Abstract
In several species, the rusty color of hair or feathers is due to pheomelanin pigments, whose adaptive function is unknown. Pheomelanin may be costly because it is phototoxic and its production consumes a key intracellular antioxidant. Pheomelanin-based traits are, however, positively associated with individual quality in several bird species, where they have thus been suggested to have evolved through sexual selection. Here we investigated the signaling potential of the pheomelanin-based coloration of the crown feathers in the blue petrel. Although this pelagic seabird is nocturnal at the breeding colony and breeds within deep burrows, it might use visual communication when settled on the water during daytime. We tested the correlation between crown color and several fitness-related traits, and we found that higher-quality females displayed less-orange crown than poorer-quality females. This result is inconsistent with an adaptive function of pheomelanin-based coloration in inter-, or intra-, sexual selection in females. We suggest that it might, however, be in line with a signaling function of eumelanin-based coloration, if inter-individual variations in orange coloration are mainly due to eumelanin-to-pheomelanin ratio, rather than to pheomelanin quantity. In contrast to females, we did not find strong evidence for associations between melanin-based coloration and individual quality in males, suggesting sex-specific selective pressures on melanin-based traits in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanin is the most common pigment in higher vertebrates, occurring in two main chemical forms: eumelanin, which produces black, gray and dark brown colors, and pheomelanin, which is responsible for reddish and yellowish colors. Eumelanin-based traits have been suggested to play a role in signaling, thermoregulation, camouflage to predators, and protection from UV radiation, mechanical damage or feather-degrading parasites (Mcgraw 2006; Galván and Solano 2016). In contrast, the adaptive value of pheomelanin remains unclear, as it is phototoxic and its synthesis in melanocytes requires the consumption of cysteine via glutathione (GSH), the most important intracellular antioxidant (Galván et al. 2012b; Panzella et al. 2014). Accordingly, in humans, red-haired individuals are more prone to skin cancers (Gerstenblith et al. 2010), and in Asian barn swallows (Hirundo rustica gutturalis), European nuthaches (Sitta europaea), boars (Sus scrofa) and lab mice, pheomelanic morphs have lower viability and higher oxidative stress (Galván et al. 2012a; Mitra et al. 2012; Galván 2017; Arai et al. 2018). Pheomelanin can confer crypsis (Nachman et al. 2003; Negro et al. 2009; Singaravelan et al. 2010), but not all pheomelanin-based traits are involved in concealment pattern.
Recently, pheomelanin has been suggested to have evolved because of its potential role in removing excess cysteine (Galván et al. 2012b). Although cysteine is an essential component of GSH, thereby being important for antioxidant protection, it can cause a variety of problems when in excess, including poor growth, pregnancy complication and brain damage (Olney and Ho 1970; Orth et al. 1992; El-Khairy et al. 2003; Galván and Alonso-Alvarez 2017). Therefore, under low levels of environmental oxidative stress—i.e., when cysteine is not needed for GSH-mediated antioxidant protection and can potentially be in excess—, pheomelanogenesis may be advantageous as it consumes toxic cysteine (Galván et al. 2012b). In contrast, pheomelanogenesis may represent a physiological cost under high environmental stress, when cysteine is needed for antioxidant protection (Galván and Solano 2009). In line with this hypothesis, recent studies have shown that, in some species, pheomelanin synthesis is increased under exposure to excess cysteine, and decreased under exposure to high environmental oxidative stress (Galván et al. 2017; Rodríguez-Martínez et al. 2019).
According to the handicap principle (Zahavi 1975), the cost associated with pheomelanogenesis in environments with high oxidative stress may have promoted the evolution of pheomelanin-based color as honest signals of quality (Galván and Solano 2009; Galván 2018), because only individuals with a high antioxidant capacity may be able to generate large pheomelanin-based signals. In agreement, in several bird species, pheomelanin-based traits are positively associated with individual quality and may thus play a role in sexual selection (Jawor and Breitwisch 2003). For instance, in eastern bluebirds (Sialia sialis), males with larger pheomelanin-based breast patches fledge heavier offspring (Siefferman and Hill 2003), and in American barn swallows (Hirundo rustica rustica), males with brighter pheomelanin-based throat have higher reproductive success (Safran and Mcgraw 2004).
The blue petrel (Halobaena caerulea) is a Procellariidae burrowing seabird that harbors a variable amount of orange feathers in the crown (Fig. 1), which is likely due to variation in the quantity of pheomelanin. Blue petrels being pelagic, the orange coloration is unlikely to play a role in camouflage. Like many other procellariidaes, this seabird is nocturnal on the breeding ground (Warham 1996), and breeds within deep burrows, which have lead to the suggestion that color signals are not essential to communicate (Bretagnolle 1996). However, petrels are active during daytime at sea, where they can aggregate in very dense foraging flocks (Van Franeker et al. 2002), or settle in groups on the calm water (Tickell 1962). Little is known about vision in procellariidaes. Burrow-nesting petrels do not have exceptional nocturnal vision, and seem to have lower visual acuity than surface-nesting petrels (Brooke 1989; Mitkus et al. 2016). However, in the leach-storm petrel (Oceanodroma leucorhoa), a species with similar ecology as the blue petrel, the smallest object that an individual may see in daylight at a distance of 2 m is 16 mm in diameter for low-contrast objects and 2 mm in diameter for high contrast objects (Mitkus et al. 2016). These observations suggest the potential for visual communication among individuals who are settled close to each other on the water, even in burrow-nesting petrels. In addition, males and females do not differ in foraging distribution during breeding and non breeding seasons (Phillips et al. 2009), suggesting that inter-sexual communication might occur.
