Abstract
Pollinators are important agents of selection on floral traits, including nectar sugar composition. Although it is widely assumed that the proportion of sugars (mainly sucrose, glucose and fructose) in nectar reflects pollinators’ physiological limitations and digestive efficiency, the relative impact of pollinators and abiotic factors on nectar sugar composition, as well as the generality of these associations across the angiosperms, remain unknown. We compiled data on nectar sugar composition for >1000 plant species, along with information on flower visitors, plant growth form and latitudinal climatic zone, to provide the first comprehensive assessment of correlates of variation in sugar nectar composition in the angiosperms. After assembling a phylogeny linking all species in the dataset, we estimated the amount of phylogenetic signal in the percentage of sucrose and, by applying phylogenetically-informed multiple regressions, we evaluated whether nectar composition was influenced either by the main pollinator group, plant growth form, or latitudinal climatic zone. The relative importance of each of these factors was then assessed through model selection based on Akaike information criteria and deviance partitioning analysis. Nectar was dominated by sucrose in 56.8% of all the species, glucose in 16.7%, and fructose in 5.5%. Nectar in the remaining species was characterized by similar proportions of the three sugars. Variation in the proportion of sucrose was highest (~70%) at the intrafamily level, and had a significant but low phylogenetic signal, which partially reflects phylogenetic conservatism of the pollinator niche. After controlling for phylogenetic effects, the proportion of sucrose was mainly related to pollinator type and secondarily to climate. Accordingly, this study indicates that nectar sugar composition shows high evolutionary lability and its variation reflects plant-pollinator associations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugars in nectar secreted by flowers are one of the principal food rewards for pollinators. Therefore, nectar sugar composition is expected to reflect pollinator-specific innate preferences, physiological constraints and/or digestive efficiencies (Heinrich 1975; Martínez del Rio et al. 1992; Schondube and Martínez del Rio 2004). In particular, the proportion of the disaccharide sucrose versus the two main monosaccharide components, the hexoses glucose and fructose (the three dominant sugars in nectar), has been considered a distinctive signature of adaptation to different pollinators (Baker and Baker 1983, 1990; Proctor et al. 1996; Nicolson and Thornburg 2007). Whereas sucrose-rich nectars have been linked to pollination by hummingbirds, butterflies, moths, and long-tongued bees, hexoses-rich nectars have been linked to pollination by bats, passerine birds, flies, and short-tongued bees (Baker and Baker 1983, 1990; Elisens and Freeman 1988; Freeman et al. 1991; Stiles and Freeman 1993; Bruneau 1997; Dupont et al. 2004; Chalcoff et al. 2006; Wolff 2006; Schmidt-Lebuhn et al. 2007; Krömer et al. 2008). Therefore, shifts in plant-pollinator associations during angiosperm evolution may have resulted in changes in nectar sugar proportions.
Nectar sugar composition is one of the traits that define the so-called “pollination syndromes” (i.e. convergent evolution of a correlated set of floral traits driven by adaptation to the most efficient pollinator, Faegri and van der Pijl 1979), which also include other nectar characteristics, such as sugar concentration, and other floral characteristics, such as corolla shape, flower color, and presence and type of scents. However, few studies have addressed the generalization of pollination syndromes in a phylogenetic context (Ornelas et al. 2007; Pérez et al. 2007; Smith et al. 2008; Martén-Rodríguez et al. 2010; Sakai et al. 2013; Rosas-Guerrero et al. 2014; Gómez et al. 2014), or, more specifically, have evaluated the role of pollinators as selective agents on nectar sugar composition by considering shared evolutionary history among angiosperm species (Bruneau 1997; Dupont et al. 2004; Petanidou 2005; Ackermann and Weigend 2006; Wolff 2006; Schmidt-Lebuhn et al. 2007; Johnson and Nicolson 2008; Krömer et al. 2008; Witt et al. 2013). Furthermore, these latter studies focused on isolated factors affecting nectar composition (e.g. pollinators, flower morphology, or flowering period) and on single plant communities or lineages. Consequently, the overall role of pollinators as selective agents on nectar sugar composition within a macroevolutionary context has not been yet thoroughly established.
In addition to pollinators, other factors may also play important roles in explaining nectar composition variation. Since nectar sugars are derived from photosynthates, environmental factors affecting plant physiology, such as temperature, light, CO2 levels, and water availability may directly or indirectly affect nectar composition (Pacini et al. 2003; Nicolson and Thornburg 2007). For instance, water stress has been related to changes in nectar composition (Freeman and Head 1990; Villarreal and Freeman 1990; Petanidou 2005), although the mechanism behind this effect remains unclear. Moreover, it has been demonstrated that the enzyme invertase, which hydrolyzes sucrose to hexoses, is more active at low temperatures (Sturm and Tang 1999), and thus, nectars from species occurring at higher latitudes or altitudes might contain higher proportions of hexoses as compared to sucrose. Environmental effects could both determine variation in nectar sugar composition at geographical scales or within local communities in association with, for instance, growth form, an intrinsic plant trait that can be linked to specific physiological characteristics and particular microclimatic conditions (e.g. Wiens 1984; Díaz et al. 1998). Nevertheless, as far as we know, no studies have distinguished between phylogenetic versus environmental effects on nectar sugar proportions.
Accordingly, because of the fragmentary nature of existing information, the relative influence of different biotic and abiotic factors on nectar composition and, especially, the generality of these associations in an adequate phylogenetic framework, remain unknown. The need to incorporate phylogenetic information in comparative studies stems from the observation that phenotypic traits of closely related species may be similar due to phylogenetic closeness (i.e. phylogenetic signal), and therefore are not statistically independent (Harvey and Pagel 1991). But resemblance among closely related species in a phenotypic trait may result not solely from common ancestry (i.e. be non-adaptive), but also may be further adaptively enhanced by phylogenetic niche conservatism (PNC). This occurs when closely related species are phenotypically more similar than expected by phylogeny alone because a clade’s ancestor and their descendants occupy the same ecological niche (Losos 2008, 2011). Therefore, it is important not only to estimate the presence and magnitude on any phylogenetic signal but also the role of PNC in comparative analyses.
