Abstract
Plant populations can be locally adapted and the strength of local adaptation is predicted to increase with increasing environmental distance, e.g. to be larger across than within regions. Meta-analyses comparing reciprocal transplant studies across various taxa confirmed this pattern, whereas single studies including various spatial scales are rare. We transplanted plants among locations of six populations of the herbaceous plant Anthyllis vulneraria in the European Alps. We assessed survival and measured aboveground biomass, reproductive allocation and flowering propensity to test for local adaptation at two spatial scales: within and between two climatically contrasting regions in the Eastern and Western Swiss Alps. Performance of transplanted Anthyllis vulneraria varied between spatial scales. Transplant survival did not show patterns of local adaptation. However, total aboveground biomass, reproductive allocation and flowering propensity were lowest when plants were transplanted to another region, compared with transplantations within regions and to the site of origin. These results indicate local adaptation of populations across regions, but not within regions. Our findings suggest that environmental variation across alpine regions, potentially the contrasting precipitation pattern, is a strong driver of local adaptation. A previous microsatellite study suggested that gene flow is restricted even within populations; therefore, the absence of local adaptation within regions is likely due to weak environmental variation rather than to gene flow counteracting local adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Local adaptation can arise from spatial variation in environmental conditions and is expected when populations of a species experience consistent divergent selection, when they are sufficiently genetically isolated, and have adequate genetic variation (Endler 1977, 1987; Conner and Hartl 2004). Accordingly, local adaptation should be strong in plant populations across large geographical and environmental distances, which create strong selection pressures and limit gene flow among them (Joshi et al. 2001; Kawecki and Ebert 2004; Becker et al. 2006; Banta et al. 2007; Gonzalo-Turpin and Hazard 2009). In contrast, at small geographic distances, gene flow may counteract the process of local adaptation by homogenizing allele frequencies (Conner and Hartl 2004), and at small environmental distances local adaptation may not be necessary for population survival due to low environmental variation (Becker et al. 2006).
To investigate local adaptation in natural populations, the reciprocal-transplant design has become the gold standard (Clausen et al. 1941; Kawecki and Ebert 2004). However, reciprocal transplant experiments have rarely been performed at several spatial scales to specifically study the strength of local adaptation and the relative roles of geographic and environmental distances. Amongst the few studies that investigated the spatial scale of adaptive evolution, Galloway and Fenster (2000) found little evidence for local adaptation in an annual legume, except when geographic distances exceeded 1000 km. Similarly, Toräng et al. (2014) found local adaptation in arctic–alpine Arabis alpina when populations were seperated by more than 3000 km, but not within smaller spatial scales. In contrast, Hamann et al. (2016) showed that a common alpine fodder grass was locally adapted across regions separated by only 200 km, and also found evidence for adaptive differentiation within regions at geographic distances below 20 km. Although these and other studies (Sambatti and Rice 2006; Hereford and Winn 2008; Anderson et al. 2015; Peterson et al. 2016) did not specifically investigate environmental variables, it seems likely that the strength of local adaptation increases with spatial scale due to increasingly diverging conditions among transplant sites, but not necessarily with geographic distance (Bischoff et al. 2006). Indeed, two meta-analyses of reciprocal transplant studies across many taxa confirmed that the strength of local adaptation increased with increasing ecological distance (Hereford 2009) and did not correlate with geographic distance (Leimu and Fischer 2008). Yet, more studies are still needed to test for local adaptation across multiple ecological scales within single reciprocal transplant experiments to improve our understanding of the conditions under which local adaptation evolves.
In this context, mountain landscapes are particularly suitable for the study of local adaptation because of strong environmental heterogeneity across relatively small spatial scales (Stöcklin et al. 2009). Within a mountain system, different regions may be governed by particular regional climates (Ozenda 1988) leading to divergent selective pressures across larger geographic disctances. At smaller spatial scales within those regions environmental heterogeneity—e.g. in microclimate, soil conditions and biotic interactions—may also be strong due to variation in elevation, aspect, slope and other factors (Körner 2003). While studies in mountain systems usually find considerable phenotypic differentiation among plant populations (e.g. Frei et al. 2011), there is mixed evidence for the prevalence of local adaptation (Galen et al. 1991; Angert and Schemske 2005; Geber and Eckhart 2005; Byars et al. 2007; Gonzalo-Turpin and Hazard 2009; Anderson et al. 2015; Sedlacek et al. 2015; Hamann et al. 2016; Hirst et al. 2016; Halbritter et al. 2018). In mountain landscapes, the divergent climatic conditions and the virtual absence of gene flow across regions should increase the prevalence of local adaptation across larger spatial scales. However, with environmental heterogeneity being strong at small spatial scales, local adaptation may also prevail over short geographic distances when adaptive evolution is not hindered by strong gene flow (Gonzalo-Turpin and Hazard 2009; Hirst et al. 2016).