We investigate the signaling potential of the pheomelanin-based coloration of the crown feathers of blue petrels, by testing the correlation between color expression and individual quality, which we define as a composite measure of multiple phenotypic traits putatively related to fitness (Wilson and Nussey 2010). If orange pheomelanin-based coloration is a quality signal used in sexual selection, we expect good-quality individuals to display more intense orange crown feathers. In contrast, a negative association would preclude a signaling role of pheomelanin color. In this species, males and females provide similar incubation and parental care (Chaurand and Weimerskirch 1994b), thus we predict that crown coloration reflects individual quality in both sexes and that breeding pairs show assortative mating in relation to this trait. We also explore whether variation in crown coloration is potentially linked to oxidative stress, by testing the correlation between color expression and oxidative stress in plasma. We expect blue petrels with high oxidative stress to display less-intense orange crown coloration, because oxidative stress may be a physiological trait related to individual quality (Bize et al. 2008; Cohen Alan et al. 2008; Hill 2014), and because it is suggested to downregulate pheomelanin production, at least in some species (Galván 2018). We measure oxidative stress at the time of breeding and not at the time of molt when pheomelanin is deposited into feathers. However, although oxidative status is known to change over time, a few studies showed that differences in oxidative status among individuals are consistent over a few week period, as well as across breeding seasons (Costantini et al. 2007; Hau et al. 2015; Herborn et al. 2016).
Materials and methods
Study site
A colony of about 80 nest burrows of blue petrels was studied at île Verte in the Kerguelen archipelago (southern Indian Ocean; 49°510 S, 70°050 E). 16 adult males and 23 adult females (including 12 breeding pairs) were included in the analyses. Burrows were checked every 2–4 days to check for bird, egg and chick presence. Birds were captured during incubation, and tarsus length and body mass were recorded. For a subset of birds (n = 4 males and 11 females), a blood sample was collected from the brachial vein and plasma was stored at − 20 °C until analyses.
Color measurements
Crown coloration was measured from digital photographs. Pictures were taken at approximately 40 cm using a digital camera (Panasonic, DMC-TZ30). For each photograph, the same color swatch (QpCARD 201) was placed next to the bird to standardize subsequent measurements. Low quality pictures (due to ambient lighting variations) were removed from the dataset. All pictures were analyzed using the software Adobe Photoshop v7.0 and the CIELAB color space. The CIELAB color space is a perceptually uniform color space designed to provide estimates of human luminance and chromatic perception. It has been suggested to be more appropriate than the RGB color space that is non-uniform and cannot represent all perceivable colors even to humans (Stevens and Cuthill 2005). In the CIELAB color space, L* represents the achromatic signal, while a* and b* represent two chromatic channels representing green–red and blue–yellow respectively. The average components of L*a*b* were recorded in a standardized area of the crown (length of the standardized area: 2 cm measured from where the top of the skull angles to the frontal zone; width of the standardized area: the width of the skull at the angle between the top of the skull and the frontal zone; Fig. 1). The L*a*b* values of each integument were corrected according to the L*a*b* values of the color swatch, by using the residuals of a linear regression between the values of the crown and the values of the color swatch. In our study, color varied from black (lower values of L*, a* and b*) to orange (higher values of L*, a* and b*; Fig. S1). In addition, we recorded the standard deviation of L* (L*std), which represented the patchiness of crown coloration. L*, a*, b* and L*std values were highly correlated (Pearson correlation tests: all r > 0.66, and all P > 0.0001; except for the correlation between L*std and a, and L*std and b: r = 0.46, P = 0.001 and r = 0.43, P = 0.003 respectively). Therefore, the color of the crown was described by the first two principal components of a PCA on these four color variables (hereafter referred to as PC1color and PC2color). PC1color accounted for 74% of the variation observed among color variables, and higher PC1color indicated lighter, patchier and more orange crown (eigenvectors: L*: 0.91, a*: 0.88, b*: 0.89 and L*std: 0.76). PC2color accounted for 19% of the variation observed among color variables, and higher PC2color mainly indicated patchier crown (eigenvectors: L*: 0.26, a*: − 0.39, b*: − 0.40 and L*std: 0.61).
In animal color studies, color scores obtained from digital photographs have been suggested to be less appropriate to than those obtained with a reflectance spectrophotometer and visual models, because they do not consider the perception of color by the receiver’s visual system (Stevens et al. 2009). However, information obtained from digital pictures has revealed patterns and effects of biological meaning in several bird species (Pérez-Rodríguez and Viñuela 2008; Laucht et al. 2010; Leclaire et al. 2011). In addition, in tawny owls (Strix aluco), who show variations in pheomelanin coloration in feathers, coloration scores obtained from pictures correlate with those obtained with a spectrophotometer and with the concentration of pheomelanin pigments in feathers (Gasparini et al. 2009).
