, < 5 meters from the pond edge) and within nearby woods (> 10 meters from the pond edge, in bushy patches under tree cover). Selecting sunny days only, we slowly walked along the edge of the pond and woods and captured damselflies using an insect sweep net (Khan 2015, 2018). During a single sample event, we collected males for 25–35 min in either habitat and then counted the number of red, yellow, and newly emerged yellow males captured at the pond and woods. We identified newly emerged males by their shiny wings (Khan 2020; Khan and Herberstein 2020a). We released red and yellow males after counting them, however we kept newly emerged males for 2–4 h to allow their wings to harden before release. We marked the wings of damselflies with a dot before releasing them to avoid counting the same individuals more than once. We calculated damselfly frequencies in 2017 (n = 7 days), 2018 (n = 6 days), and 2019 (n = 3 days) between September and October when juveniles and adults co-occurred. Number of sampling days varied in different studied years because of logistical constraints.
Male–male interaction
X. erythroneurum females and sexually immature males are non-aggressive to conspecifics. Therefore, we only considered response of the adult males when assessing male–male interactions. Our interest is in understanding the driver of habitat selection of yellow males. Thus we experimentally investigated if yellow males received less male–male interactions (male approaches, male aggression, male–male copulation attempts) in the woods than by the pond using a modified damsel-on-a-dowel technique (Fincke et al. 2007; Khan and Herberstein 2020b). Using UHU™ glue, we glued the legs of live yellow males to dowels. In such a condition, restrained males still were able to exhibit mating refusal and aggressive display by raising their abdomen like free-flying damselflies. We placed the dowels at the edge of the pond and in the woods, and measured the interactions from conspecific males while sitting approximately one meter away from the dowel. This distance allowed us to follow the interactions clearly without disturbing regular movements of the approaching damselflies. An approaching male can detect the focal male when it passes within 10 cm of the focal male (Fincke 2015). We counted numbers of approaches when intruder males passed within 10 cm of focal males on the right or left. An approach can result in an aggressive response, a non-aggressive response or a mating attempt. If approaching males passed focal males without any physical contact, we recorded it as a non-aggressive response. When approaching males bit the focal males, we counted it as aggressive response. Finally, when approaching males tried to clasp the focal male (moving their cerci to the focal male’s prothorax), or formed a tandem (physically connected to the focal male with their cerci) we counted it as a mating attempt. We measured interactions for a focal male for 10 min (one trial). We performed all trials (n = 41) on sunny days and used each male only once. At the end of the experiment, males were unglued, body length was measured and their wings were marked before they were released. As our hypothesis did not pertain to the habitat selection of red males, we did not include them in this set up.
Statistical analyses
We calculated the proportions of red, yellow, and newly emerged males collected from the pond and woods. The proportions of a male colour type can vary from 0 to 1 in their habitats. These type data such as proportions and rates are best fitted by beta regression model (Cribari-Neto and Zeileis 2010). We fitted a beta regression model to determine whether males prefer a certain habitat type. Beta regression models cannot deal with extreme values of 1 and 0, so we transformed male proportions by using the equation y * (n − 1) + 0.5)/n, where y is the calculated proportion and n is the sample size (Smithson and Verkuilen 2006). We fitted beta regression models using proportions of the three male categories as response variables, and habitat (pond or woods) as a covariate.
We estimated population density at the pond and woods as the number of damselflies collected per minute (Iserbyt et al. 2013). We applied a linear mixed effect model (LMM) using male density as the response variable and habitat (pond or woods) as a fixed factor. We used study days as a random factor to account for abiotic factors (temperature, cloud cover, wind speed, and humidity) that might affect damselfly assemblage and density. We used the r.squaredGLMM function of the R package ‘MuMIn’ to determine the effect size of the model (Johnson 2014).
We fitted a generalized linear model (GLM) using overdispersed Poisson distribution (quasipoisson) to determine effects of habitat on the number of approaches received by focal males. We tested possible models with habitat and total body length as covariates and selected the best model using quasi Akaike’s information criterion (QAIC). To account for zero inflation and overdispersion, we fitted zero-inflated generalized linear mixed models (ZIGLMM) to determine aggression and mating attempts received by focal males at the pond and woods. We tested a number of models with habitat (pond and woods) and total body length of the focal damselfly as fixed effects, and experimental days as a random effect. We choose the best-fitting models using Akaike’s information criterion corrected for small sample sizes (AICc) (see supplementary material for model selection). All models were fitted in R v 3.5.2 using the packages ‘betareg’ (Cribari-Neto and Zeileis 2010), ‘lme4’ (Bates et al. 2019), and ‘glmmADMB’ (Fournier et al. 2012).
