Abstract
Virtually all eukaryotes host microbial symbionts that influence their phenotype in many ways. In a host population, individuals may differ in their symbiotic complement in terms of symbiont species and strains. Hence, the combined expression of symbiont and host genotypes may generate a range of phenotypic diversity on which selection can operate and influence host population ecology and evolution. Here, we used the pea aphid to examine how the infection with various symbiotic complements contributes to phenotypic diversity of this insect species. The pea aphid hosts an obligate symbiont (Buchnera aphidicola) and several secondary symbionts among which is Hamiltonella defensa. This secondary symbiont confers a protection against parasitoids but can also reduce the host’s longevity and fecundity. These phenotypic effects of H. defensa infection have been described for a small fraction of the pea aphid complex which encompasses multiple plant-specialized biotypes. In this study, we examined phenotypic differences in four pea aphid biotypes where H. defensa occurs at high frequency and sometimes associated with other secondary symbionts. For each biotype, we measured the fecundity, lifespan and level of parasitoid protection in several aphid lineages differing in their symbiotic complement. Our results showed little variation in longevity and fecundity among lineages but strong differences in their protection level. These differences in protective levels largely resulted from the strain type of H. defensa and the symbiotic consortium in the host. This study highlights the important role of symbiotic complement in the emergence of phenotypic divergence among host populations of the same species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Symbionts are omnipresent in the living world and are ‘hidden players’ in many ecological and evolutionary processes (Frago et al. 2012). Symbiotic micro-organisms can be either obligate or accessory for their hosts and are often heritable. They may influence host phenotype through the combined expression of host and symbiont genomes (i.e. ‘extended phenotype’ termed by Dawkins 1982). Recent reviews have compiled evidence for the implication of symbionts on many traits of their hosts (Sachs et al. 2011; Feldhaar 2011; Friesen et al. 2011) such as nutrition (Douglas 2009), protection against natural enemies (Haine 2008; Oliver et al. 2014) or reproduction (Engelstädter and Hurst 2009). Symbionts can thus bring to their hosts novel biological properties and new phenotypes and so can play a crucial role in species ecology and evolution (Moran et al. 2008; Oliver and Martinez 2014).
Not only symbiont species but also strains of the same symbiont can influence the host’s phenotype. The well-known bacterial symbiont Wolbachia which infects 20–60 % of insect species (Werren et al. 1995; Werren and Windsor 2000; Hilgenboecker et al. 2008) was initially described as a reproductive parasite inducing a variety of sex manipulations (Werren et al. 2008). These reproductive alterations are seen as strategies for Wolbachia to invade host populations. But an alternative strategy for a microbial symbiont to spread is to provide a fitness benefit to their host individuals (Oliver and Martinez 2014). Recent years have seen rapid accumulation of evidence for Wolbachia conferring fitness improvements to their hosts through different mechanisms (Zug and Hammerstein 2015). This diversity in Wolbachia-mediate phenotypes is thought to result from complex interactions between host genotypes, symbiont strains and environmental conditions during the course of evolution (Zug and Hammerstein 2015).