Pigment analyses
To obtain firm evidence of the pigment nature of the orange crown feathers of blue petrels, we collected feathers from three adult birds. The feathers were analyzed by micro-Raman spectroscopy, as pheomelanin and eumelanin exhibit distinctive Raman signals that can be used to identify them (Galván et al. 2013). We used a Thermo Fisher DXR confocal dispersive Raman microscope (Thermo Fisher Scientific, Madison, WI, USA) with a point-and-shoot Raman capability of 1 µm spatial resolution and using a near-infrared excitation laser of 780 nm. Laser power was set at 7 mW, integration time at 3 s and number of accumulations at 8. The spectra were obtained using a 50 × confocal objective and a slit aperture of 50 µm. The system was operated with Thermo Fisher OMNIC 8.1 software. Calibration and alignment of the spectrograph were checked using pure polystyrene. We obtained two Raman spectra from the feathers of each individual bird, and then computed the average spectrum. We only obtained Raman signal of pheomelanin, as the spectra showed the three distinctive Raman bands of this pigment at about 500, 1500 and 2000 cm−1 (Fig. S2) (Galván et al. 2013), thus confirming that the orange feathers in the crown of blue petrels are colored by pheomelanin.
Index of individual quality
We assessed individual quality by conducting a principal components analysis (PCA) on several life-history traits commonly related to individual fitness: tarsus length, body mass index, date of laying, egg volume, number of days spent incubating the egg over the whole incubating period and hatching success. Four females and one male, for whom we did not have the incubation duration, were excluded from these analyses (sample size for the analyses on individual quality: n = 19 females and 15 males).
In blue petrels, body mass and number of days spent incubating the egg have been shown to be related to reproductive success (Chaurand and Weimerskirch 1994a). Once the single egg is laid, males and females take individual incubating bouts in the nest, that can last up to 12 days and during which parents lose body mass (Chaurand and Weimerskirch 1994a). Individuals in higher body condition at the start of the incubation bout are able to take longer incubation bouts, thereby increasing hatching success (Chaurand and Weimerskirch 1994a). We therefore calculated a body mass index as the residual of a linear regression between body mass and duration of the incubating bout until capture. Because this correlation tended to depend on sex (interaction between sex and bout duration until capture: F1,35 = 3.72, P = 0.06), we performed the linear regression for each sex separately (correlation between body mass at date of capture and bout duration until capture: males: r = − 0.82, P = 0.0001; females: r = − 0.78, P < 0.0001). In a high number of studies, body condition is calculated by controlling body mass for body size effects (Schulte-Hostedde et al. 2005; Peig and Green 2009). However, in our study, body mass and corrected body mass index were not related to tarsus length (females: r = 0.10, P = 0.65 and r = 0.14, P = 0.53; males: r = − 0.43, P = 0.10 and r = − 0.20, P = 0.46). The other measures included in the PCA (i.e., tarsus length, date of laying and egg volume) have not yet been associated with reproductive success in blue petrels, but they are related to fitness in numerous other bird species (Kingsolver and Pfennig 2004; Verhulst and Nilsson 2008; Krist 2011).
Individual quality was described by the first three principal components of a PCA on traits related to individual fitness (hereafter referred to as PC1quality, PC2quality and PC3quality). PC1quality, PC2quality and PC3quality accounted for 32%, 23% and 15% of the variation observed among life-history traits. Individuals with higher PC1quality had higher body condition, spent more days incubating their egg and had higher hatching success (eigenvectors: 0.66, 0.84 and 0.68 respectively). Individuals with higher PC2quality had longer tarsus and larger egg (eigenvectors: 0.72 and 0.74 respectively). The egg of individuals with higher PC3quality was laid earlier (eigenvectors related to laying date: − 0.79).
Oxidative stress analyses
Oxidative stress was measured in plasma samples using the d-reactive oxygen metabolites (d-ROM) and the oxy-adsorbent tests (Diacron International, Grosseto, Italy) as previously described in birds including blue petrels (Costantini and Bonadonna 2010). The d-ROM test measures plasmatic hydroperoxydes, a reactive oxygen metabolite (ROM) resulting from the attack of reactive oxygen species on organic substrates (carbohydrates, lipids, amino acids, proteins, nucleotides), while the oxy-adsorbent test measures the total plasma anti-oxidant capacity.
Statistical analyses
To test the relationships between color expression in crown feathers (PC1color and PC2color) and individual quality (PC1quality, PC2quality and PC3quality), we used linear mixed models (package lme4, version 1.1-21, in R; Bates et al. 2014). PC1quality, PC2quality, PC3quality, sex, and the two-way interactions between sex and each PCquality were included in the models as fixed effects. Because pheomelanin color can fade with time (Arai et al. 2015), we included date of capture as a fixed effect also. Burrow identity was included as a random factor.
We tested the association between color scores and ROM and OXY levels using linear models. Since ROM and OXY levels were not correlated within individuals (Pearson correlation test: r = 0.002, P = 0.99), they were both included as fixed effects in the models. In addition, because the sample size was very low for males (n = 4 males), we restricted these analyses on females.