In the current study, we reciprocally transplanted six populations of Anthyllis vulneraria L. across two spatial scales; within and between regions in the Eastern and Western Swiss Alps, which differ in precipitation and temperature regime. Anthyllis vulneraria is a widespread and fast-growing perennial species that inhabits a range of environmental conditions, making it ideal for testing predictions about local adaptation over different spatial scales. Furthermore, a previous study revealed substantial among-population differentiation at neutral microsatellite markers (Kesselring et al. 2015), suggesting low gene flow, which could facilitate local adaptation. Individuals from each of six alpine populations (three in each region) were transplanted to their site of origin (local), to another site in the same region (INTRAregional), and to a site in the other region (INTERregional). We monitored plant survival over two growing seasons and measured aboveground biomass and flowering propensity as indicators of plant performance. We tested for local adaptation using the sympatric versus allopatric criterion, i.e. comparing the average performance of plants transplanted to their local site versus the average performance of plants in INTRAregional and INTERregional transplants (Blanquart et al. 2013). We further compared results from our phenotypic data with previously investigated genetic differentiation of these same populations (Kesselring et al. 2015) to infer on the role of gene flow in the adaptive evolution of these populations. Specifically, our study addresses the following questions: (1) is there evidence for local adaptation among alpine populations of Anthyllis vulneraria? (2) does the geographic scale of transplantation (i.e. INTRA- versus INTERregional) explain the occurrence and/or strength of local adaptation? (3) can the previously investigated neutral genetic differentiation explain patterns of local adaptation?
Materials and methods
Study species
Anthyllis vulneraria L. sensu lato (s.l.; Fabaceae) is a clade of self-compatible short-lived perennial rosette plants common throughout Europe. It grows preferably on calcareous grassland and scree up to 3000 m a.s.l. (Hegi 1975). Plants grow to a height of ca. 15–45 cm. Each plant comprises a variable number of shoots, of which each bears 2–6 inflorescences. Each inflorescence contains a number of 7–19 mm long white to yellow, sometimes claret to red flowers arranged in a capitulum (Hegi 1975; Navarro 1999a). Selfed and geitonogamous offspring may be produced due to the spatial co-location of self-pollen and stigma and the asynchronous flower ripening across capitula and shoots. Populations of Anthyllis vulneraria may be exclusively selfing (Couderc 1971) or may be protandrous to a degree where selfing is effectively prevented (Navarro 1999b). Anthyllis vulneraria s.l. is a particularly polymorphic taxon with unclear infraspecific classification (Nanni et al. 2004; Köster et al. 2008). We have assigned the alpine populations studied here to Anthyllis vulneraria ssp. alpestris (Schult.) Asch. and Graben, and to Anthyllis vulneraria ssp. valesiaca (Beck) Guyot (Lauber et al. 2012). Two populations in the Western region (Findelgletscher and Findelwald) belong to Anthyllis vulneraria ssp. valesiaca while the other four populations belong to Anthyllis vulneraria ssp. alpestris.
Reciprocal transplantations
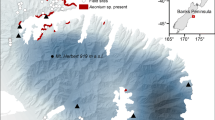
Our study spanned two regions in the Swiss Alps, namely the region Zermatt, located in the Canton of Valais in the Western Swiss Alps, and the region Davos, located in the Canton of Grisons in the Eastern Swiss Alps. Six sites, three from each region, were chosen for their Anthyllis vulneraria populations of at least 200 individuals, and for their comparable elevations, ranging between 2010 and 2650 m a.s.l (Fig. 1b). The distance between regions approximates 180 km, and distances between populations within regions range from 2 to 18 km (Fig. 1a). For each region we retrieved climate data from two nearby MeteoSwiss (2015) weather stations from similar elevations as our sites and averaged long-term climate data (i.e. 1984–2010). For the three Western sites near Zermatt we estimated an average annual temperature of 2.3 °C, an annual rainfall sum of 1046 mm and an annual snowfall depth of 672 cm at an average elevation of 1960 m a.s.l (Fig. 2a). For the three Eastern sites near Davos we estimated an annual average temperature of 0.8 °C, an annual precipitation sum of 1216 mm and an annual depth of snowfall of 725 cm at an average elevation of 2142 m a.s.l. (Fig. 2b). As such, the Western region is warmer and drier than the Eastern region (MeteoSwiss 2015). The differences between regions are even more pronounced when comparing temperature and precipitation patterns solely during the growing season (June-October). The Western sites have 1.5 °C warmer temperatures while receiving ca. 30% less precipitation than the Eastern sites (Fig. 2a, b).