To improve our understanding on how microbial symbionts are implicated in the evolutionary trajectories of their host species, one challenge is to assess both the diversity of the symbiont-associated phenotypes and their impacts on host populations and species. The pea aphid Acyrthosiphon pisum Harris is the host of heritable bacterial symbionts, including the obligate endosymbiont Buchnera aphidicola and several secondary symbionts (Gauthier et al. 2015). This insect forms a complex of plant adapted biotypes each specialized on one or few legume species and associated with a specific symbiont community (Peccoud et al. 2009, 2015). One famous secondary symbiont of the pea aphid is Hamiltonella defensa which is known to protect its hosts against one of its main enemies the parasitic wasp Aphidius ervi Haliday (Oliver et al. 2003; Ferrari et al. 2004; Oliver et al. 2005) through interaction with the phage APSE (Acyrthosiphon Pisum Secondary Endosymbiont; Moran et al. 2005; Oliver et al. 2009). Some studies have shown that infection with this protective symbiont could come with a fitness reduction for the host either directly through physiological costs (i.e., survival and fecundity reduction) or indirectly through ecological costs (i.e., higher vulnerability to predators; Oliver et al. 2008; Simon et al. 2011; Dion et al. 2011a; Polin et al. 2014, 2015). However, the phenotypes of individuals infected with H. defensa have been studied mostly in one of the 15 biotypes reported so far in the pea aphid (Peccoud et al. 2015): the Medicago sativa Linnaeus (alfalfa) biotype which has a worldwide distribution and is highly infected with H. defensa (Simon et al. 2003; Oliver et al. 2006; Frantz et al. 2009; Ferrari et al. 2012; Henry et al. 2013; Smith et al. 2015). Besides the M. sativa biotype, H. defensa is also found in high proportion or even fixed in four other biotypes: Lotus pedunculatus Cavanilles, Ononis spinosa Linnaeus (Ferrari et al. 2004, 2012; Henry et al. 2013), Genista tinctoria Linnaeus and G. sagittalis Linnaeus (Peccoud et al. 2015).
In this study, we examined the range of phenotypes associated with H. defensa in biotypes of the pea aphid with contrasted genetic and ecological differences, with this idea that phenotypic variation under symbiont influence would be greater between than within biotypes. For each of the four pea aphid biotypes in which H. defensa is highly prevalent or even fixed, we measured two life-history traits (total fecundity and lifespan) and the level of parasitism protection in several aphid lineages differing in their symbiotic complement (free of any secondary symbionts, infected with H. defensa singly or in co-infection with another secondary bacterial symbiont). In parallel, the strains of H. defensa found in the tested pea aphid lineages were genetically characterized in order to assess the link between host phenotype and strain type of H. defensa.
Materials and methods
Aphid lineages
The Acyrthosiphon pisum Harris lineages (i.e. a clone of aphid with its own symbiont composition) considered in the biological experiments were selected according to both their biotype and symbiotic status. Four A. pisum biotypes (M. sativa Linnaeus, O. spinosa Cavanilles, G. tinctoria Linnaeus and G. sagittalis Linnaeus) and three symbiotic statuses (without secondary symbiont, with H. defensa singly or with H. defensa in coinfection) were studied here (Table 1). ‘Pea aphid biotypes’ and ‘symbiotic status’ were not crossed factors here. Indeed, for both G. sagittalis and G. tinctoria biotypes, no natural individuals free of H. defensa were considered as this bacterial symbiont is fixed in Genista natural populations (Peccoud et al. 2015). Also, in natural populations of pea aphids, co-infection status differs between biotypes: H. defensa is frequently found in association with the secondary symbiont PAXS in the M. sativa biotype and with Serratia symbiotica in the two Genista biotypes; by contrast, co-infections involving H. defensa are rare in the Ononis biotype (Ferrari et al. 2012; Henry et al. 2013). All the pea aphid lineages were sampled in 2014 and 2015 in France, except for two lineages from M. sativa biotype naturally lacking H. defensa (in France, pea aphids from the M. sativa biotype free of any secondary symbiont are scarce (Simon et al. 2003; Frantz et al. 2009). In addition, to establish more directly the phenotypes conferred by H. defensa to the biotypes where this symbiont is fixed or close to fixation (i.e., O. spinosa, G. tinctoria and G. sagittalis biotypes), we attempted to eliminate this symbiont in the single H. defensa infected natural lineages by antibiotic treatments following the protocol described in McLean and Godfray (2015). Despite several attempts, we failed to eliminate H. defensa in the three G. sagittalis lineages and in one O. spinosa lineage (i.e., Os_Hd + 3). On contrary, H. defensa was successfully eliminated in the three G. tinctoria biotype lineages and the two other O. spinosa biotype lineages. A total of 29 natural pea aphid lineages were used for aphid life-history traits measurement and 40 lineages (i.e., 35 natural and 5 artificial) were analyzed in parasitism protection experiments (Table 1). Every aphid lineage (except those deriving from antibiotic treatments) was genetically distinct at seven microsatellite markers following (Peccoud et al. 2008) to avoid measuring biological traits in several representatives of the same clone. Aphid lineages were maintained individually in parthenogenetic reproduction (18 °C, 16 h of daylight) on the universal host plant Vicia faba.