To test for assortative mating according to crown feather coloration, we used Pearson correlation tests to correlate female coloration with feather color expression of the breeding partner. All statistical analyses were carried out with the R software (R version: 3.6.0; R Core Team 2017).
Results
Color and individual quality
Plasma ROM levels were positively related to PC2quality in females (F1,6 = 6.92, P = 0.039, n = 8 females for whom we had ROM levels and all quality-related variables; Fig. S3), but not to PC1quality and PC3quality (F1,5 = 2.21, P = 0.20 and F1,5 = 0.00, P = 0.97).This result suggests that oxidative damage is related to some aspects of individual quality in females, namely larger eggs and longer tarsi. Plasma OXY levels were related to none of the PCquality (all P > 0.10).
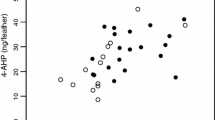
PC1color varied with the interactions between sex and PC1quality (χ21 = 4.29, P = 0.038; Fig. 2), and sex and PC2quality (χ21 = 4.15, P = 0.042; Fig. 2). In females, PC1color decreased with both PC1quality and PC2quality (F1,15 = 8.14, P = 0.012 and F1,15 = 4.94, P = 0.042; Fig. 2). In contrast, in males, PC1color did not vary with PC1quality (F1,13 = 0.00, P = 0.98; Fig. 2), and tended to increase with PC2quality (F1,13 = 3.46, P = 0.085; Fig. 2). PC1color decreased with PC3quality in both sexes (χ21 = 4.63, P = 0.031; Fig. 3). PC1color tended to decrease with date (χ21 = 2.75, P = 0.097). In females, PC1color decreased with ROM levels in plasma (F1,9 = 5.89, P = 0.038; Fig. 4), but it did not vary with OXY levels in plasma (F1,8 = 0.015, P = 0.91). PC1color therefore decreased with oxidative stress, which was calculated as ROM levels/OXY levels (F1,9 = 5.65, P = 0.041).
PC2color varied with none of the variables included in the models related to individual quality (i.e., the three PCquality and date: all P > 0.10), and neither with ROM nor OXY levels (all P > 0.15).
Sexual dichromatism and assortative mating
On average, males had higher PC1color than females (χ21 = 5.82, P = 0.016). No sex-differences in PC2color were detected (χ21 = 0.18, P = 0.89). PC1color was positively correlated among breeding pairs (r = 0.57, P = 0.047; Fig. 5), while PC2color was not (r = − 0.36, P = 0.23).
Discussion
The orange color of crown feathers, which is produced by pheomelanin, was related to fitness traits and oxidative stress in incubating blue petrels, especially in females. Females with more-intense orange coloration (i.e., higher PC1color) were poor-quality females (i.e., lower PCquality being related to lower body mass, shorter tarsi, smaller egg, shorter time spent incubating the egg, lower reproductive success and delayed laying date). Similar negative relationships between pheomelanin-based coloration and individual-quality traits were found in nestling Eurasian nuthatches (Sitta europaea) and tawny owls (Strix aluco), where individuals with more intense pheomelanin-based feathers are in poorer condition (Galván 2017) and have lower viability during adverse environmental conditions (Karell et al. 2011), respectively. Pheomelanin production has been suggested to be costly because it is phototoxic and depletes glutathione stores in melanocytes (Kinnaert et al. 2004; Napolitano et al. 2014). It thereby increases oxidative stress (Roulin et al. 2011; Mitra et al. 2012; Napolitano et al. 2014), which is known to impair numerous cell functions and reduce survival and fecundity (Bize et al. 2008). Thus the negative association between pheomelanin-based coloration and individual quality in blue petrel females may be due to the important physiological costs associated with pheomelanin expression. Non exclusively, pheomelanin-based coloration might be related to individual quality because of the functional role of pheomelanogenesis in cysteine detoxification (Galván et al. 2012b). Poor-quality females may develop more intense pheomelanin-based coloration of the crown if they have higher need for cysteine detoxification, due for instance to higher cysteine levels in the diet or higher susceptibility to cysteine toxicity. To further evaluate this hypothesis, studies are needed to determine whether excess cysteine can occur in blue petrels and cause health problems.
Whatever the physiological mechanisms linking pheomelanin expression to individual quality, the negative association between these two traits suggests that, in blue petrels, pheomelanin-based coloration may not have evolved as a social or sexual signal of individual quality in females. In several species, melanin-based coloration is determined not only by the total amount of pigments, but also by the eumelanin-to-pheomelanin ratio (Ito and Wakamatsu 2003; Singaravelan et al. 2010; Morales-Guerrero et al. 2019). Several molecules such as agouti and glutathione that trigger the production of pheomelanin have an inhibitory effect on eumelanin production, whereas other hormones, such as melanocortins, have the opposite effect (Benedetto et al. 1982; Lu et al. 1994; Furumura et al. 1996; Le Pape et al. 2008). It is therefore possible that blue petrel females with more-intense orange coloration have lower eumelanin expression in the crown. Under this hypothesis, good-quality females may be able to deposit more eumelanin into feathers than poor-quality females. Eumelanin traits are associated with variation in physiological and behavioral traits in several species (Vágási et al. 2010; Jacquin et al. 2011; Roulin et al. 2011; Arai et al. 2019). In particular, eumelanism is associated with higher fitness in females in barn owls (Roulin and Altwegg 2007), tawny owls (Strix aluco) (Roulin et al. 2003) and Eurasian kestrels (Falco tinnunculus) (Vergara et al. 2009). Interestingly, similar to barn owls (Roulin 2003), where eumelanin-based coloration is a sexual signal of quality in females only (Roulin and Altwegg 2007), blue petrel females are on average less orange-colored than blue petrel males. To determine whether eumelanin-based coloration of females’ crown can signal individual quality, studies incorporating analyses of eumelanin and pheomelanin quantities in feathers are necessary.