Population and site information for the six Anthyllis vulneraria populations used in this study: a map of the population locations in close proximity to the villages of Zermatt (in black) and Davos (in grey) in Switzerland, b table of population abbreviations, their coordinates (WGS84), and their elevation. The last column shows which populations were used for the local transplant, for the INTRAregional transplant, and for the INTERregional transplant, respectively at each site
Climate diagram for a Western sites located near Zermatt and b Eastern sites located near Davos with mean monthly temperatures indicated by the solid line and left y-axes and monthly precipitation sum indicated by the bars and the right y-axes. The diagrams were constructed by averaging climate data (MeteoSwiss from 1981–2010) from the two weather stations closest to our sites in each region, and representative for the average elevation of our study sites. For the Western sites, we used climate data from Zermatt village (1638 m a.s.l.) and Gütsch ob Andermatt (2283 m a.s.l.) and calculated climate for an average elevation of 1960 m a.s.l. For the Eastern sites, we used climate data from Davos village (1594 m a.s.l.) and Weissfluhjoch (2691 m a.s.l.) and calculated climate for an average elevation of 2142 m a.s.l
Seeds of Anthyllis vulneraria were collected in the second week of August 2012. From each of the six populations, seeds were collected from 45 individual maternal plants spaced at a minimum distance of 4 m to avoid sampling closely related individuals. One week later, seeds were scarified and germinated on wet filter paper in Petri dishes in the greenhouse. In an effort of raising enough seedlings for each transplant type, a minimum of three seedlings per maternal plant from each population were potted in multitrays (54-pots of 4 cm ø and 5 cm depth) filled with low nutrient soil (Anzuchterde, Ökohum GmbH, Herrenhof, Switzerland). Seedlings were established in the greenhouse for four weeks under natural daylight, with temperatures ranging between 16–20 °C during the day and 8–10 °C during the night.
Transplantations to field sites were performed mid-September 2012 before the onset of adverse autumn conditions. Following the same design as in our previous work (Hamann et al. 2016), the transplant experiment consisted of planting seedlings from maternal plants from each population to their site of origin (local), to one site within the same region (INTRAregional) and to one site in the other region (INTERregional). Thereby, each site received individuals originating from maternal plants of its local population, individuals from a foreign population from the same region, and individuals from one population from the other region. Combinations of foreign populations and sites were chosen randomly, with the restriction that each population was only used once in each transplant type (see detail for each site in Fig. 1b). This transplant design was chosen rather than transplanting all populations to all sites, because a full-factorial design would lead to the over-representation of allopatric transplant combinations (i.e. INTRAregional and INTERregional) compared to sympatric transplant combinations (i.e. local) (Blanquart et al. 2013). Individuals were directly transplanted from the multitrays into the native soil at field sites by digging a small hole the size of the root ball, while leaving the surrounding native vegetation intact. To further avoid root disturbance, plants were transplanted with the low nutrient potting soil, and air spaces were filled with loosened soil from the field sites. Finally, each plant was watered with 200 mL of water to facilitate root contact with the local soil and plant establishment. Ten seedlings were planted in one row, alternating between individuals from the local, INTRAregional, and INTERregional transplant type, and spacing individuals at 20 cm distance from each other. Each site received 135 individuals (i.e. 45 individuals representing offspring from 45 distinct maternal plants from each of the three transplanted populations) that were arranged in 14 rows (i.e. rows 1–13 with 10 individuals and row 14 with 5 individuals). Additional replicates (57 in total) that were raised per mother plant as potential substitutes were not discarded but added to the appropriate transplant sites to increase overall sample size so that a total of 867 Anthyllis vulneraria individuals were transplanted across the six sites.
Survival was monitored twice at all sites, in September 2013 and at harvest, in September 2014, to assess yearly survival (i.e. September 2012–2013, September 2013–2014). At two of the sites (Monstein and Findelgletscher), we also monitored survival in July 2013 to assess initial transplant shock and winter survival (i.e. September 2012–July 2013) as well as survival during the growing period (July–September 2013).
At the end of the second growing season in September 2014, flowering status (0/1) was assessed for each individual plant. Aboveground biomass of all Anthyllis vulneraria plants was harvested and divided into reproductive and vegetative biomass, before being dried in the oven at 80 °C for 48 h and weighed. Total aboveground biomass was calculated by summing the reproductive and vegetative biomass, and reproductive allocation was calculated as the reproductive biomass divided by the total aboveground biomass.