Parasitoids
The parasitic wasp Aphidius ervi Haliday which is the main parasitoid of the pea aphid was used in experiments aimed at assessing how infections with secondary symbionts affect the parasitism protection. A mass-rearing was established in 2015 in the laboratory conditions (19 ± 2 °C, 60 ± 10 % humidity, 16 h of daylight) from fifty individuals produced by Koppert Biological Systems©. Before experiments, parasitoids were reared during several generations on a mixed-age culture of A. pisum feeding on Vicia faba and free of any known secondary symbionts. For the oviposition experimental parasitoid females were standardized: 2–3 days-old, mated and fed (honey and water). Before each experiment, the parasitoid female was exposed to one third-instar aphid larva for oviposition experience.
Aphid life-history traits measurement
For each of the 29 aphid lineages (Table 1), twenty adults were isolated and placed by two on a Vicia faba plant. After 1 day, the two adults were removed from the plant and only one first-instar offspring was monitored. Every 5 days, each individual was checked for survival and its fecundity (i.e. the number of offspring produced by the aphid) was recorded from onset of reproduction. Offspring were removed from the plant to avoid overcrowding. Total fecundity and lifespan were assessed for each aphid. To measure fitness for each aphid lineage, ten replicates were performed.
Parasitism assays
The experimental set-up consisted of introducing a single parasitoid female in a 4-cm diameter glass Petri dish containing 15 third-instar aphids from the tested lineage. During the experiment, an aphid was removed from the arena once attacked by the parasitic wasp. A parasitoid attack corresponds to an ovipositor insertion which, in this species, leads to a single egg injection into the host (McBrien and Mackauer 1990). The experiment ended when a minimum of 10 aphids were attacked. The attacked aphids were transferred onto a V. faba plant. Twelve days later, attacked aphids were inspected in order to measure the parasitism rate. This latter was estimated by dividing the number of aphid mummies (i.e., dead aphid containing a parasitoid immature) among the total number of attacked aphids transferred onto the V. faba plant. For each aphid lineage, five experimental replicates were performed (i.e., 50–60 aphid individuals attacked; Table 1).
Molecular characterization of H. defensa strains and detection of the APSE phage
Symbiont composition was checked prior life-history traits and parasitism experiments using diagnostic PCR based on the 16S ribosomal RNA gene according to (Peccoud et al. 2014). In the 26 aphid lineages infected with H. defensa (Table 1), a multilocus sequence-typing (MLST) was performed for strain characterization with housekeeping genes accD and murE sequences (Henry et al. 2013). Fragments were amplified by PCR using H. defensa-specific primers and cycling conditions described in (Henry et al. 2013). Amplicons were sent to Genoscreen for Sanger sequencing. Sequences obtained were then cleaned and aligned using Geneious® v.7.1.5 (Kearse et al. 2012). For each sample, accD and murE sequences were concatenated and used to build a phylogenetic tree using the Neighbor Joining method (Tamura-Nei distance). Bootstrap values were computed for each branch node (N = 1000). The presence of APSE phage was checked by using the same PCR protocol about symbiont composition based on two primers: P3 (forward) 5′-TCGGGCGTAGTGTTAATGAC-3′ (reverse) 5′-TTCCATAGCGGAATCAAAGG-3′ and P51 (forward) 5′-AGGTGCGATTACCCTGTTTG-3′ (reverse) 5′-GATAAAACATCGCCGTTTGC-3′ (Degnan and Moran 2008).