We found that, during incubation, high-quality females (and thus females with less-orange crowns) had higher oxidative stress, as a result of higher oxidative damage, than poor-quality females. This positive association between oxidative damage and individual quality in females seems surprising, as oxidative stress, leading to a plethora of deleterious effects on homeostasis (Jones 2006), is generally thought to be negatively related to individual quality and fitness (Bize et al. 2008). Oxidative stress and its sensitivity are known, however, to vary with several ecological factors and life-history traits, including reproductive effort (Costantini 2008; Metcalfe and Alonso-Alvarez 2010). In addition, long periods of starvation lead to elevated oxidative stress in several species (Pascual et al. 2003; Wasselin et al. 2014). The higher oxidative stress observed in good-quality females may thus be due to their increased reproductive effort and longer time spent fasting in the nest. As a result, we cannot exclude that, outside the breeding period, e.g., at the time of molt when pheomelanin pigments are deposited into feathers, high-quality females face lower oxidative stress than poor-quality females. Further studies investing oxidative stress levels during crown-feather formation are needed to further evaluate the potential for a mechanism associated with oxidative stress to be at the bases of crown color in blue petrels.
We did not find strong evidence for associations between crown color and individual quality in males. Sex-specific selective pressures on melanin-based traits may be due to differences between sexes in their sensitivity to environmental stress or to excess cysteine (Galván and Alonso-Alvarez 2009; Galván et al. 2012b). However, the sample size of our study is relatively low (n = 15 males) and further studies should include a higher sample size to draw firm conclusion on the potential association between melanin-based crown coloration and individual quality in males.
In blue petrels, males and females seem to pair assortatively by crown coloration. This type of pairing has been observed in other melanin-colored species (Bortolotti et al. 2008; Rowe and Weatherhead 2011; Indykiewicz et al. 2017). Assortative pairing can occur when there is mutual mate choice for similar ornaments (Holveck and Riebel 2009). However, the lack of association between color and individual quality in male blue petrels suggests that directional preference is not a plausible mechanism in this species. In numerous species, the behavioral compatibility of mates is a major factor determining reproductive success (Spoon et al. 2006; Ariyomo and Watt 2013; Mariette and Griffith 2015). Because of the pleiotropic effects of genes regulating melanogenesis, variation in behavior is often associated with melanin-based coloration (reviewed in Ducrest et al. 2008). Therefore, homotypic preference for behavioral traits might result in an apparent assortative mating by crown coloration in blue petrels. Assortative mating by age has also been recorded in a wide range of avian species (reviewed in Jiang et al. 2013), and it is widely acknowledged that melanin-based coloration varies with age (e.g., Potti and Montalvo 1991; Budden and Dickinson 2009; Galván and Møller 2009). Assortative mating by age might thus drive, at least to some extent, positive correlation in crown color among mates in blue petrels.
In conclusion, our result provides evidence for an association between individual quality and melanin-based color of crown feathers in female blue petrels. However, females with less-orange crowns were higher-quality females, which is inconsistent with an adaptive function of pheomelanic coloration in inter-, or intra-, sexual selection in this species. Our findings therefore open new doors for further studies that may comprehensively investigate how pheomelanin expression is adaptive in light of natural selection in petrel species.