Here, we conducted a comparative analysis to assess the relative contribution of pollinator-type, plant growth form and latitudinal climatic zone in explaining variation in the proportion of sucrose in nectar on, to our knowledge, the largest existing database on nectar composition gathered until today. To the extent that the most efficient pollinator represents the main factor shaping nectar characteristics, and that there are frequent shifts in plant-pollinator associations within lineages, we predicted (1) the dominance of pollinator type as the most important among all ecological factors explaining variation in nectar sugar composition, and, therefore, (2) a weak influence of shared evolutionary history beyond that explained by shared pollinator type (i.e. PNC; Losos 2008, 2011).
Materials and methods
We compiled data on nectar sugar composition (proportion of sucrose, glucose and fructose) for a total of 1214 species (representing a 20% of all angiosperm families and a 50% of all angiosperm orders), from a literature search on the Scopus online database (www.scopus.com). During early 2013 we searched for studies published using the keywords “nectar” and “sucrose” or “hexoses”, retrieving a total of 53 articles published since 1981, each of which contained information on nectar content of sucrose, glucose, and fructose for at least one plant species. Average nectar sugar composition was calculated when more than one value was reported for a particular species. We excluded additional sugars (i.e. xylose, present in 49 species) from the calculations of the proportion of sucrose versus hexoses. The entire data set is reported in Table S1.
We analyzed the role of three factors in explaining interspecific variation in nectar sugar composition: main pollinator type (PT), plant growth form (GF), and latitudinal climatic zone (CZ). These factors were previously hypothesized to affect nectar sugar composition, as introduced above (see also Pacini et al. 2003; Nicolson and Thornburg 2007). Plant species were categorized, according to the taxonomic identity of the main pollinator, into one out of 11 pollinator types: four types of vertebrates, i.e. hummingbirds (HUM), passerine birds (PAS), bats (BAT), and rodents (ROD); four of invertebrates, i.e. hymenopterans (HYM), dipterans (DIP), lepidopterans (LEP), and coleopterans (COL); and also three types of “generalists”, i.e. plants pollinated by more than one order of insects (GI), plants pollinated by more than one order of vertebrates (GV), and plants pollinated by at least one order of vertebrates and one order of insects (GIV). Although HYM and LEP include insects that might belong to different taxonomic and functional groups of pollinators (i.e. bees and wasps or long- and short-tongued bees in the first case, and butterflies and moths in the second one), we decided to pool these insects into these categories since in many instances we were unable to separate them using the available information. Pollinator-type assignment was based on data or descriptions from the published sources reporting nectar composition data, and was reinforced, in many cases, with information withdrawn from other reliable online sources, including published databases and other articles. Since we avoided inferring ourselves the most probable pollinator/s based on floral traits associated with “pollination syndromes” (Faegri and van der Pijl 1979), 214 plant species in our dataset, for which pollinator information was not available, were excluded from the regression analyses.
Moreover, we classified plant species into six categories of growth form: herbs, subshrubs (including giant herbs exceeding 1.5 m height), shrubs (including treelets), epiphytes (in a broad sense, including aerial hemiparasites), climbers, and trees. Finally, plant species were categorized according to latitudinal climatic zone, which mainly relates to temperature, based on the geographical latitudinal belt where their collection sites were located: tropical (between 0° and 23.5° in each hemisphere), subtropical (between 23.5° and 38° in each hemisphere), and temperate (between 38° and 66.5° in each hemisphere). This classification was based on the Köppen climate classification system, with the exception that temperate zones were further subdivided into subtropical and truly temperate (McKnight and Hess 2000). For an ancillary analysis of altitudinal changes in nectar composition, tropical species (n = 305) were further categorized according to their altitudinal position, as inferred from the collection sites, in truly tropical (n = 236), altitudinal-subtropical (n = 38), and altitudinal-temperate (n = 31) zones. Following Rebetez et al. (2004), we have considered that in tropical latitudes the lowering of temperature with altitude results in subtropical environments between 2000 and 3000 m, and in temperate environments between 3000 and 5000 m.
Statistical analyses
Variation in nectar composition
Because sucrose hydrolyzes into the hexoses glucose and fructose, most of the variation in nectar sugar composition can be characterized by the relative contributions of sucrose versus these two hexoses considered together (i.e. glucose + fructose). For each species, we used the proportion of sucrose [p = sucrose/(sucrose + glucose + fructose], rather than the sugar ratio [r = sucrose/(glucose + fructose)] proposed by Baker and Baker (1983), to classify nectars according to sugar composition. This was motivated by the fact that the sugar ratio implies a transition from hexoses- to sucrose-rich nectars occurring at the counterintuitive value of 33%, whereas when using the proportion of sucrose this transition occurs at the more intuitive value of 50% (see also Nicolson and Thornburg 2007). Furthermore, to comply with assumptions of normality and homoscedasticity for statistical tests, we used a logit transformation (i.e. ln[p/(1 − p)]) of the proportion of sucrose (p), which allows this variable to vary continuously with no upper or lower distribution limits (Warton and Hui 2011).
We estimated variance components for sucrose proportions among taxonomic levels (i.e. among species within genera, among genera within families, among families within orders, among orders within classes and between classes) using hierarchical analysis of variance (Gelman and Hill 2007). This statistical analysis was conducted with the lme function from R package nlme (Pinheiro et al. 2014; R Development Core Team 2015).
Phylogenetic tree construction
Phylogenetic relationships were inferred among all species in the database for which we obtained data for all the three covariates mentioned before (1000 species, Table S1). We used the Phylomatic v3 online tool (http://phylodiversity.net/phylomatic/; Webb and Donoghue 2005), and chose the R20120829 megatree assembled by the Angiosperm Phylogeny Group III (APGIII 2009). We manually resolved several polytomies in the resulting tree using published molecular phylogenies when available (Table S2), and we calibrated the tree by running the bladj (branch length adjustment) algorithm from the Phylocom 4.2 software (Webb et al. 2008), extracting the estimated node ages from Wikström et al. (2001). The complete megatree (Figure S1) and corresponding Nexus format file (File S1) are available in the Supplementary Material.