Statistical methods
The first analysis aimed at identifying the effect of the symbiotic complement on host phenotypes within each pea aphid biotype. By considering each biotype separately, both the aphid life-history traits and the parasitism protection level were tested against both the aphid symbiotic status and the aphid lineage, the aphid lineage factor being nested in the symbiotic status factor. Aphid life-history traits (lifespan and total fecundity) were analyzed using general linear models (LM), after validation of the Normal distribution of these dependent variables, while the analysis of the parasitism rates was performed by fitting generalized linear mixed models (GLMMs) by assuming a Binomial error and a logit link function. As one parasitoid female attacked several aphid individuals, the parasitoid individual was considered as a random factor in the parasitism rate statistical modelling. After the statistical modelling, pairwise comparisons between aphid lineages within each symbiotic status modality were performed by using either least-squares means for LM and contrast method for GLMM. After this first set of analyses, the second analysis aimed at testing whether the effects of symbiotic complement on host phenotypes depended on the pea aphid biotypes. For this purpose, data from all biotypes were pooled and both the aphid life-history traits and the parasitism protection level were tested against the interaction term between biotype and symbiotic status. Finally, to test whether the parasitism protection phenotype was related to H. defensa phylogeny, we used a Mantel test with Kendall method (Legendre and Legendre 2012) to calculate the correlation between the matrix of molecular distances between H. defensa strains harbored by the pea aphid lineages and the matrix of phenotypic distances between all pairwise combinations of these lineages. The molecular distance was calculated using the Juke-Cantor distance (Jukes and Cantor 1969) while the phenotypic distance corresponded to the absolute value of the difference between the parasitism protection levels for each pair of aphid lineages. All statistics were performed in R (Team 2014) using the package lme4 for GLMMs, lsmeans and DoBy for pairwise comparisons, vegan for Mantel test and GrapheR for graphics.
Results
Aphid life-history traits
In the M. sativa and O. spinosa pea aphid biotypes, the total fecundity did not differ between aphid individuals infected with H. defensa, singly or in coinfection, or free of H. defensa (Fig. 1; F2,81 = 2.41, p = 0.096; F1,69 = 3.70, p = 0.058; respectively). In the G. sagittalis and G. tinctoria biotypes, the number of offspring produced was lower in aphid individuals coinfected with H. defensa and S. symbiotica than aphids with a single infection of H. defensa (F1,51 = 4.04, p = 0.050; F1,72 = 6.11, p = 0.016; respectively). Within the M. sativa biotype, the total fecundity varied significantly between lineages coinfected with H. defensa and PAXS (Fig. 1). For all other modalities, no fecundity variation between aphid lineages has been observed. Concerning the aphid life span, the symbiotic complement had no effect in three out of the four biotypes (Fig. 2, M. sativa: F2,81 = 0.36, p = 0.698; O. spinosa: F1,69 = 0.04, p = 0.844; G. sagittalis: F1,51 = 0.03, p = 0.869). In the G. tinctoria biotype, aphids co-infected with H. defensa and S. symbiotica had a shorter lifespan (F1,72 = 21.72, p = 1.41 × 10−5). Within the O. spinosa biotype, the aphid lifespan varied significantly between lineages infected with H. defensa (Fig. 2). For all other modalities, no lifespan variation between lineages was observed.