References
Arai E, Hasegawa M, Nakamura M, Wakamatsu K (2015) Male pheomelanin pigmentation and breeding onset in barn swallows Hirundo rustica gutturalis. J Ornithol 156:419–427
Arai E, Hasegawa M, Wakamatsu K, Ito S (2018) Males with more pheomelanin have a lower oxidative balance in Asian barn swallows (Hirundo rustica gutturalis). Zool Sci 35:505–514
Arai E, Hasegawa M, Sato M, Sakai H, Ito S, Wakamatsu K (2019) Eumelanin levels in rufous feathers explain plasma testosterone levels and survival in swallows. Ecol Evol 9:2755–2764
Ariyomo TO, Watt PJ (2013) Disassortative mating for boldness decreases reproductive success in the guppy. Behav Ecol 24:1320–1326
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv:1406.5823
Benedetto J-P, Ortonne J-P, Voulot C, Khatchadourian C, Prota G, Thivolet J (1982) Role of thiol compounds in mammalian melanin pigmentation. II. Glutathione and related enzymatic activities. J Investig Dermatol 79:422–424
Bize P, Devevey G, Monaghan P, Doligez B, Christe P (2008) Fecundity and survival in relation to resistance to oxidative stress in a free-living bird. Ecology 89:2584–2593
Bortolotti GR, González LM, Margalida A, Sánchez R, Oria J (2008) Positive assortative pairing by plumage colour in Spanish imperial eagles. Behav Process 78:100–107
Bretagnolle V (1996) Acoustic communication in a group of nonpasserine birds, the petrels. Ecol Evol Acoust Commun Birds 160:177
Brooke MDL (1989) Determination of the absolute visual threshold of a nocturnal seabird, the common diving petrel Pelecanoides urinatrix. Ibis 131:290–294
Budden AE, Dickinson JL (2009) Signals of quality and age: the information content of multiple plumage ornaments in male western bluebirds Sialia mexicana. J Avian Biol 40:18–27
Chaurand T, Weimerskirch H (1994a) Incubation routine, body mass regulation and egg neglect in the blue petrel Halobaena caerulea. Ibis 136:285–290
Chaurand T, Weimerskirch H (1994b) The regular alternation of short and long foraging trips in the blue petrel Halobaena caerulea: a previously undescribed strategy of food provisioning in a pelagic seabird. J Anim Ecol 63:275–282
Cohen Alan A, McGraw Kevin J, Wiersma P, Williams Joseph B, Robinson WD, Robinson Tara R et al (2008) Interspecific associations between circulating antioxidant levels and life-history variation in birds. Am Nat 172:178–193
Core Team R (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Costantini D (2008) Oxidative stress in ecology and evolution: lessons from avian studies. Ecol Lett 11:1238–1251
Costantini D, Bonadonna F (2010) Patterns of variation of serum oxidative stress markers in two seabird species. Polar Res 29:30–35
Costantini D, Coluzza C, Fanfani A, Dell’Omo G (2007) Effects of carotenoid supplementation on colour expression, oxidative stress and body mass in rehabilitated captive adult kestrels (Falco tinnunculus). J Comp Physiol B 177:723–731
Ducrest A-L, Keller L, Roulin A (2008) Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol Evol 23:502–510
El-Khairy L, Vollset SE, Refsum H, Ueland PM (2003) Plasma total cysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine Study. Am J Clin Nutr 77:467–472
Furumura M, Sakai C, Abdel-Malek Z, Barsh GS, Hearing VJ (1996) The interaction of agouti signal protein and melanocyte stimulating hormone to regulate melanin formation in mammals. Pigment Cell Res 9:191–203
Galván I (2017) Condition-dependence of pheomelanin-based coloration in nuthatches Sitta europaea suggests a detoxifying function: implications for the evolution of juvenile plumage patterns. Sci Rep 7:9138
Galván I (2018) Predation risk determines pigmentation phenotype in nuthatches by melanin-related gene expression effects. J Evol Biol 31:1760–1771
Galván I, Alonso-Alvarez C (2009) The expression of melanin-based plumage is separately modulated by exogenous oxidative stress and a melanocortin. Proc R Soc B 276:3089–3097
Galván I, Alonso-Alvarez C (2017) Individual quality via sensitivity to cysteine availability in a melanin-based honest signaling system. J Exp Biol 220:2825–2833
Galván I, Møller AP (2009) Different roles of natural and sexual selection on senescence of plumage colour in the barn swallow. Funct Ecol 23:302–309
Galván I, Solano F (2009) The evolution of eu-and pheomelanic traits may respond to an economy of pigments related to environmental oxidative stress. Pigment Cell Melanoma Res 22:339–342
Galván I, Solano F (2016) Bird integumentary melanins: biosynthesis, forms, function and evolution. Int J Mol Sci 17:520
Galván I, Alonso-Alvarez C, Negro JJ (2012a) Relationships between hair melanization, glutathione levels, and senescence in wild boars. Physiol Biochem Zool 85:332–347
Galván I, Ghanem G, Møller AP (2012b) Has removal of excess cysteine led to the evolution of pheomelanin? Pheomelanogenesis as an excretory mechanism for cysteine. BioEssays 34:565–568
Galván I, Jorge A, Ito K, Tabuchi K, Solano F, Wakamatsu K (2013) Raman spectroscopy as a non-invasive technique for the quantification of melanins in feathers and hairs. Pigment Cell Melanoma Res 26:917–923
Galván I, Inácio Â, Romero-Haro AA, Alonso-Alvarez C (2017) Adaptive downregulation of pheomelanin-related Slc7a11 gene expression by environmentally induced oxidative stress. Mol Ecol 26:849–858