To account for the effect of phylogenetic uncertainty due to the presence of polytomies in the megatree described before, we applied a procedure which simultaneously resolved polytomies and adjusted branch lengths using an evolutionary constant rate birth–death model. First, we ran the PolytomyResolver R script (Kuhn et al. 2011), which produced an input file for the BEAST software (Drummond and Rambaut 2007) containing topological (known topology) and chronological (known node ages) constraints. We set the topology of the megatree and the node ages mentioned above as topological and chronological constraints, respectively, and kept the default settings as described in Kuhn et al. (2011). Then, with this input file we ran BEAST so as to obtain a posterior distribution of randomly resolved dated trees. We ran Markov Chain Monte Carlo (MCMC) analyses for 116 iterations, sampling trees every 103 iterations, and then we used the TreeAnnotator software (Drummond and Rambaut 2007) to discard a 25% burnin and to summarize the remaining trees in a maximum clade fully-resolved credibility tree (File S2). Finally, we computed matrices of pairwise phylogenetic distances from 100 randomly-selected trees from BEAST posterior distribution, from the maximum clade credibility tree, and from the megatree containing polytomies. We compared the degree of resemblance between the randomly-resolved trees (including the maximum clade credibility tree) and the polytomic megatree by means of Mantel correlation tests performed with R package vegan. The correlation between matrices was extremely high (Mantel r statistic = 0.995, p ≪ 0.001 for the maximum clade credibility tree; range of Mantel r’s = 0.972–0.996 for the 100 individual randomly-selected trees), and, thus, we concluded that the effect of phylogenetic uncertainty reflected in the soft polytomies on our conclusions is expected to be negligible.
Phylogenetic signal and phylogenetic niche conservatism
Phylogenetic signal in nectar composition was estimated with Blomberg’s K (Blomberg et al. 2003), which quantifies the amount of phylogenetic signal in the data relative to a Brownian Motion (BM) model of trait evolution, with K = 1 reflecting a BM pattern, and K = 0 a random distribution of the trait along the phylogeny. K-values were calculated with the phylosig function from the R package phytools (Revell 2012) and its statistical significance (p values <0.05) assessed by generating 10,000 randomized values of K.
We assessed the relevance of PNC in explaining the observed variation in nectar composition by comparing the magnitude of the phylogenetic signal in sucrose content estimated as explained above (Ksucrose hereafter) with the phylogenetic signal in the residuals obtained from four different General Linear Models (GLMs): one multiple GLM including all three predictors (KPT+GF+CZ hereafter), and three single GLM including each predictor separately (KPT, KGF, and KCZ hereafter). Any phylogenetic signal, albeit weak, should decrease after removing the effect of any ecological factor (e.g. pollinator type) that could have been preserved along some lineages, even in those subtended by terminal nodes. Accordingly, a significant decrease in the phylogenetic signal in the residuals of the multiple linear models would suggest that at least part of the observed phylogenetic effect can be attributed to conservation of the ecological niche, as defined by the model’s predictors, while the same pattern arising from the residuals from the simple linear models would identify the most influential niche dimension/s. Statistically significant decreases in the magnitude of the phylogenetic signals were assessed by comparing the overlap between 95% confidence intervals of the K values.
Phylogenetic regression models
To analyze whether (logit-transformed) sucrose proportion in nectar was influenced either by the main pollinator type, growth form of the species, or the latitudinal climatic zone of occurrence, we applied Phylogenetic Generalized Least Squares (PGLS) models. PGLS is an extension of the GLS method in which the autocorrelation among species arising from phylogenetic non-independence is controlled by incorporating a phylogeny-based variance–covariance matrix to account for structure in the error term of the model (Grafen 1989; Martins and Hansen 1997). This matrix can be constructed either by assuming a BM model of trait evolution, or by incorporating different branch length transformations accounting for departures from BM. We used functions in the R package caper (Orme et al. 2013) to conduct PGLS regressions, considering a statistical significance level of 0.05. We evaluated full models optimizing either λ (lambda), κ (kappa) or δ (delta) parameters of branch length transformation; the best fit was obtained by optimizing λ and thus, we only report results from these models. Given the categorical nature of the explanatory variables, we also tested for main effects by running the command anova on the fitted PGLS full model.
Furthermore, we determined the relevance of each ecological factor (pollinator type, growth form, and latitudinal climatic zone) in explaining variation in nectar sugar composition. First we compared, in terms of Akaike Information Criteria (AIC), the fit of our data to all possible PGLS main-effect models obtained by combining additively the three ecological covariates (i.e. one-factor, two-factor, and three-factor analyses). Secondly, we carried out a deviance partitioning analysis to quantify the percentage of deviance explained by each ecological factor, using the code developed by González-Moreno et al. (2013) which uses functions from the R package vegan v 2.2-1 (Oksanen et al. 2015). This analysis was performed on the residuals obtained from the null PGLS model (i.e. the model in which all factors, except the intercept, were excluded) so as to control for phylogenetic non-independence.
An ancillary analysis was restricted to the 305 species from the tropical latitudinal zone and focused on comparing nectar sugar composition across altitudinal belts (i.e. truly tropical, subtropical, and temperate). Because of the more limited data set, we included “hummingbird” (i.e. “yes” or “no”) as the only additional factor, given that many high-altitudinal species in the Neotropics are pollinated by hummingbirds (Table S1) and hummingbird-pollinated plants tend to produce sucrose-rich nectars (see Fig. 1A). In this analysis, in addition to test for an overall effect of altitude, we tested for differential responses in sucrose proportion between hummingbird and non-hummingbird plants across altitudinal zones.
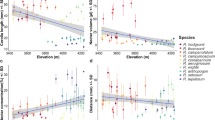
Nectar sugar composition in relation to pollinator type (A), the latitudinal climatic zone where species occur (B) and species growth form (C). Depicted values are back-transformed (i.e. 100*inverse logit) adjusted means (+1 SE and −1 SE). Different lowercase letters depict significantly different groups (see Table S3 for p values of contrast analyses) and numbers in brackets are the sample size (i.e. number of species) for each category of predictor variables. For pollinator type abbreviations see “Materials and methods” section
Ornstein–Uhlenbeck models
We fitted Ornstein–Uhlenbeck (OU hereafter) models (Hansen 1997; Butler and King 2004; Beaulieu et al. 2012) to assess whether pollinator type and latitudinal climatic zone (i.e. the two factors that showed significant associations with nectar sugar composition; see “Results” section) could constitute selective regimes modeling nectar evolution. The OU model is an extension of the BM model in which, apart from a stochastic term reflecting the effect of random drift (represented by the σ2 parameter), a parameter describing the strength of selection (α) is added, which pulls continuous character evolution towards one or multiple discrete adaptive peaks (θi). To carry out this analysis, we applied the make.simmap function from R package phytools (Revell 2012) to build stochastic character mapped reconstructions on each of 100 trees sampled randomly from BEAST posterior distribution of fully-bifurcating trees (see Phylogenetic tree construction). We then obtained 100 stochastic ancestral character reconstructions for pollinator type, and 100 stochastic reconstructions for latitudinal climatic zone. As the size of the phylogeny (1000 terminals) and the amount of parameters to be estimated (especially for the OU model with 11 adaptive peaks defined by the different pollinator types, see below) make OU models highly demanding on computer memory, we randomly sampled 50 stochastic reconstructions for each type of ancestral selective regime, which were then used to fit BM and OU models with the R package OUwie (Beaulieu and O’Meara 2016). We fitted single rate BM models (“BM1”) and OU (“OUM”) models with single rates of stochastic motion and selection strength, and with multiple optima represented by the different pollinator types (11 peaks) and, alternatively, by the different latitudinal climatic zones (3 peaks). Model fit was evaluated using AICc values. To assess selection arising solely from pollinators and climate, we used residuals from simple linear models so as to remove variation due to latitudinal climatic zone and pollinator type, respectively.