Statistical analysis
We used linear mixed-effects models for the analysis of total aboveground biomass and reproductive allocation based on the sympatric versus allopatric contrast to test for local adaptation (Blanquart et al. 2013). We use the term local to refer to individuals transplanted in sympatry, i.e. naturally occurring site by population combinations, and INTRAregional and INTERregional to refer to individuals transplanted in allopatry, i.e. to experimentally created site by population combinations within and between the two regions, respectively. We specified models in the lmerTest package (Kuznetsova et al. 2013) for R (R Development Core Team 2017), which included the factors site, population and transplant type, the latter describing whether a combination of site and population was local, INTRAregional or INTERregional. While transplant type was specified as a fixed effect, site and population were specified as random effects because individuals were never transplanted to all six sites and sites never received all six populations. Initially, it was intended to include mother plant identity (i.e. seed families) in the models as a random effect, but this term was omitted because extensive mortality was leading to highly unbalanced family memberships in the transplant types. We used Type 3 sums of squares and Satterthwaite approximations for degrees of freedom as implemented in the lmerTest package. Likelihood ratio tests were performed to assess significance levels of the random effects of site and population, and F-tests to assess significance levels of the fixed effect of transplant type. No data transformation was needed for total biomass and reproductive allocation to comply with model assumptions.
Significant site or population terms indicate differences in the performance due to site effects such as soil fertility or microclimate and due to intrinsic population quality, respectively. A significant transplant type factor indicates that populations performed differently depending on whether they were transplanted locally, INTRAregionally or INTERregionally. If transplant type was significant, we used the lsmeans package (Lenth 2016) to perform post-hoc pairwise comparisons using least-squares means to reveal which transplant types differed. Significantly higher performance in local than in INTRAregional transplant types would indicate local adaptation at the scale of populations within regions. Significantly higher performance in local compared to INTERregional transplant types would indicate local adaptation at the regional scale.
Survival and flowering propensity were analysed using generalized linear mixed-effects models of the lme4 package (Bates et al. 2015) with a binomial distribution and the logit link function. Identical model specifications were used as for the linear mixed-effects models above. Likelihood ratio tests were performed to assess significance levels of all factors, and least-squares means were used as above for pairwise comparisons of transplant types.
Results
Survival after transplantation and after the first winter was monitored in July 2013 at one site per region (Table 1). 74% of individuals survived at the Monstein site in Davos, compared to 42% at the Findelgletscher site in Zermatt. In September 2013, survival was monitored after the first growing season at all sites. Only two individuals had died during the growing season (between July and September 2013) at the Findelgletscher site and five at the Monstein site, suggesting that most mortality occurred right after transplantation or during the first winter before the growing season at these sites. Across all sites, 475 individuals had survived from the start of the experiment until September 2013 (55%; Table 1). While survival from initial transplanting into the field until the end of the first growing season did not depend on the transplant type or population (df = 2, LR = 2.25, P(χ2) = 0.52; df = 1, LR = 1.01, P(χ2) = 0.31, respectively; Table 2), the site factor was significant (df = 1, LR = 141.1, P(χ2) < 10−4; Table 2). This site effect was mainly due to an uncommonly low survival at the Stafelalp site in Zermatt (14%).
Survival throughout the second year was higher than during the first year (Table 1): out of the 475 plants living at all six sites in September 2013, 315 (66%) survived throughout the second winter and the growing season before the final harvest in September 2014. Again, survival in 2013–2014 was neither dependent on transplant type nor on population (df = 2, LR = 0.22, P(χ2) = 0.89; df = 1, LR = 0.33, P(χ2) = 0.56, respectively; Table 2), but differed between sites (df = 1, LR = 60.1, P(χ2) < 10−4; Table 2). The lowest survival occurred at the Findelwald site in Zermatt (11%).
Individuals at the Monstein site were very small and none flowered due to intense cattle grazing. Hence, this site was excluded from analysis of total aboveground biomass, reproductive allocation and flowering propensity.
Total aboveground biomass of Anthyllis vulneraria varied between sites and populations (df = 1, LR = 106.3, P(χ2) < 10−4, df = 1, LR = 10.9, P(χ2) < 10−4, respectively; Table 3). The transplant type was also significant (df = 2, F-ratio = 15.5, P(F) < 10−4; Table 3). The average total aboveground biomass across the experiment was lower in INTERregional plants compared to local plants and compared to INTRAregional plants (Fig. 3a; see also Fig. S1a for population by site averages). However, total aboveground biomass did not differ significantly between local and INTRAregional transplant types (Fig. 3a).
Reproductive allocation of Anthyllis vulneraria varied significantly across sites but not between populations (df = 1, LR = 25.4, P(χ2) < 10−4, df = 1, LR = 2.9, P(χ2) = 0.09, respectively; Table 3). Moreover, a significant transplant type effect was found for reproductive allocation (df = 2, F-ratio = 8.8, P(F) < 10−4; Table 3). Reproductive allocation decreased significantly when populations were transplanted across regions. The INTERregional transplants allocated the least resources to reproductive structures compared to the local transplant type, while INTRAregional transplant types did not significantly differ from local or INTERregional transplant types (Fig. 3b; see also Fig. S1b for population by site averages).