Effect of the symbiotic status and aphid lineages on total fecundity in four different biotypes of the pea aphid (N = 10/treatment). Aphids without secondary symbiont in white (HD−), infected with H. defensa singly in grey (HD+) or infected with H. defensa in coinfection with another secondary symbiont in hatched (COINF). Error bars show the standard error. Different letters indicate significant differences between lineages for each symbiotic status in each biotype. Stars or NS indicates difference or not between symbiotic statuses within each biotype
Effect of the symbiotic status and lineages on lifespan in four different biotypes of the pea aphid (N = 10/treatment). Aphids without secondary symbiont in white (HD−), with H. defensa singly in grey (HD+) or with H. defensa in coinfection with another secondary symbiont in hatched (COINF). Error bars show the standard error. Different letters indicate significant differences between lineages for each symbiotic status in each biotype. Stars or NS indicates difference or not between symbiotic statuses within each biotype
The statistical analysis of the interaction term between pea aphid biotype and symbiotic status showed that the effect of a single infection with H. defensa on the host’s life-history traits did not differ between biotypes (aphid fecundity: F1,114 = 2.51, p = 0.072; aphid lifespan: F1,114 = 0.262, p = 0.609). However, the effect of the coinfection with H. defensa and another secondary symbiont on aphid’s life-history traits could differ between biotypes (aphid fecundity: F2,177 = 0.06, p = 0.933; aphid lifespan: F2,177 = 9.757, p = 9.55 × 10−5).
Parasitism protection
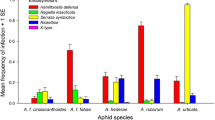
In the M. sativa and O. spinosa biotypes, parasitism rate was lower when aphids were single infected with H. defensa (Fig. 3, χ2 = 19.04, df = 1, p = 1.28 × 10−5; χ2 = 6.24, df = 1, p = 0.013 respectively). In the M. sativa biotype, high parasitism variation was observed between the different aphid lineages having the same symbiont status (Fig. 3). For instance, the parasitism rate strongly varied between M. sativa lineages single infected with H. defensa (from 0 to 74 %). In the O. spinosa biotype, parasitism rate variation was also observed between the lineages single infected with H. defensa (from 36 to 90 %) and the significance of the symbiotic status effect on the parasitism rate was mainly due to the results observed on the Os_HD3 lineage (Fig. 3). Multiple symbiotic infections also influenced the A. ervi parasitism rate in the pea aphid. In the M. sativa biotype, all aphid lineages co-infected with H. defensa and PAXS showed complete protection against A. ervi parasitism. In the G. sagittalis biotype, the parasitism rate was high (about 80 %) but aphids co-infected with H. defensa and S. symbiotica were significantly less parasitized than singly infected aphids (χ2 = 8.60, df = 1, p = 0.003). In the G. tinctoria biotype, no aphid mummies were observed regardless of the symbiotic status. Surprisingly, all attacked aphids became swollen, whitish and after dissection, the presence of a dead parasitoid larva was observed systematically (Fig. 5). For both Genista biotypes, no lineage variation was recorded for each symbiotic status (Fig. 3).
Effect of the symbiotic status and lineages on parasitism rate in four different biotypes of the pea aphid (N = 5 parasitoids/treatment). Aphids without secondary symbiont in white (HD−), with H. defensa singly in grey (HD+) or with H. defensa in coinfection with another secondary symbiont in hatched (COINF). Error bars show the standard error. Different letters indicate significant differences between lineages for each symbiotic status in each biotype. Stars or NS indicates difference or not between symbiotic statuses within each biotype. “0” when no mummy was observed
The statistical analysis of the interaction term between pea aphid biotype and symbiotic status showed that the effect of the single infection with H. defensa on parasitism rate was similar between biotypes (χ2 = 2.65, df = 1, p = 0.103) while the effect of multiple symbiotic coinfection on parasitism rate differed between biotypes (no test as only null values for two biotypes among the three co-infected with secondary symbionts but see Fig. 3).
The two artificial lineages from the O. spinosa biotype cured for H. defensa showed no difference in parasitism rate compared to the same symbiont-infected lineages. For the G. tinctoria biotype, aphids artificially deprived of H. defensa showed a high parasitism rate (more than 80 %) for the three lineages tested while the infected lineages from which they derived were fully protected (Figs. 4, 5).