Results
Nectar composition patterns, phylogenetic signal and phylogenetic niche conservatism
The 1214 species included in the database belonged to 452 genera from 87 families in 29 orders of angiosperms, including both monocots (23%) and dicots (77%). The sampled species occurred in all continents, with 60% of the species belonging to the Americas (11 countries), 20% to Africa (2), 13% to Europe (9), 6% to Asia (3) and 1% to Oceania (1). Taxonomically, the database was dominated by the orders Lamiales (302 species), Fabales (131), Poales (128), Asparagales (105), Gentianales (93), Ericales (80) and Asterales (78), wherein the largest families were Bromeliaceae (114 species), Fabaceae (88), Acanthaceae (76), Gesneriaceae (72) and Rubiaceae (56) (Table S1).
Out of these 1214 species, 689 contained nectar with more than 50% of sucrose, 201 with more than 50% glucose, 67 with more than 50% of fructose, while in the remaining 257 species no single sugar exceeded 50% (Figure S2). The analysis of variance components showed an unequal partitioning of total variation in sugar proportion among taxonomic levels. Negligible variation (<1%) occurred between classes, 14.6% occurred among orders, 13.8% among families, 35.6% among genera, and 36.0% among species. Accordingly, over two thirds of the variation (~70%) occurred at the intra-family level. Phylogenetic signal in sucrose (i.e. Ksucrose) reflected this pattern, being low (K = 0.149) but significantly higher than the values expected under completely random trait distribution (p < 0.001; see also Table 1). Furthermore, we found some evidence of phylogenetic niche conservatism defined by one or more of the ecological variables considered here, given that a significant decrease in phylogenetic signal was observed in the residuals from the multiple regression including all ecological factors (Table 1). Indeed, significant reduction in phylogenetic signal as compared with Ksucrose occurred only in residuals from regression models including pollinator type as factor (i.e. KPT and KPT+GF+CZ; Table 1). This pattern suggests that phylogenetic signal in nectar sucrose content might in part reflect conservatism of the pollinator niche.
Ecological correlates of nectar
The best transformation of the off-diagonal elements of the variance–covariance matrix incorporated in the PGLS regression was achieved by using Pagel’s λ, with λ = 0.77 (95% CI [0.70–0.83]). The PGLS multiple regression showed that sugar composition was influenced primarily by pollinator identity (F10, 982 = 17.49, p < 0.0001) and secondarily by climatic zone of occurrence (F2, 982 = 3.17, p = 0. 04). On the other hand, growth form did not have a significant influence on sugar composition (F5, 982 = 0.43, p = 0. 83).
Plant species principally pollinated by hummingbirds (HUM) had the sucrose-richest nectar (>50% sucrose) followed, in decreasing order, by the more balanced nectars (i.e. approximately 50% of sucrose) of dipteran-pollinated species (DIP), lepidopteran-pollinated species (LEP), and hymenopteran-pollinated species (HYM). Species producing sucrose-poor nectars (<50% sucrose) were, in decreasing order, those pollinated by diverse insect assemblages (GI), generalist plant species pollinated by insects and vertebrates (GIV), bats (BAT), rodents (ROD), diverse vertebrate assemblages (GV), passerines (PAS), and coleopterans (COL) (Fig. 1A). Rather than being clustered in discrete groups, nectar sucrose content seemed to vary gradually across these pollinator-type categories.
In terms of abiotic factors, the sucrose content of nectar decreased significantly across latitudinal climatic zones from plants with ≥40% sucrose nectars in tropical and subtropical zones, to <40% sucrose nectars in temperate ones (Fig. 1B). This pattern was not biased by the large proportion of tropical hummingbird-pollinated species in our dataset (32%), which had sucrose-rich nectars, because the same trend remained even after removing all hummingbird-pollinated species from the analysis (F2, 664 = 5.7, p = 0.003; back-transformed adjusted mean sucrose content: tropical = 49.0%, subtropical = 41.2%, and temperate = 32.8%). Also, the ancillary analysis showed that this latitudinal trend (i.e. the decrease of sucrose content of nectar in temperate zones) was partially mimicked across altitudinal belts within the tropical latitudinal zone, although the trend was not statistically significant (F2, 299 = 0.82, p = 0.44; back-transformed adjusted mean sucrose content: truly tropical = 50.3%, altitudinal-subtropical = 53.5% and altitudinal-temperate = 41.3%). However, a more pronounced decline in the altitudinal-temperate zone was hidden by the constancy of hummingbird-pollinated species to secrete sucrose-rich nectar independent of altitude, as revealed by a significant hummingbird × altitude-zone interaction (F2, 299 = 3.42, p = 0.034; Figure S3). Finally, in the main PGLS analysis growth form had non-significant effect on nectar composition, and no significant differences between its categories were observed (Fig. 1C). Results of the contrast analyses for the three ecological factors are shown in Table S3.
Both the PGLS model comparison and the deviance partitioning analyses showed that pollinator type (PT) was the most important factor explaining nectar sucrose content, followed by latitudinal climatic zone (CZ). The best-fitting PGLS models contain both PT and CZ as predictors (Fig. 2). Also, models containing PT as predictor (i.e. PT, PT + GF, PT + CZ, and PT + GF + CZ) fitted always better than those models excluding this factor (i.e. GF, CZ, GF + CZ and NULL; Fig. 2). The same trend was observed with the deviance partitioning analysis, where the models including PT as factor accounted, together, for 83.4% out of total variance (23.3% for PT + GF + CZ model, 22.1% for PT + CZ model, 19.5% for PT + GF model, and 18.5% for PT model; Fig. 2). Together, these results suggest that pollinator type was the most important factor, among those chosen in this study, in explaining nectar sucrose content, followed by climatic zone.