Gasparini J, Bize P, Piault R, Wakamatsu K, Blount JD, Ducrest AL et al (2009) Strength and cost of an induced immune response are associated with a heritable melanin-based colour trait in female tawny owls. J Anim Ecol 78:608–616
Gerstenblith MR, Shi J, Landi MT (2010) Genome-wide association studies of pigmentation and skin cancer: a review and meta-analysis. Pigment Cell Melanoma Res 23:587–606
Hau M, Haussmann MF, Greives TJ, Matlack C, Costantini D, Quetting M et al (2015) Repeated stressors in adulthood increase the rate of biological ageing. Front Zool 12:4
Herborn KA, Daunt F, Heidinger BJ, Granroth-Wilding HMV, Burthe SJ, Newell MA et al (2016) Age, oxidative stress exposure and fitness in a long-lived seabird. Funct Ecol 30:913–921
Hill GE (2014) Cellular respiration: the nexus of stress, condition, and ornamentation. Integr Comp Biol 54:645–657
Holveck M-J, Riebel K (2009) Low-quality females prefer low-quality males when choosing a mate. Proc R Soc B 277:153–160
Indykiewicz P, Podlaszczuk P, Surmacki A, Kudelska K, Kosicki J, Kamiński M et al (2017) Scale-of-choice effect in the assortative mating by multiple ornamental and non-ornamental characters in the black-headed gull. Behav Ecol Sociobiol 71:183
Ito S, Wakamatsu K (2003) Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res 16:523–531
Jacquin L, Lenouvel P, Haussy C, Ducatez S, Gasparini J (2011) Melanin-based coloration is related to parasite intensity and cellular immune response in an urban free living bird: the feral pigeon Columba livia. J Avian Biol 42:11–15
Jawor JM, Breitwisch R (2003) Melanin ornaments, honesty, and sexual selection. Auk 120:249–265
Jiang Y, Bolnick DI, Kirkpatrick M (2013) Assortative mating in animals. Am Nat 181:E125–E138
Jones DP (2006) Redefining oxidative stress. Antioxid Redox Signal 8:1865–1879
Karell P, Ahola K, Karstinen T, Valkama J, Brommer JE (2011) Climate change drives microevolution in a wild bird. Nat Commun 2:208
Kingsolver JG, Pfennig DW (2004) Individual-level selection as a cause of Copes’ rule of phyletic size increase. Evolution 58:1608–1612
Kinnaert E, Duez P, Morandini R, Dubois J, Van Houtte P, Ghanem G (2004) Cysteine but not glutathione modulates the radiosensitivity of human melanoma cells by affecting both survival and DNA damage. Pigment Cell Res 17:275–280
Krist M (2011) Egg size and offspring quality: a meta-analysis in birds. Biol Rev 86:692–716
Laucht S, Kempenaers B, Dale J (2010) Bill color, not badge size, indicates testosterone-related information in house sparrows. Behav Ecol Sociobiol 64:1461–1471
Le Pape E, Wakamatsu K, Ito S, Wolber R, Hearing VJ (2008) Regulation of eumelanin/pheomelanin synthesis and visible pigmentation in melanocytes by ligands of the melanocortin 1 receptor. Pigment Cell Melanoma Res 21:477–486
Leclaire S, Bourret V, Wagner RH, Hatch SA, Helfenstein F, Chastel O et al (2011) Behavioral and physiological responses to male handicap in chick-rearing black-legged kittiwakes. Behav Ecol 22:1156–1165
Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T et al (1994) Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371:799
Mariette MM, Griffith SC (2015) The adaptive significance of provisioning and foraging coordination between breeding partners. Am Nat 185:270–280
McGraw KJ (2006) Mechanics of melanin-based coloration. In: Hill GE, McGraw KJ (eds) Bird coloration. I. Mechanisms and measurements. Harvard University Press, Cambridge
Metcalfe NB, Alonso-Alvarez C (2010) Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct Ecol 24:984–996
Mitkus M, Nevitt GA, Danielsen J, Kelber A (2016) Vision on the high seas: spatial resolution and optical sensitivity in two procellariiform seabirds with different foraging strategies. J Exp Biol 219:3329–3338
Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J et al (2012) An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature 491:449
Morales-Guerrero B, Gendron D, Martinez-Levasseur LM, Acevedo-Whitehouse K (2019) Blue whale (Balaenoptera musculus) skin contains eumelanin and pheomelanin. Aquat Mamm 45:88–98
Nachman MW, Hoekstra HE, D’Agostino SL (2003) The genetic basis of adaptive melanism in pocket mice. Proc Natl Acad Sci USA 100:5268–5273
Napolitano A, Panzella L, Monfrecola G, d’Ischia M (2014) Pheomelanin-induced oxidative stress: bright and dark chemistry bridging red hair phenotype and melanoma. Pigment Cell Melanoma Res 27:721–733
Negro JJ, Bortolotti GR, Mateo R, García IM (2009) Porphyrins and pheomelanins contribute to the reddish juvenal plumage of black-shouldered kites. Comp Biochem Physiol B 153:296–299
Olney JW, Ho O-L (1970) Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature 227:609–611
Orth MW, Bai Y, Zeytun IH, Cook ME (1992) Excess levels of cysteine and homocysteine induce tibial dyschondroplasia in broiler chicks. J Nutr 122:482–487
Panzella L, Leone L, Greco G, Vitiello G, D’Errico G, Napolitano A et al (2014) Red human hair pheomelanin is a potent pro-oxidant mediating UV-independent contributory mechanisms of melanomagenesis. Pigment Cell Melanoma Res 27:244–252