Results
Male proportions and population density in pond and woods
Proportions of newly emerged males were greater at the pond than the woods (beta regression: estimate = 2.01 ± 0.04, z = 48.68, p < 0.001, pseudo R2 = 0.98; Fig. 1c). By contrast, proportions of juvenile yellow males were higher in the woods than the pond (beta regression: estimate = 1.88 ± 0.04, z = 39.40, p < 0.001, pseudo R2 = 0.97; Fig. 1d). The proportion of adult red males, on the other hand, was higher at the pond habitat than the woods (beta regression: estimate = 1.04 ± 0.23, z = 5.04, p < 0.001, pseudo R2 = 0.59; Fig. 1e). Population density of damselflies was higher at the pond than the woods (GLM: estimate = 1.10 ± 0.18, t = 5.85, p < 0.001, R2 = 0.46; Fig. 1f).
Male–male interactions
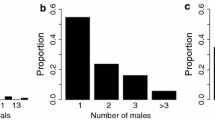
The juvenile focal males received more approaches from the intruder males at the pond than in the woods (GLM: estimate = 2.33 ± 0.32, t = 7.22, p < 0.001, R2 = 0.78; Fig. 2a). Similarly, the focal juvenile males at the pond received more aggression than those in the woods (ZIGLMM: estimate = 2.88 ± 0.48, z = 5.99, p < 0.001; Fig. 2b). The focal males also received fewer mating attempts in the woods than the pond (ZIGLMM: estimate = 3.24 ± 1.06, z = 3.05, p < 0.01). Body size of the focal male, however, did not have a significant effect on received aggression (ZIGLMM: estimate = 0.18 ± 0.12, z = 1.50, p = 0.13) or mating attempts (ZIGLMM: estimate = 0.14 ± 0.36, z = 0.39, p = 0.69).
Male–male interactions at the pond and the woods. a number of approaches and b aggression rate (number of attacks/number of approaches) received by the focal males at the pond (n = 19) and the woods (n = 22). Bold lines indicate medians. Boxes enclose 25th to 75th percentiles. Error bars enclose the data range, excluding outliers. Dots are data points; dots that are vertically outside the error bars are outliers, > 1.5 times the interquartile range; * indicates p < 0.05
Discussion
Ontogenetic habitat shifts can occur due to nutritional requirements, to avoid predation, and to reduce intra- and interspecific antagonistic interactions (Kleeck et al. 2018; Nokelainen et al. 2019; Sánchez-Hernández et al. 2019). We determined ontogenetic habitat preference in X. erythroneurum damselfly and showed that the proportion of juvenile males was higher in the woods, whereas adult males occurred in higher proportions at the pond. We further measured adult male aggression towards, and mating attempts with juvenile males and showed that juvenile males received less aggression and fewer mating attempts in the woods than the pond. We conclude that male antagonistic interactions (aggression and mating attempts) at the pond are significant contributors to the observed ontogenetic habitat shifts in X. erythroneurum damselflies.
Sexually mature X. erythroneurm males assemble in a mating arena (pond) and engage in scramble competition where they aggressively attack conspecific males (Khan and Herberstein 2020b). Such aggressive male–male interactions at the pond have been shown to reduce male fitness and longevity in damselflies (Gering 2017). The juvenile males, on the other hand, do not benefit from occupying the pond as they are not sexually mature and do not mate (Khan and Herberstein 2020a). Thus, by moving to a different habitat, juvenile males could reduce adult aggression. Accordingly, our results showed that juvenile males can effectively evade male aggression by shifting to the woods. The mechanisms for this are two-fold: firstly, fewer aggressive red males occur in the woods and secondly, overall population density, and therefore male interactions, are lower in the woods than the pond. Other possible mechanisms that we did not test here include a lower detection probability of yellow males due to greater background matching in the woods.
In addition to aggressive interactions, male–male mating attempts frequently occur in damselflies, especially in high male density assemblages (Miller 1987). These male–male mating attempts are costly in terms of time, energy and unsuccessful mating attempts and reduce male fitness (Gering 2017). Male morphological traits, such as blue abdominal bands, conspicuous body colouration, and behaviour such as refusal display, can reduce male–male mating interactions (Sherratt and Forbes 2001; Khan and Herberstein 2019, 2020b). Our experiment showed that juvenile males that reside at the pond habitat are likely to incur male–male mating attempts. The adult males probably recognise juvenile males as potential mates because of the body colour similarities between juvenile males and juvenile females. Body colour similarity between males and females increase male–male mating interactions in other damselflies (Beatty et al. 2015; Gering 2017). Since adult males and females are similarly red in colour, recognition error among adult males is also possible and reduced due to the male-limited abdominal blue bands that function as antiharassment aposematic signals and reduce male–male mating interactions (Beatty et al. 2015; Khan and Herberstein 2019). While juvenile males also have these blue bands, they are much paler (personal observation) and likely to be less effective than habitat shifts.