During the second growing season (2013–2014), populations of Anthyllis vulneraria in local combinations of populations and sites had 76% flowering propensity, INTRAregional combinations 66%, and INTERregional only 62% (Fig. 3c). Transplant type significantly affected flowering propensity (df = 2, LR = 6.96, P(F) = 0.03; Table 3). The INTERregional transplant type had a significantly lower flowering propensity than the local transplant type, while the flowering propensity in INTRAregional transplant types did not significantly differ from the local or INTERregional transplant types (Fig. 3c). However, flowering propensity was neither affected by site nor by population (df = 1, LR = 0.79, P(χ2) = 0.37, df = 1, LR = 0.85, P(χ2) = 0.35, respectively; Table 3; see also Fig. S1c for population by site averages).
Discussion
Determining the scale of local adaptation in widespread plant species is important for understanding their ecology and evolution. In the current study, in which we transplanted six alpine populations of Anthyllis vulneraria within their sites of origin and across two spatial scales, we found a signature of local adaptation; total aboveground biomass, reproductive allocation and flowering propensity decreased with increasing spatial scale of transplants.
When populations of Anthyllis vulneraria were transplanted to an allopatric site, their average performance decreased, and more strongly so when transplanted across regions (INTERregionally) compared to within regions (INTRAregionally). Total aboveground biomass, reproductive allocation and flowering propensity all reflected local adaptation of Anthylllis vulneraria populations across regions, whereas these traits never reflected local adaptation within regions although a trend was visible for reproductive allocation and flowering propensity (Fig. 3). These results suggest that the regional environmental differences are stronger than those within regions, causing stronger local adaptation between than within regions. The observed pattern is in accordance with a QST–FST study on the same populations with plants reared in a common garden (Kesselring et al. 2015), which showed that flowering phenology (but not biomass traits) has been under past selection and showed stronger differences between than within regions.
Previous transplant studies showed regional differences over similar as well as over much larger distances (Gonzalo-Turpin and Hazard 2009; Toräng et al. 2014; Sedlacek et al. 2015; Hamann et al. 2016) and mostly failed to show local adaptation at smaller spatial scales (Becker et al. 2006; Hirst et al. 2016; Hamann et al. 2017). Similarly, we could not detect significant local adaptation within regions in our study. The decreasing reproductive allocation and flowering propensity with increasing transplant distance may suggest that the strength of environmental selection is generally weaker or spatially or temporally less consistent within than between regions (Becker et al. 2006; Hereford and Winn 2008) or that phenotypic plasticity of functional traits is strong enough to adjust to the environmental heterogeneity experienced by plants within regions (Hamann et al. 2017).
An alternative explanation would be that the strength of environmental selection is similar within and between regions but that the difference in the strength of gene flow among populations is responsible for the observed patterns of local adaptation, with strong gene flow counteracting local adaptation at smaller spatial scales (Stanton and Galen 1997; Lenormand 2002; Sambatti and Rice 2006; Sexton et al. 2011). Kesselring et al. (2015) showed that differentiation at neutral microsatellite loci accorded with the spatial distribution of populations, with stronger differentiation across (FST-range: 0.038–0.127) than within regions (FST-range Davos: 0.014–0.084; Zermatt: 0.000–0.052), and thus mirrored the observed patterns of local adaptation. Nevertheless, the pairwise population differentiation was almost always significant, indicating restricted gene flow even among populations within regions. Thus, we think that gene flow between and within regions did not hamper phenotypic differentiation and local adaptation. Furthermore, it is well established that population size is generally positively related with the strength of local adaptation (Leimu and Fischer 2008). However, we do not think that population size affected our results since all study populations had at least 200 individuals and were therefore unlikely to have a restricted genetic diversity and ability to adapt to local environmental conditions. Since local adaptation was probably not limited by population size or counteracted by gene flow, we suggest that regional adaptation may have been caused by environmental differences.
As we did not specifically set out to test the effect of certain environmental variables on local adaptation in Anthyllis vulneraria, it is difficult to determine the responsible selective agents. Although the European Alps are environmentally heterogeneous at small spatial scales, environmental differences can be even greater across regions, for instance due to regionally varying climate and soil characteristics (Ozenda 1988). When comparing the two regions used in this study, the Western region of Zermatt has substantially warmer and drier summers and less snowfall during winter than the Eastern region of Davos (Fig. 2). It is well supported that climatic factors play a major role in plant local adaptation (Macel et al. 2007; Ågren and Schemske 2012; Manel et al. 2012; Toräng et al. 2014). In our study, the strong precipitation difference and possibly also the temperature difference may have resulted in stronger drought resistance in populations from the Western region compared to the Eastern region, allowing plants to grow and reproduce better in their local and INTRAregional transplant sites than in their INTERregional transplant sites. However, we cannot exclude that other abiotic or biotic factors have acted as selective agents. An alternative possibility for the observed local adaptation might be plant-soil feedback (Macel et al. 2007; Alexander et al. 2015), but lack of data in our study system precludes any inferences on this.