Relative importance of pollination type (PT), latitudinal climatic zone (CZ) and growth form (GF) in explaining variation in nectar sugar composition, assessed by comparing ΔAIC values (above) and also as a result of a deviance partitioning analysis (below). The NULL in ΔAIC corresponds to a null model in which all of the terms except the intercept were excluded. In both graphs, models were sorted by increasing fit from best to worst across the Y-axis
Finally, the comparison between BM and OU models suggests that both pollinator type and climate impose selective regimes on nectar sucrose content, as both the OU model with 11 optima for the different pollinator types and the OU model with three optima for the different latitudinal climatic zones performed better than the BM models with a single stochastic rate of evolution (Table 2). Parameters from both OU models are shown in Table S4.
Discussion
Here we report the first comprehensive comparative study assessing the relative importance of different ecological factors in determining nectar sugar composition across angiosperms. Variation in nectar sucrose proportion was highest (70%) at the intrafamily level (i.e. among genera and species) and, accordingly, the phylogenetic signal in this trait was low. Pollinator type, and to a lesser extent latitudinal climate zone of occurrence, were the main factors associated with relative sucrose content. Plant growth form did not seem to play any important role in determining nectar sugar composition.
Nectar sugar composition and pollinators
Despite the widespread use of phylogenetic comparative methods, attempts to address the classical hypothesis about the effect of pollinator type on nectar-related traits within a phylogenetic framework are surprisingly scarce and either included scattered taxonomic groups or geographically circumscribed floras. Comparative studies have been conducted within the genus Erythrina (Bruneau 1997), in the families Acanthaceae (Schmidt-Lebuhn et al. 2007), Bromeliaceae (Krömer et al. 2008), and Caryophyllaceae (Witt et al. 2013); across the Canarian flora (Dupont et al. 2004) and Mediterranean flora (Petanidou 2005); and between bird-pollinated species from Africa and the Americas (Johnson and Nicolson 2008). Although these studies have shown an association between pollinator types and nectar sugar composition, conflicting results (e.g. Wolff 2006 in Gentianales) still maintain alive the debate on the role exerted by pollinators on the evolution of this trait. Therefore, our study is key for resolving this historical debate, because, by including a broad taxonomic representation and by accounting for phylogenetic relationships, it provides robust evidence in favor of the general association between different pollinator types and nectar sugar composition across the angiosperms.
Nectar sugar composition, in terms of proportion of sucrose versus hexoses, differed among plant species pollinated by different pollinator types. Values reported here were mainly in agreement with those from previous studies (Baker and Baker 1983, 1990; Elisens and Freeman 1988; Freeman et al. 1991; Stiles and Freeman 1993; Bruneau 1997; Dupont et al. 2004; Petanidou 2005; Chalcoff et al. 2006; Wolff 2006; Nicolson 2007; Schmidt-Lebuhn et al. 2007; Krömer et al. 2008). In general, sucrose-rich nectars (i.e. nectars containing more than 50% sucrose) prevailed in flowers pollinated by hummingbirds, butterflies and moths, and sucrose-poor nectars (i.e. nectars containing more than 50% hexoses) in flowers pollinated by bats, beetles, and passerine birds. Nectar from hymenopteran-pollinated flowers were reported to be sucrose-rich for long-tongued bees and sucrose-poor for short-tongued bees (e.g. Baker and Baker 1983). However, we could not separate hymenopterans into these two categories (i.e. long- and short-tongued), because of lack of information on bee identity in many of the published sources, and interestingly, nectar reported here for hymenopteran-pollinated species contained almost equal proportions of sucrose and hexoses.
Nectar sugar adjustment to the main pollinator type emphasizes the prevalence of the ecological and the evolutionary role of functional specialization in plant-pollinator interactions, at least in terms of a main pollinator type, and challenges the proposed view of widespread generalization in pollination systems (Waser et al. 1996; Johnson and Nicolson 2008). Instead, it seems to support the view that floral traits, including nectar characteristics, are being selected by the main pollinator type (Rosas-Guerrero et al. 2014), even if a given “type of pollinator” includes several species from a high taxonomic category like family or order. Even more, we found differences in nectar composition among different categories of generalist plant species as defined here (i.e. GI, GV, and GIV). The mean sucrose content was 36% for generalist insect-pollinated plants (GI), being similar to the average over all groups of insect-pollinated species (37.5%), while the sucrose content for species pollinated both by insects and vertebrates (GIV) was 49%, being only somewhat higher than the average across all groups (approx. 41%). In contrast, the mean for the generalist vertebrate-pollinated plants (GV) was much lower (21.5%) than the average across all vertebrate-pollinated plants (45%), although this value must be taken with caution given the low number of species within this category (i.e. 18 species). These results suggest the possibility of macroevolutionary divergences in nectar traits along the evolution of lineages of generalist plants. This scenario clearly relates to recent results by Gómez et al. (2015a, b) on floral diversification in a genus of generalist plants in the mustard family, where extant plant species differ in the quantitative composition of their respective pollinator assemblages.
Although our results are consistent with an adaptive scenario of evolutionary response to pollinator selection, sugar composition seems to reflect, at the same time, some phylogenetic inertia, as revealed by a significant, albeit weak, phylogenetic signal. For instance, although excluded from the analysis, the monosaccharide xylose appeared only in nectars of species pollinated by beetles and rodents, which in our dataset were mostly restricted to the genus Protea (Table S1). This infrequent sugar has been associated to mice-pollination (Johnson et al. 2001; Nicolson 2007), although its occurrence is not universal among mice-pollinated flowers (Wester et al. 2009; Turner et al. 2011; and Liparia here). Therefore, its presence in nectars from Protea and Faurea, both Proteaceae, could result from common ancestry rather than be the result of adaptation to mice-pollination (Van Wyk and Nicolson 1995; Steenhuisen and Johnson 2012). In short, nectar sugar composition may reflect mainly pollinator selection in some lineages (leading to convergent evolution when different lineages respond to selection imposed by the same type of pollinator), and common ancestry (i.e. similarity by descent) in others.