While our study makes a convincing case that juvenile males enjoy a selective benefit from habitat shifts based on reduced aggression and male–male mating attempts, we cannot, however, exclude the possibility that the woods additionally provide selective benefits such as food resources or protection from predators. Damselflies feed on other smaller insects including Diptera and Lepidoptera (Corbet 1999). Bushes and woods with more complex vegetation structure are more suitable habitats than pond sites, thereby offering more food resources (Hughes et al. 2000). Furthermore, woods probably provide better camouflage for damselflies thus reducing predation risks. Further studies are required to test these mutually non-exclusive hypotheses.
Ontogenetic habitat shifts are common in many vertebrates and invertebrates often meet the ecological requirements at different developmental stages. In fishes and reptiles, ontogenetic habitat shift is primarily driven by nutritional demands and interspecific interactions such as predation risk (Dahlgren and Eggleston 2000; Keren-Rotem et al. 2006). Here, in damselflies, we showed that adult male aggressions and mating attempts towards juvenile males selects for ontogenetic habitat shift. The sexually immature males shift from pond site to the woods where they stay until sexual maturity and after gaining sexual maturity return to the pond again where they mate. Our study highlights the contribution of intraspecific interactions in habitat dispersion and provides experimental evidence for the role of intraspecific interactions in ontogenetic habitat shift.
Data accessibility
All raw data are available via github https://github.com/KhanKawsar/Habitat-shift.
References
Bates D, Maechler M, Bolker B, et al (2019) lme4: linear mixed-effects models using “Eigen” and S4. Version 1.1-21. https://CRAN.R-project.org/package=lme4
Beatty CD, Andrés JA, Sherratt TN (2015) Conspicuous coloration in males of the Damselfly Nehalennia irene (Zygoptera: Coenagrionidae): Do males signal their unprofitability to other males? PLoS ONE 10:e0142684. https://doi.org/10.1371/journal.pone.0142684
Corbet PS (1999) Dragonflies: behaviour and ecology of odonata. Cornell University Press, Ithaca
Cribari-Neto F, Zeileis A (2010) Beta regression in R. J Stat Softw 34:1–24. https://doi.org/10.18637/jss.v034.i02
Dahlgren CP, Eggleston DB (2000) Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology 81:2227–2240. https://doi.org/10.1890/0012-9658(2000)081%5b2227:EPUOHS%5d2.0.CO;2
Delaney DM, Warner DA (2017) Effects of age- and sex-specific density on behaviour and survival in a territorial lizard (Anolis sagrei). Anim Behav 129:31–41. https://doi.org/10.1016/j.anbehav.2017.04.014
Fincke OM (2015) Trade-offs in female signal apparency to males offer alternative anti-harassment strategies for colour polymorphic females. J Evol Biol 28:931–943. https://doi.org/10.1111/jeb.12623
Fincke OM, Fargevieille A, Schultz TD (2007) Lack of innate preference for morph and species identity in mate-searching Enallagma damselflies. Behav Ecol Sociobiol 61:1121–1131. https://doi.org/10.1007/s00265-006-0345-3
Fournier DA, Skaug HJ, Ancheta J et al (2012) AD model builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim Methods Softw 27:233–249. https://doi.org/10.1080/10556788.2011.597854
Gering EJ (2017) Male-mimicking females increase male–male interactions, and decrease male survival and condition in a female-polymorphic damselfly. Evolution 71:1390–1396. https://doi.org/10.1111/evo.13221
Grof-Tisza P, Holyoak M, Antell E, Karban R (2015) Predation and associational refuge drive ontogenetic niche shifts in an arctiid caterpillar. Ecology 96:80–89. https://doi.org/10.1890/14-1092.1
Hawkins GL, Hill GE, Mercadante A (2012) Delayed plumage maturation and delayed reproductive investment in birds. Biol Rev 87:257–274. https://doi.org/10.1111/j.1469-185X.2011.00193.x
Hughes JB, Daily GC, Ehrlich PR (2000) Conservation of insect diversity: a habitat approach. Conserv Biol 14:1788–1797. https://doi.org/10.1111/j.1523-1739.2000.99187.x
Iserbyt A, Bots J, Van Gossum H, Sherratt TN (2013) Negative frequency-dependent selection or alternative reproductive tactics: maintenance of female polymorphism in natural populations. BMC Evol Biol 13:139. https://doi.org/10.1186/1471-2148-13-139