Survival was low and was partially explained by site conditions, population origin did not explain survival, and populations were not locally adapted through differential survival. This is in contrast to many other transplant studies, which found local adaptation via differential survival (Galloway and Fenster 2000; Bischoff et al. 2006; Sambatti and Rice 2006; Giménez-Benavides et al. 2007; Toräng et al. 2014), but counterexamples with a random pattern of survival exist (Bischoff et al. 2006; Hamann et al. 2016). Our results suggest that mortality was mainly due to transplant shock and possibly other unsystematic effects, such as damage by herbivores. We were able to assess survival in more detail at two sites (Monstein and Findelgletscher). At these sites, survival was lowest during winter (74% and 42%, respectively) as compared to the growing period (July–September 2013; 95% and 97%, respectively), suggesting that universally adverse winter conditions possibly in combination with transplant shock were responsible for mortality.
Although our study effectively revealed patterns of local adaptation across regions, we acknowledge a few technical limitations that should be considered to improve future studies. Our transplant design was not fully reciprocal but transplantation to each site included the original population, another population from within the same region and a third population from the other region. In this way, the design was more balanced with respect to sympatric versus allopatric transplant combinations and therefore optimized the statistical power for a restricted number of transplanted plants (Blanquart et al. 2013). A fully reciprocal design would have been most powerful, and although this would have doubled the size of the experiment (1620 instead of 810 plants) making it logistically challenging, it may have strengthened the weak signal of local adaptation within regions.
Anthyllis vulneraria is a perennial species, so the performance measures taken over the course of this experiment reflect only part of the individuals’ lifetime performance. A long-term study, ideally capturing lifetime fitness, would therefore have been more informative (Shaw et al. 2008; Bennington et al. 2012). Nevertheless, our observations over the course of two growing seasons proved sufficient to reveal expected patterns of local adaptation, at least in response to divergent selection pressures between regions. The investigated performance traits span important aspects of a plant’s fitness, ranging from survival and biomass accumulation to the investment into reproductive structures. Except for survival, all traits point in the same direction, suggesting strong local adaptation, presumably to regional climatic conditions.
Another limitation of our study is that the experiment started with seedlings and therefore precluded the germination and seedling establishment phase, which has repeatedly been found to be the most vulnerable life cycle stage of plants and often requires local adaptation to environmental conditions (Waser and Price 1985; Giménez-Benavides et al. 2007; Anderson et al. 2015). Similar experiments should therefore ideally include a parallel reciprocal seed sowing experiment to assess this. Finally, more data on environmental variables at the different transplant sites could have helped identifying drivers of local adaptation.
Conclusions
We performed a transplant experiment with Anthyllis vulneraria to test for local adaptation at two spatial scales. We found that aboveground biomass, reproductive biomass and flowering propensity decreased when transplanted at INTERregional scale but not at INTRAregional scale. This is possibly due to divergent environmental conditions across regions, especially precipitation and temperature, whereas stronger gene flow within regions is unlikely to have played a role in counteracting local adaptation.
Data availability
All data generated or analysed during this study are included in the supplementary information files Table S1 and Table S2.
References
Ågren J, Schemske DW (2012) Reciprocal transplants demonstrate strong adaptive differentiation of the model organism Arabidopsis thaliana in its native range. New Phyt 194:1112–1122
Alexander JM, Diez JM, Levine JM (2015) Novel competitors shape species' responses to climate change. Nature 525(7570):515–518
Anderson JT, Perera N, Chowdhury B et al (2015) Microgeographic patterns of genetic divergence and adaptation across environmental gradients in Boechera stricta (Brassicaceae). Am Nat 186:S60–S73
Angert AL, Schemske DW (2005) The evolution of species’ distributions: reciprocal transplants across the elevation ranges of Mimulus cardinalis and M. lewisii. Evolution 59(8):1671–1684
Banta JA, Dole J, Cruzan MB et al (2007) Evidence of local adaptation to coarse-grained environmental variation in Arabidopsis thaliana. Evolution 61(10):2419–2432