Effect of the symbiotic status and lineages on parasitism rate in two different biotypes of the pea aphid (N = 5 parasitoids/treatment). Artificial cured aphids in white (HD−) and naturally infected by H. defensa alone in grey (HD+). Error bars show the standard error. Different letters indicate significant differences between lineages in each biotype. “0” when no mummy was observed
Healthy aphid of G. tinctoria biotype non-exposed to parasitoid (left). Aphid individuals from the M. sativa biotype (middle) and from the G. tinctoria biotype (right) 12 days after A. ervi parasitoid attack. Presence of dead parasitoid larva (‘p’) inside a G. tinctoria aphid individual dissected 12 days after parasitoid attack (up right)
Hamiltonella defensa strains and APSE phage detection
We detected the APSE phage for all aphid lineages infected with H. defensa. The analysis of concatenated sequences of murE and accD genes revealed six different haplotypes among the 26 strains of H. defensa (Fig. 6) and the NJ tree clustered these sequences into 4 clades supported by bootstrap values >80 %. Compared to the strains of H. defensa harbored by the two Genista biotypes, strains of H. defensa from the M. sativa and O. spinosa biotypes were spread in different clades. Note that H. defensa strains in coinfection with PAXS in M. sativa biotype belonged to the same haplotype. Clade I was associated with a mixture of protective levels against A. ervi parasitoid but complete protection in this clade is only observed for coinfection with PAXS. Clade II comprises a single strain with complete protection. Clade III includes the two strains from O. spinosa that conferred low protection. Clade IV gathers strains associated with protection >95 %. Finally, the difference of parasitism protection level across the pea aphid lineages was related to the molecular distance between the H. defensa strains (Mantel statistic r = 0.2613, p = 0.015).
Neighbor joining tree based on concatenated sequences of genes murE and accD from 26 strains of H. defensa. Bootstrap values are indicated on branch nodes. *: new protective phenotype. Strain code: ‘Ms’: Medicago sativa biotype; ‘Os’: Ononis spinosa biotype; ‘Gt’: Genista tinctoria biotype; ‘Gs’: Genista sagittalis biotype; ‘Hd’: H. defensa in monoinfection; ‘Coinf’: H. defensa in coinfection with another secondary symbiont
Discussion
In this study, we measured the phenotype of aphid lineages infected or not with H. defensa, singly or in coinfection, and from different biotypes of the pea aphid complex. A wide range of phenotypes was observed, especially for the level of protection against the A. ervi parasitism. This phenotypic variation would strongly depend on both the co-infection status and the bacterial strains of H. defensa associated with aphid lineages and biotypes.
A symbiont not so costly
In both O. spinosa and M. sativa biotypes, pea aphids infected with H. defensa presented similar life-history traits compared to individuals free of secondary symbionts. In natural populations of these two biotypes, H. defensa occurs at intermediate frequencies in Europe (Simon et al. 2003; Frantz et al. 2009; Ferrari et al. 2012; Henry et al. 2013) and North America [for M. sativa biotype only (Oliver et al. 2006; Smith et al. 2015)] despite the protection against parasitoids this symbiont confers (Oliver et al. 2003). Such an ecological paradox could be explained by a cost-benefit balance associated with H. defensa (Kwiatkowski and Vorburger 2012; Vorburger et al. 2013; Oliver et al. 2014). In few studies, a fitness reduction has been observed in aphids harboring H. defensa from the M. sativa biotype of A. pisum (Oliver et al. 2008; Simon et al. 2011) and in Aphis fabae (Vorburger and Gouskov 2011). These fitness costs of H. defensa infection have been mostly detected in manipulated lines generated after injection or removal of H. defensa. These artificial symbiotic associations have thus not been exposed to natural selection, which may explain the contrast with our results. For both Genista biotypes, aphids with two secondary symbionts had lower fecundity than those with H. defensa alone. In various systems, multiple infections often induce higher fitness costs to the hosts (Mouton et al. 2004; Oliver et al. 2006; Leclair et al. 2016). Besides these direct costs, H. defensa infection could reduce aphid fitness through other effects such as ecological costs (Dion et al. 2011a; Polin et al. 2015), which have not been studied here.