Nectar sugar composition and the environment
Because of nectar’s photosynthetic origin, environmental factors are also expected to affect nectar sugar composition (Pacini et al. 2003; Nicolson and Thornburg 2007). Accordingly, we found a significant association between climatic zone of occurrence and nectar sucrose proportion, with sucrose-poor nectar being associated with higher latitudes and, consequently, decreasing temperatures. This decrease in sucrose-rich nectars was also found at high altitudes in the tropical zone, after accounting for hummingbird pollination. The association between low sucrose content and low temperatures was also observed elsewhere (Stiles and Freeman 1993; Galetto and Bernardello 2003; Petanidou 2005; Paiaro et al. 2012), although this is the first study reporting the generality of this association. Both intrinsic (e.g. plant physiology) and extrinsic (e.g. pollinator preferences associated with climatic conditions) factors may explain this pattern.
One plausible explanation for this environmental association could relate to the temperature-dependent activity of the enzyme invertase, which cleaves, irreversibly, sucrose into fructose and glucose. This enzyme has been called a “cold-induced sweetening enzyme” because it is more active at lower temperatures (Sturm and Tang 1999). This enzymatic response may even be adaptive, since an increase in hexoses, from which energy can be obtained more straightforwardly than from sucrose, could respond to the higher energy requirements of many pollinators from higher latitudes (Paiaro et al. 2012). On the other hand, in an experimental study, the relative amount of hexoses in nectar of Ipomopsis longiflora (Polemoniaceae) increased with higher temperatures (Freeman and Head 1990; Villarreal and Freeman 1990). However, this pattern may reflect water stress associated with high temperatures, rather than the effect of temperature per se (Petanidou 2005), which in turn relates to differences in osmotic pressures between sucrose- and hexoses-rich nectars (Nicolson and Thornburg 2007). In any case, we cannot assert that the latitudinal effect on nectar composition is adaptive, since it may also constitute a non-adaptive plastic response to prevailing environmental factors. However, strong selection on nectar sugars exerted by pollinators, such as hummingbirds, might overcome any environmental influence (Fig. S3). Finally, the fact that growth form did not affect nectar sugar composition might indicate that large (e.g. climate) rather than small-scale environmental factors are, by far, the most important abiotic determinant in nectar sugar composition.
Other factors
Other biological and non-biological factors, not considered in this study, could also increase variability in the balance between sucrose versus hexoses. For instance, the presence of yeasts in nectar could influence nectar sugar composition, as a postsecretory process, due to differential glucose consumption (Canto and Herrera 2012 and references therein). However, at best we only found a weak signature of glucose deficiency that could be attributed to yeast nectar infection (41% of the species had nectars with more glucose than fructose, whereas 49% had nectars with more fructose than glucose; Figure S2). Furthermore, variation in the conditions under which nectar was sampled (e.g. temperature differences), measured, and analyzed across studies, represents an undeniable and unmeasurable source of variability. This is expected to inflate species-level variability, although more than half of the sampled genera including more than one species came from the same bibliographic source (117 of 202 multispecies genera). At the same time, the large amount of variance found at the genus level may reflect differences among studies. Nevertheless, the influence of the among-study factor on nectar sucrose variation should be limited since data for 85 multispecies genera came from independent sources. In any event, differences among sources did not introduce any bias in relation to the role of pollinator type or latitudinal climatic zone on nectar composition, because the inclusion of bibliographic source as an explanatory factor in the PGLS regression analysis reported above (see Results) did not alter the importance of any of these factors (Table S5).
Evolutionary lability of nectar sugar composition
During the last three decades, the development of comparative approaches accounting for the phylogenetic relationships among taxa allowed to address adaptive hypotheses within a macroevolutionary framework, as well as to evaluate the importance of phylogenetic effects (Felsenstein 1985; Harvey and Pagel 1991; Blomberg and Garland 2002). Our study showed that sucrose proportion seems to be a labile trait, such as other pollinator-related floral characteristics (e.g. Bruneau 1997; Dupont et al. 2004; Ornelas et al. 2007; Pérez et al. 2007; Smith et al. 2008; Sakai et al. 2013; Witt et al. 2013, Gómez et al. 2014). Low phylogenetic signal could result from several scenarios, such as convergent evolution, high rates of divergent selection, adaptive radiation in which relatives diversify rapidly to fill new niches, or causes such as random distribution of plastic traits across the phylogeny (Blomberg and Garland 2002; Blomberg et al. 2003; Ives et al. 2007; Revell et al. 2008; Kamilar and Cooper 2013). Nevertheless, low phylogenetic signal can also be due to sampling errors, as those mentioned above, and other statistical issues (Blomberg and Garland 2002; Ives et al. 2007). Although nectar sugar composition might be conserved within some lineages, the low magnitude of phylogenetic signal in sucrose content and the associations of this trait with biotic and abiotic factors suggest that this trait might be evolutionarily labile, changing rapidly under novel selection regimes, such as a switch in pollinator type or, to a lesser extent, under changes in environmental conditions.
Most variation in nectar sucrose percent (~70%) seemed to occur at the intrafamily level, a taxonomic level within which floral evolution in relation to changes in plant-pollinator interactions is common (van der Niet and Johnson 2012). Thus, the low, albeit significant, phylogenetic signal we report here is consistent with a weak and probably lineage-dependent degree of phylogenetic conditioning of nectar sugar composition. The fact that part of the estimated phylogenetic patterning in this trait could be explained by conservatism of the pollinator niche, probably along lineages subtended by more terminal nodes, strengthens our proposal that the sucrose versus hexoses balance in nectar could respond rapidly to transitions in plant-pollinator systems.
Concluding remarks
In agreement with the pattern found in other floral traits involved in animal pollination, nectar sugar composition showed evolutionary lability, and its variation was mostly associated with pollinator preferences. Indeed, the low phylogenetic signal in this trait could result, in part, from conservation of the pollinator niche, although it might also reflect similarity by descent within some clades. As a final conclusion, our study provides substantial evidence that pollinator type, and secondarily climate, are two important factors that may have shaped variation in nectar sugar composition across the angiosperms.