Bates D, Mächler M, Bolker B et al (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Becker U, Colling G, Dostal P et al (2006) Local adaptation in the monocarpic perennial Carlina vulgaris at different spatial scales. Oecologia 150:506–518
Bennington CC, Fetcher N, Vavrek MC et al (2012) Home site advantage in two long-lived arctic plant species: results from two 30-year reciprocal transplant studies. J Ecol 100:841–851
Bischoff A, Crémieux L, Smilauerova M et al (2006) Detecting local adaptation in widespread grassland species—the importance of scale and local plant community. J Ecol 94:1130–1142
Blanquart F, Kaltz O, Nuismer SL et al (2013) A practical guide to measuring local adaptation. Ecol Lett 16(9):1195–1205
Byars SG, Papst W, Hoffmann AA (2007) Local adaptation and cogradient selection in the alpine plant, Poa hiemata, along a narrow altitudinal gradient. Evolution 61(12):2925–2941
Clausen J, Keck DD, Hiesey WM (1941) Regional differentiation in plant species. Am Nat 75:231–250
Conner JK, Hartl DL (2004) A primer of ecological genetics. Sinauer Associates, Sunderland
Couderc H (1971) Etude expérimental de la reproduction de l'Anthyllis vulneraria L. Bull de la Societé Botanique de France 118:359–374
Endler JA (1977) Geographic variation, speciation and clines. Princeton University Press, Princeton
Endler JA (1987) Natural selection in the wild. Monographs in Population Biology, vol 21. Princeton University Press, Princeton
Frei ES, Scheepens JF, Armbruster GFJ et al (2011) Phenotypic differentiation in a common garden reflects the phylogeography of a widespread Alpine plant. J Ecol 100(2):297–308
Galen C, Shore JL, Deyoe H (1991) Ecotypic divergence in alpine Polemonium viscosum: genetic structure, quantitative variation, and local adaptation. Evolution 45(5):1218–1228
Galloway LF, Fenster CB (2000) Population differentiation in an annual legume: local adaptation. Evolution 54(4):1173–1181
Geber and Eckhart (2005) Geber MA, Eckhart VM (2005) Experimental studies of adaptation in Clarkia xantiana: II. Fitness variation across a subspecies border. Evolution 59(3):521–531
Giménez-Benavides L, Escudero A, Iriondo JM (2007) Local adaptation enhances seedling recruitment along an altitudinal gradient in a high mountain mediterranean plant. Ann Bot 99:723–734
Gonzalo-Turpin H, Hazard L (2009) Local adaptation occurs along altitudinal gradient despite the existence of gene flow in the alpine plant species Festuca eskia. J Ecol 97(4):742–751
Halbritter AH, Fior S, Keller I et al (2018) Trait differentiation and adaptation of plants along elevation gradients. J Evol Biol 31(6):784–800
Hamann E, Kesselring H, Armbruster GFJ et al (2016) Evidence of local adaptation to fine- and coarse-grained environmental variability in Poa alpina in the Swiss Alps. J Ecol 104(6):1627–1637
Hamann E, Scheepens JF, Armbruster GFJ et al (2017) High intraspecific phenotypic variation, but little evidence for local adaptation in Geum reptans populations in the Central Swiss Alps. Alp Bot 127:121–132
Hegi G (1975) Illustrierte Flora von Mitteleuropa. Verlag Paul Parey, Berlin und Hamburg, Deutschland
Hereford J (2009) A quantitative survey of local adaptation and fitness trade-offs. Am Nat 173(5):579–588
Hereford J, Winn AA (2008) Limits to local adaptation in six populations of the annual plant Diodia teres. New Phyt 178(4):888–896
Hirst MJ, Sexton JP, Hoffmann AA (2016) Extensive variation, but not local adaptation in an Australian alpine daisy. Ecol Evol 6:5459–5472
Joshi J, Schmid B, Caldeira MC et al (2001) Local adaptation enhances performance of common plant species. Ecol Lett 4:536–544
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7(12):1225–1241
Kesselring H, Armbruster GFJ, Hamann E et al (2015) Past selection explains differentiation in flowering phenology of nearby populations of a common alpine plant. Alp Bot 125(2):113–124
Körner Ch (2003) Alpine plant life. Springer, Heidelberg
Köster E, Bitocchi E, Papa R et al (2008) Genetic structure of the Anthyllis vulneraria L. s. l. species complex in Estonia based on AFLPs. Central Eur J Biol 3(4): 442–450
Kuznetsova A, Brockhoff PB, Christensen RHB (2013) lmerTest: tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). https://cran.r-project.org/web/packages/lmerTest/index.html. Accessed 13 Jan 2014
Lauber K, Wagner G, Gygax A (2012) Flora Helvetica. Haupt Verlag, Bern
Leimu R, Fischer M (2008) A meta-analysis of local adaptation in plants. PLoS ONE 3(12):e4010
Lenormand T (2002) Gene flow and the limits to natural selection. Trends Ecol Evol 17(4):183–189
Lenth RV (2016) Least-squares means: The R package lsmeans. J Stat Softw 68:1–33
Macel M, Lawson CS, Mortimer SM (2007) Climate vs. soil factors in local adaptation of two common plant species. Ecology 88(2):424–433
Manel S, Gugerli F, Thuiller W et al (2012) Broad-scale adaptive genetic variation in alpine plants is driven by temperature and precipitation. Mol Ecol 21(15):3729–3738