A symbiont not always protective
Hamiltonella defensa has been repeatedly reported as a protective symbiont in the pea aphid (Oliver et al. 2003; Ferrari et al. 2004; Oliver et al. 2005). Here, we observed a wide range of variation in protective level between and within A. pisum biotypes. We confirmed that H. defensa is not always protective in the well-studied M. sativa biotype (Oliver et al. 2003, 2005; Ferrari et al. 2004), this being dependent on symbiont strain (Oliver et al. 2005) and its associated phage (Moran et al. 2005; Oliver et al. 2009). Note that one aphid lineage free of H. defensa of the M. sativa biotype presented a strong A. ervi parasitism resistance confirming that the resistant phenotype can also be observed in absence of any secondary symbiont (Martinez et al. 2014) and due to aphid genetic background (Hufbauer and Via 1999). Compared to the M. sativa biotype, no protection due to H. defensa is suggested in the O. spinosa biotype (McLean and Godfray, 2015). Here, a partial protection from A. ervi parasitism was observed in one H. defensa infected lineage (i.e., Os_Hd + 3) among the three we tested. Unfortunately, as we failed to eliminate H. defensa in this lineage, the role of the secondary symbiont on this protective phenotype cannot be confirmed. Furthermore, elimination of this symbiont in the two other lineages of the O. spinosa biotype showed that aphids with or without H. defensa were parasitized at same rate. The level of parasitism protection did not spread randomly in the phylogenetic tree built on gene sequences of H. defensa and a significant correlation between protective phenotype and genetic distances among the 26 H. defensa strains was found. This indicates that the wide range of protective phenotype would be mainly related to molecular differences between strains.
Aphids from the G. tinctoria biotype were totally protected against the A. ervi parasitism. Interestingly, we reported a new protective phenotype displayed by all individuals exposed to parasitoids: they became swollen and sterile, a whitish mass was visible inside and after dissection, a dead parasitoid larva was systematically found and all aphid embryos looked in decay. Despite the strong protection against parasitoids, this peculiar phenotype can be considered as a ‘dead end’ for the attacked aphid as no offspring is produced. At the aphid population level, it could however be advantageous as parasitoid immatures are killed into their hosts. Here, we succeeded to eliminate H. defensa in three different lineages of the G. tinctoria biotype. By comparing the same lineage with or without the symbiont, we could demonstrate that H. defensa is indeed responsible for this new kind of protective phenotype.
By contrast, the infection with H. defensa did not confer any protection in the G. sagittalis biotype as aphids attacked by A. ervi presented a high rate of parasitism (>90 %). A slight decline in parasitism rate has been however observed when aphids were coinfected with H. defensa and S. symbiotica (>80 %). The lack of protection despite the infection with H. defensa could be due to the phage (APSE) which is a key player in parasitism resistance (Moran et al. 2005; Oliver et al. 2009). Here, the APSE phage has been detected in all aphid lineages harboring H. defensa singly or in co-infection. However, APSE variants differ in the toxins they produced and therefore in the protection level against parasitoids (Oliver et al. 2005; Degnan and Moran 2008; Oliver et al. 2009). Molecular characterization of the phage associated with the different strains of H. defensa is needed to assess the link between phage variants and the protection phenotype.
Together, our results indicate high variation in parasitism protection within the pea aphid complex and this would generate a spatial heterogeneity in resistance to A. ervi: some pea aphid biotypes are good quality sources while other are sinks for these natural enemies. Experimental evolution approaches show that A. ervi parasitoid populations facing aphids harboring H. defensa strains conferring a strong protection level readily evolve counter-adaptations to the symbiont-mediated resistance of their aphid hosts (Dion et al. 2011b; Rouchet and Vorburger 2014). In nature, Aphidius ervi populations are unlikely to be specialized on different pea aphid biotypes (Bilodeau et al. 2013; Derocles et al. 2016; Zepeda-Paulo et al. 2016) and then, the differential protection levels between pea aphid biotypes may impose different evolutionary challenges to parasitoid populations and may particularly alter counter-adaptation by A. ervi.