Johnson PCD (2014) Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods Ecol Evol 5:944–946. https://doi.org/10.1111/2041-210X.12225
Keren-Rotem T, Bouskila A, Geffen E (2006) Ontogenetic habitat shift and risk of cannibalism in the common chameleon (Chamaeleo chamaeleon). Behav Ecol Sociobiol 59:723–731. https://doi.org/10.1007/s00265-005-0102-z
Khan MK (2015) Dragonflies and damselflies (Insecta: Odonata) of the northeastern region of Bangladesh with five new additions to the Odonata fauna of Bangladesh. J Threat Taxa 7:7795–7804. https://doi.org/10.11609/JoTT.o4314.7795-804
Khan MK (2018) Odonata of eastern Bangladesh with three new records for the country. J Threat Taxa 10:12821–12827. https://doi.org/10.11609/jott.3819.10.13.12821-12827
Khan MK (2020) Female pre-reproductive colouration reduces mating harassment in damselflies. Evolution. https://doi.org/10.1111/evo.14048
Khan MK, Herberstein ME (2019) Sexually dimorphic blue bands are intra-sexual aposematic signals in non-territorial damselflies. Anim Behav 156:21–29. https://doi.org/10.1016/j.anbehav.2019.07.011
Khan MK, Herberstein ME (2020a) Ontogenetic colour change signals sexual maturity in a non- territorial damselfly. Ethology 126:51–58
Khan MK, Herberstein ME (2020b) Male–male interactions select for conspicuous male colouration in damselflies. bioRxiv 2020.06.24.168823. https://doi.org/10.1101/2020.06.24.168823
Kleeck MJV, Smith TAH, Holland BS (2018) Paedophagic cannibalism, resource partitioning, and ontogenetic habitat use in an invasive lizard. Ethol Ecol Evol 30:497–514. https://doi.org/10.1080/03949370.2018.1441190
Martin AE, Hoover TM, Richardson JS (2013) Modeling the role of stage-structured agonistic interactions in ontogenetic habitat shifts. Behav Ecol 24:355–365. https://doi.org/10.1093/beheco/ars171
Miller PL (1987) An examination of the prolonged copulations of Ischnura elegans (Vander Linden) (Zygoptera: Coenagrionidae). Odonatologica 16:37–56
Miller TEX, Rudolf VHW (2011) Thinking inside the box: community-level consequences of stage-structured populations. Trends Ecol Evol 26:457–466. https://doi.org/10.1016/j.tree.2011.05.005
Moran NA (1994) Adaptation and constraint in the complex life cycles of animals. Annu Rev Ecol Syst 25:573–600. https://doi.org/10.1146/annurev.es.25.110194.003041
Morris DW (2003) Toward an ecological synthesis: a case for habitat selection. Oecologia 136:1–13. https://doi.org/10.1007/s00442-003-1241-4
Nokelainen O, Maynes R, Mynott S et al (2019) Improved camouflage through ontogenetic colour change confers reduced detection risk in shore crabs. Funct Ecol 33:654–669. https://doi.org/10.1111/1365-2435.13280
Sánchez-Hernández J, Nunn AD, Adams CE, Amundsen P-A (2019) Causes and consequences of ontogenetic dietary shifts: a global synthesis using fish models. Biol Rev 94:539–554. https://doi.org/10.1111/brv.12468
Sherratt TN, Forbes MR (2001) Sexual differences in coloration of Coenagrionid damselflies (Odonata): A case of intraspecific aposematism? Anim Behav 62:653–660. https://doi.org/10.1006/anbe.2001.1789
Shine R, Shine T, Shine B (2003) Intraspecific habitat partitioning by the sea snake Emydocephalus annulatus (Serpentes, Hydrophiidae): the effects of sex, body size, and colour pattern. Biol J Lin Soc 80:1–10. https://doi.org/10.1046/j.1095-8312.2003.00213.x
Smithson M, Verkuilen J (2006) A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods 11:54–71. https://doi.org/10.1037/1082-989X.11.1.54
Theischinger G, Hawking J (2016) The complete field guide to dragonflies of Australia, 3rd edn. CSIRO Publishing, Victoria
Acknowledgements
We thank Jim McLean for his comments on the initial version of the manuscripts and Sukanya Hasan for the illustrations. MKK thanks Payal Barua and Leelaboti Khona for all of their support.
Funding
MKK was supported by International Macquarie University Research Excellence Scholarship and Macquarie University research support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khan, M.K., Herberstein, M.E. Ontogenetic habitat shifts reduce costly male–male interactions. Evol Ecol 34, 735–743 (2020). https://doi.org/10.1007/s10682-020-10064-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-020-10064-y