References
Ackermann M, Weigend M (2006) Nectar, floral morphology and pollination syndrome in Loasaceae subfam. Loasoideae (Cornales). Ann Bot 98:503–514
Baker HG, Baker I (1983) Floral nectar sugar constituents in relation to pollinator type. In: Jones CE, Little RJ (eds) Handbook experimental pollination biology. Scientific and Academic Editions, New York, pp 117–141
Baker HG, Baker I (1990) The predictive value of nectar chemistry to the recognition of pollinator types. Isr J Bot 39:157–166
Beaulieu J, O’Meara B (2016) OUwie: analysis of evolutionary rates in an OU framework. R package version 1.50. https://CRAN.R-project.org/package=OUwie
Beaulieu JM, Jhwueng D-C, Boettiger C, O’Meara BC (2012) Modeling stabilizing selection: expanding the Ornstein–Uhlenbeck model of adaptive evolution. Evolution 66:2369–2383. doi:10.1111/j.1558-5646.2012.01619.x
Blomberg SP, Garland T (2002) Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods. J Evol Biol 15:899–910. doi:10.1046/j.1420-9101.2002.00472.x
Blomberg SP, Garland T, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745. doi:10.1111/j.0014-3820.2003.tb00285.x
Bruneau A (1997) Evolution and homology of bird pollination syndromes in Erythrina (Leguminosae). Am J Bot 84:54–71
Butler MA, King AA (2004) Phylogenetic comparative analysis: a modeling approach for adaptive evolution. Am Nat 164:683–695. doi:10.1086/426002
Canto A, Herrera CM (2012) Micro-organisms behind the pollination scenes: microbial imprint on floral nectar sugar variation in a tropical plant community. Ann Bot 110:1173–1183. doi:10.1093/aob/mcs183
Chalcoff VR, Aizen MA, Galetto L (2006) Nectar concentration and composition of 26 species from the temperate forest of South America. Ann Bot 97:413–421
Díaz S, Cabido M, Casanoves F (1998) Plant functional traits and environmental filters at regional scale. J Veg, Sci, p 9
Drummond A, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Dupont YL, Hansen DM, Rasmussen JT, Olesen JM (2004) Evolutionary changes in nectar sugar composition associated with switches between bird and insect pollination: the Canarian bird-flower element revisited. Funct Ecol 18:670–676. doi:10.1111/j.0269-8463.2004.00891.x
Elisens WJ, Freeman C (1988) Floral nectar sugar composition and pollinator type among new world genera in tribe Antirrhineae (Scrophulariaceae). Am J Bot 75:971–978
Faegri K, van der Pijl L (1979) The principles of pollination ecology. Pergamon Press, New York
Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125:1–15
Freeman C, Head KC (1990) Temperature and sucrose composition of floral nectars in Ipomopsis longiflora under field conditions. Sowthwest Nat 35:423–426
Freeman C, Worthington RD, Jackson MS (1991) Floral nectar sugar compositions of some south and southeast Asian species. Biotropica 23:568–574
Galetto L, Bernardello G (2003) Sugar nectar composition in Angiosperms from Chaco and Patagonia (Argentina): an animal visitor’s matter. Plant Syst Evol 238:69–86
Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, New York
Gómez JM, Perfectti F, Klingenberg CP (2014) The role of pollinator diversity in the evolution of corolla-shape integration in a pollination-generalist plant clade. Philos Trans R Soc Lond B Biol Sci 369:20130257. doi:10.1098/rstb.2013.0257
Gómez JM, Perfectti F, Abdelaziz M et al (2015a) Evolution of pollination niches in a generalist plant clade. New Phytol 205:440–453. doi:10.1111/nph.13016
Gómez JM, Perfectti F, Lorite J (2015b) The role of pollinators in floral diversification in a clade of generalist flowers. Evolution 69:863–878. doi:10.1111/evo.12632
González-Moreno P, Pino J, Carreras D et al (2013) Quantifying the landscape influence on plant invasions in Mediterranean coastal habitats. Landsc Ecol 28:891–903
Grafen A (1989) The phylogenetic regression. Philos Trans R Soc Lond B Biol Sci 326:119–157
Hansen TF (1997) Stabilizing selection and the comparative analysis of adaptation. Evolution 51:1341. doi:10.2307/2411186
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford
Heinrich B (1975) Energetics of pollination. Annu Rev Ecol Syst 6:139–170
Ives AR, Midford PE, Garland T (2007) Within-species variation and measurement error in phylogenetic comparative methods. Syst Biol 56:252–270
Johnson SD, Nicolson SW (2008) Evolutionary associations between nectar properties and specificity in bird pollination systems. Biol Lett 4:49–52. doi:10.1098/rsbl.2007.0496
Johnson SD, Pauw A, Midgley J (2001) Rodent pollination in the African lily Massonia depressa (Hyacinthaceae). Am J Bot 88:1768–1773
Kamilar JM, Cooper N (2013) Phylogenetic signal in primate behaviour, ecology and life history. Philos Trans R Soc Lond B Biol Sci 368:20120341. doi:10.1098/rstb.2012.0341
Krömer T, Kessler M, Lohaus G, Schmidt-Lebuhn AN (2008) Nectar sugar composition and concentration in relation to pollination syndromes in Bromeliaceae. Plant Biol 10:502–511. doi:10.1111/j.1438-8677.2008.00058.x
Kuhn TS, Mooers AØ, Thomas GH (2011) A simple polytomy resolver for dated phylogenies. Methods Ecol Evol 2:427–436. doi:10.1111/j.2041-210X.2011.00103.x
Losos JB (2008) Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett 11:995–1003. doi:10.1111/j.1461-0248.2008.01229.x
Losos J (2011) Seeing the forest for the trees: the limitations of phylogenies in comparative biology. Am Nat 177:709–727
Martén-Rodríguez S, Fenster CB, Agnarsson I et al (2010) Evolutionary breakdown of pollination specialization in a Caribbean plant radiation. New Phytol 188:403–417. doi:10.1111/j.1469-8137.2010.03330.x
Martínez del Rio C, Baker HG, Baker I (1992) Ecological and evolutionary implications of digestive processes: bird preferences and the sugar constituents of floral nectar and fruit pulp. Experientia 48:544–551
Martins EP, Hansen TF (1997) Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat 149:646–667
McKnight T, Hess D (2000) Climate zones and types: the Köppen system. Prentice Hall, Englewood Cliffs
Nicolson SW (2007) Nectar consumers. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Amsterdam, pp 289–342
Nicolson SW, Thornburg RW (2007) Nectar chemistry. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Amsterdam, pp 215–264