Pascual P, Pedrajas JR, Toribio F, López-Barea J, Peinado J (2003) Effect of food deprivation on oxidative stress biomarkers in fish (Sparus aurata). Chem Biol Interact 145:191–199
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Pérez-Rodríguez L, Viñuela J (2008) Carotenoid-based bill and eye ring coloration as honest signals of condition: an experimental test in the red-legged partridge (Alectoris rufa). Naturwissenschaften 95:821–830
Phillips RA, Bearhop S, Mcgill RAR, Dawson DA (2009) Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160:795–806
Potti J, Montalvo S (1991) Male colour variation in Spanish pied flycatchers Ficedula hypoleuca. Ibis 133:293–299
Rodríguez-Martínez S, Márquez R, Inácio Â, Galván I (2019) Changes in melanocyte RNA and DNA methylation favour pheomelanin synthesis and may avoid systemic oxidative stress after dietary cysteine supplementation in birds. Mol Ecol 28:1030–1042
Roulin A (2003) Geographic variation in sexual dimorphism in the barn owl Tyto alba: a role for direct selection or genetic correlation? J Avian Biol 34:251–258
Roulin A, Altwegg R (2007) Breeding rate is associated with pheomelanism in male and with eumelanism in female barn owls. Behav Ecol 18:563–570
Roulin A, Ducret B, Ravussin P-A, Altwegg R (2003) Female colour polymorphism covaries with reproductive strategies in the tawny owl Strix aluco. J Avian Biol 34:393–401
Roulin A, Almasi B, Meichtry-Stier KS, Jenni L (2011) Eumelanin- and pheomelanin-based colour advertise resistance to oxidative stress in opposite ways. J Evol Biol 24:2241–2247
Rowe KM, Weatherhead PJ (2011) Assortative mating in relation to plumage traits shared by male and female American robins. The Condor 113:881–889
Safran RJ, McGraw KJ (2004) Plumage coloration, not length or symmetry of tail-streamers, is a sexually selected trait in North American barn swallows. Behav Ecol 15:455–461
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass–size residuals: validating body condition indices. Ecology 86:155–163
Siefferman L, Hill GE (2003) Structural and melanin coloration indicate parental effort and reproductive success in male eastern bluebirds. Behav Ecol 14:855–861
Singaravelan N, Pavlicek T, Beharav A, Wakamatsu K, Ito S, Nevo E (2010) Spiny mice modulate eumelanin to pheomelanin ratio to achieve cryptic coloration in “Evolution Canyon,” Israel. PLoS ONE 5:e8708
Spoon TR, Millam JR, Owings DH (2006) The importance of mate behavioural compatibility in parenting and reproductive success by cockatiels, Nymphicus hollandicus. Anim Behav 71:315–326
Stevens M, Cuthill IC (2005) The unsuitability of html-based colour charts for estimating animal colours—a comment on Berggren and Merilä (2004). Front Zool 2:14
Stevens M, Stoddard MC, Higham JP (2009) Studying primate color: towards visual system-dependent methods. Int J Primatol 30:893–917
Tickell WLN (1962) The dove prion, Pachyptila desolata Gmelin. HMSO, London
Vágási CI, Pap PL, Barta Z (2010) Haste makes waste: accelerated molt adversely affects the expression of melanin-based and depigmented plumage ornaments in house sparrows. PLoS ONE 5:e14215
van Franeker JA, van den Brink NW, Bathmann UV, Pollard RT, de Baar HJW, Wolff WJ (2002) Responses of seabirds, in particular prions (Pachyptila sp.), to small-scale processes in the Antarctic Polar Front. Deep Sea Res Part 2 Top Stud Oceanogr 49:3931–3950
Vergara P, Fargallo JA, Martinez-Padilla J, Lemus JA (2009) Inter-annual variation and information content of melanin-based coloration in female Eurasian kestrels. Biol J Linn Soc 97:781–790
Verhulst S, Nilsson J-Å (2008) The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos Trans R Soc Lond B Biol Sci 363:399–410
Warham J (1996) The behaviour, population biology and physiology of the petrels. Academic Press, London
Wasselin T, Zahn S, Maho YL, Dorsselaer AV, Raclot T, Bertile F (2014) Exacerbated oxidative stress in the fasting liver according to fuel partitioning. Proteomics 14:1905–1921
Wilson AJ, Nussey DH (2010) What is individual quality? An evolutionary perspective. Trends Ecol Evol 25:207–214
Zahavi A (1975) Mate selection—selection for a handicap. J Theor Biol 53:205–214
Acknowledgements
We thank Joris Laborie for his help during fieldwork, and Felipe Ramon-Portugal and Gilles Espinasse for their help during the first stage of pigment characterization. This work was supported by the Institut Polaire Français Paul-Emile Victor (IPEV, Program No. 354 to F.B.), and by a PDOC grant from the Agence Nationale de la Recherche (No. ANR-13-PDOC-0002 to S.L.). This study was approved by the French Ethical Committee (APAFIS#: 9496-201707131540776v2), after favorable recommendation by the “comité d’éthique pour l’expérimentation animale - Languedoc-Roussillon”, and the ethical committees of “réserve naturelle des Terres Australes et Antarctiques Françaises (TAAF)” and of “Institut Polaire Rrançais Paul-Emile Victor (IPEV)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leclaire, S., Perret, S., Galván, I. et al. Pheomelanin-based coloration is related to individual quality and oxidative stress in blue petrels. Evol Ecol 33, 873–887 (2019). https://doi.org/10.1007/s10682-019-10010-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-10010-7