MeteoSwiss (2015) Federal Office of Meteorology and Climatology. https://www.meteoswiss.admin.ch/. Accessed April 2018
Nanni L, Ferradini N, Taffetani F et al (2004) Molecular phylogeny of Anthyllis spp. Plant Biol 6(4):454–464
Navarro L (1999a) Allocation of reproductive resources within inflorescences of Anthyllis vulneraria subsp. vulgaris (Fabaceae). In: Kurmann MH, Hemsley ARH (eds) The evolution of plant architecture. Royal Botanic Gardens, Kew, pp 323–330
Navarro L (1999b) Reproductive biology of Anthyllis vulneraria subsp. vulgaris (Fabaceae) in northwestern Iberian Peninsula. Nordic J Bot 19(3): 281–287
Ozenda P (1988) Die Vegetation der Alpen im Europäischen Gebirgsraum. Fischer, Stuttgart, p 353
Peterson ML, Kay KM, Angert AL (2016) The scale of local adaptation in Mimulus guttatus: comparing life history races, ecotypes, and populations. New Phyt 211(1):345–356
R Development Core Team (2017) R: a language and environment for statistical computing. R foundation for Statistical Computing, Vienna
Sambatti JBM, Rice KJ (2006) Local adaptation, patterns of selection, and gene flow in the Californian serpentine sunflower (Helianthus exilis). Evolution 60(4):696–710
Sedlacek J, Wheeler JA, Cortes AJ et al (2015) The response of the alpine dwarf shrub Salix herbacea to altered snowmelt timing: lessons from a multi-site transplant experiment. PLoS ONE 10(4):e0122395
Sexton JP, Strauss SY, Rice KJ (2011) Gene flow increases fitness at the warm edge of a species’ range. Proc Nat Acad Sci USA 108:11704–11709
Shaw RG, Geyer CJ, Wagenius S, Hangelbroek HH, Etterson JR (2008) Unifying life-history analyses for inference of fitness and population growth. Am Nat 172:35–47
Stanton ML, Galen C (1997) Life on the edge: adaptation versus environmentally mediated gene flow in the snow buttercup, Ranunculus adoneus. Am Nat 150:143–178
Stöcklin J, Kuss P, Pluess AR (2009) Genetic diversity, phenotypic variation and local adaptation in the alpine landscape: case studies with alpine plant species. Bot Helv 119:125–133
Toräng P, Wunder J, Obeso JR (2014) Large-scale adaptive differentiation in the alpine perennial herb Arabis alpina. New Phytol 206(1):459–470
Waser NM, Price MV (1985) Reciprocal transplant experiments with Delphinium nelsonii (Ranunculaceae): evidence for local adaptation. Am J Bot 72:1726–1732
Acknowledgements
We thank the Schatzalp-Bahn Davos, especially Pius App, for logistic support. Michelle Gisler has provided substantial help with sampling and germinating Anthyllis vulneraria. Constructive comments from the associate editor and anonymous reviewers improved our manuscript. This work was funded by the Swiss National Science Foundation grant no. 3100A-135611 to J.S., and the Freiwillige Akademische Gesellschaft Basel and the Basler Stiftung für biologische Forschung to H.K.
Author information
Authors and Affiliations
Contributions
JS, JFS and HK designed the experiment. HK and EH performed the experiment and analysed the data. JFS, EH and HK wrote the manuscript with contributions from the other authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10682_2019_9999_MOESM1_ESM.docx
Figure S1 (A) Total aboveground biomass, (B) reproductive allocation, and (C) flowering propensity of Anthyllis vulneraria at five transplant sites. Bars are ordered within each site by increasing distance of the transplant type from the site (i.e. local, INTRAregional, INTERregional). Error bars depict 1 standard error of the mean. Note different scales of y-axis on all panels. Sites are written in normal face and populations in italic face. Brown shaded bars are used for populations from the Eastern Swiss region, and purple shades for the Western region. Non-flowering plants were disregarded in calculating mean values of reproductive biomass (DOCX 32 kb)
10682_2019_9999_MOESM2_ESM.csv
Table S1 Data on survival of Anthyllis vulneraria at six transplant sites. Columns present plant ID, transplant site, population of origin, transplant type, and whether a plant survived (y) or not (n) till July 2013, September 2013 and September 2014. NA – not available (CSV 38 kb)
10682_2019_9999_MOESM3_ESM.csv
Table S2 Data on biomass and flowering of Anthyllis vulneraria at five transplant sites. Columns present plant ID, transplant site, population of origin, transplant type, reproductive biomass, vegetative biomass, total aboveground biomass, reproductive allocation, and flowering status (0 – not flowering; 1 – flowering). NA – not available. (CSV 17 kb)
Rights and permissions
About this article
Cite this article
Kesselring, H., Hamann, E., Armbruster, G.F.J. et al. Local adaptation is stronger between than within regions in alpine populations of Anthyllis vulneraria. Evol Ecol 33, 737–750 (2019). https://doi.org/10.1007/s10682-019-09999-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-09999-8