Oksanen J, Blanchet FG, Kindt R et al (2015) Vegan: community ecology package. R package version 2.2-1
Orme D, Freckleton R, Thomas G et al (2013) Caper: comparative analyses of phylogenetics and evolution in R. https://CRAN.R-project.org/package=caper
Ornelas JF, Ordano M, De-Nova AJ et al (2007) Phylogenetic analysis of interspecific variation in nectar of hummingbird-visited plants. J Evol Biol 20:1904–1917. doi:10.1111/j.1420-9101.2007.01374.x
Pacini E, Nepi M, Vesprini JL (2003) Nectar biodiversity: a short review. Plant Syst Evol 238:7–21
Paiaro V, Oliva GE, Cocucci AA, Sérsic A (2012) Caracterización y variación espacio-temporal del néctar en Anarthrophyllum desideratum Fabaceae): influencia del clima y los polinizadores. Bol Soc Argent Bot 47:375–387
Pérez F, Arroyo MTK, Medel R (2007) Phylogenetic analysis of floral integration in Schizanthus (Solanaceae): does pollination truly integrate corolla traits? J Evol Biol 20:1730–1738. doi:10.1111/j.1420-9101.2007.01393.x
Petanidou T (2005) Sugars in mediterranean floral nectars: an ecological and evolutionary approach. J Chem Ecol 31:1065–1088. doi:10.1007/s10886-005-4248-y
Pinheiro J, Bates D, DebRoy S, Sarkar D (2014) nlme: linear and nonlinear mixed effects models. http://cran.r-project.org/web/packages/nlme/inde
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. HarperCollins Publishers, New York
R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rebetez M, Reinhard M, Buttler A (2004) Tree physiology. Forests, tree physiology and climate. Encycl For Sci 15:419–426
Revell LJ (2012) phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223. doi:10.1111/j.2041-210X.2011.00169.x
Revell LJ, Harmon LJ, Collar DC (2008) Phylogenetic signal, evolutionary process, and rate. Syst Biol 57:591–601. doi:10.1080/10635150802302427
Rosas-Guerrero V, Aguilar R, Martén-Rodríguez S et al (2014) A quantitative review of pollination syndromes: do floral traits predict effective pollinators? Ecol Lett 17:388–400. doi:10.1111/ele.12224
Sakai S, Kawakita A, Ooi K, Inoue T (2013) Variation in the strength of association among pollination systems and floral traits: evolutionary changes in the floral traits of Bornean gingers (Zingiberaceae). Am J Bot 100:546–555. doi:10.3732/ajb.1200359
Schmidt-Lebuhn AN, Schwerdtfeger M, Kessler M, Lohaus G (2007) Phylogenetic constraints vs. ecology in the nectar composition of Acanthaceae. Flora 202:62–69
Schondube JE, Martínez del Rio C (2004) Sugar and protein digestion in flowerpiercers and hummingbirds: a comparative test of adaptive convergence. J Comp Physiol B Biochem Syst Environ Physiol 174:263–273
Smith SD, Ané C, Baum DA (2008) The role of pollinator shifts in the floral diversification of Iochroma (Solanaceae). Evolution 62:793–806. doi:10.1111/j.1558-5646.2008.00327.x
Steenhuisen S-L, Johnson SD (2012) Evidence for beetle pollination in the African grassland sugarbushes (Protea: Proteaceae). Plant Syst Evol 298:857–869. doi:10.1007/s00606-012-0589-5
Stiles FG, Freeman C (1993) Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica. Biotropica 25:191–205
Sturm A, Tang G-Q (1999) The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci 4:401–407. doi:10.1016/S1360-1385(99)01470-3
Turner RC, Midgley JJ, Johnson SD (2011) Evidence for rodent pollination in Erica hanekomii (Ericaceae). Bot J Linn Soc 166:163–170. doi:10.1111/j.1095-8339.2011.01139.x
van der Niet T, Johnson SD (2012) Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends Ecol Evol 27:353–361. doi:10.1016/j.tree.2012.02.002
Van Wyk BE, Nicolson SW (1995) Xylose is a major nectar sugar in Protea and Faurea. S Afr J Sci 91:151–153
Villarreal AG, Freeman C (1990) Effects of temperature and water stress on some floral nectar characteristics in Ipomopsis longiflora (Polemoniaceae) under controlled conditions. Bot Gaz 151:5–9
Warton DI, Hui FK (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10. doi:10.1890/10-0340.1
Waser NM, Chittka L, Price MV et al (1996) Generalization in pollination systems, and why it matters. Ecology 77:1043–1060. doi:10.2307/2265575
Webb CO, Donoghue MJ (2005) Phylomatic: tree assembly for applied phylogenetics. Mol Ecol Notes 5:181–183. doi:10.1111/j.1471-8286.2004.00829.x
Webb CO, Ackerly DD, Kembel SW (2008) Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 24:2098–2100
Wester P, Stanway R, Pauw A (2009) Mice pollinate the Pagoda Lily, Whiteheadia bifolia (Hyacinthaceae)—first field observations with photographic documentation of rodent pollination in South Africa. S Afr J Bot 75:713–719. doi:10.1016/j.sajb.2009.07.005
Wiens D (1984) Ovule survivorship, brood size, life history, breeding systems, and reproductive success in plants. Oecologia 64:47–53. doi:10.1007/BF00377542
Wikström N, Savolainen V, Chase MW (2001) Evolution of the angiosperms: calibrating the family tree. Proc Biol Sci 268:2211–2220. doi:10.1098/rspb.2001.1782
Witt T, Jürgens A, Gottsberger G (2013) Nectar sugar composition of European Caryophylloideae (Caryophyllaceae) in relation to flower length, pollination biology and phylogeny. J Evol Biol 26:2244–2259. doi:10.1111/jeb.12224
Wolff D (2006) Nectar sugar composition and volumes of 47 species of gentianales from a southern ecuadorian montane forest. Ann Bot 97:767–777
Acknowledgements
We thank Santiago Benitez-Vieyra, Rachel Dickson, Carolina Morales, Marina Strelin, and Miguel Verdú for their critical reading and for providing suggestions and comments on earlier versions of the manuscript. All authors are Scientific Researchers of the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina (CONICET). This work was partially supported by the Fondo para la Investigación Científica y Tecnológica (FONCyT, PICT-CHALCOFF-2008-1598).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chalcoff, V.R., Gleiser, G., Ezcurra, C. et al. Pollinator type and secondarily climate are related to nectar sugar composition across the angiosperms. Evol Ecol 31, 585–602 (2017). https://doi.org/10.1007/s10682-017-9887-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-017-9887-2