Incidence of coinfections
In natural populations of the pea aphid, H. defensa is often associated with other secondary symbionts (Ferrari et al. 2012). In particular, H. defensa frequently co-occurs with PAXS in the M. sativa biotype (Henry et al. 2013) and with Serratia symbiotica in G. tinctoria and G. sagittalis biotypes (Peccoud et al. 2015). Coinfection could affect the phenotype associated with H. defensa through antagonistic, additive or synergistic effects (Oliver et al. 2014). In our study, whatever the biotype considered, we observed a fitness reduction (lower fecundity and shorter lifespan) in case of coinfection between H. defensa and another secondary symbiont. However, H. defensa associated with PAXS or S. symbiotica conferred a stronger protection against parasitism. In particular, coinfection between PAXS and H. defensa is associated with a complete protection in the M. sativa biotype, as already reported in (Guay et al. 2009). By generating beneficial and detrimental effects on host fitness, multiple infections could be an important source of phenotypic variation within a host species.
Another role of H. defensa in the pea aphid complex?
Conferring a protection against natural enemies is one strategy for a microbial symbiont to spread within a host population (Zug and Hammerstein 2015). Hamiltonella defensa strains infecting the G. sagittalis biotype confer no protection against A. ervi. Surprisingly, this bacterial symbiont is fixed in natural populations for this biotype (Peccoud et al. 2015). Either the infection with H. defensa in the G. sagittalis biotype protects against different parasitoid species (McLean and Godfray 2015) or different A. ervi genotypes (Rouchet and Vorburger 2014) or it provides another benefit to their host individuals. In the whitefly Bemisia tabaci, H. defensa seems to have a nutritional function for its host (Su et al. 2013, 2015) by presumably complementing the obligate symbiont Portiera in essential nutrients for the whitefly (Rao et al. 2015). Similarly, in the aphid Cinara cedri, S. symbiotica, a secondary symbiont associated with many aphid species (Henry et al. 2015), evolved as a co-obligate symbiont by sharing with Buchnera aphidicola in providing essential nutrients (Lamelas et al. 2011). It can also be hypothesized that the fixation of H. defensa in populations of the G. sagittalis biotype could be explained by an essential nutritional role allowing aphids to exploit this specific legume species. Further experiments are then needed to determine why H. defensa is fixed in this biotype.
In this study, the host phenotypes under symbiont influence have been measured on aphids feeding on Vicia faba, the “universal” plant for all biotypes of the pea aphid complex. While this plant represents a common garden, allowing comparisons of phenotypes expressed by the different biotypes across the same environment, it is possible that phenotypes may differ when aphid biotypes feed on the plant on which they are specialized. The next step would then be to compare the extended phenotypes of the pea aphid biotypes on both their native plants and the universal host.
Conclusion
In this study, we have shown that the phenotype of individuals infected with H. defensa varies considerably according to symbiont strain, coinfection status and aphid genotype. The phenotypic variation expressed through symbiotic interactions would be the outcome of complex interactions between all involved genomes, but also with the biotic (e.g. natural enemies) and abiotic components of the environment where these interactions take place. To be even closer to the complexity of these symbiotic interactions, also the aphid host plant must be considered. Further works are needed to take into account simultaneously variation in symbionts, aphid hosts and plants for their influence on phenotypic variation in insect populations.
References
Bilodeau E, Simon JC, Guay JF et al (2013) Does variation in host plant association and symbiont infection of pea aphid populations induce genetic and behaviour differentiation of its main parasitoid, Aphidius ervi? Evol Ecol 27:165–184
Dawkins R (1982) The extended phenotype: the gene as the unit of selection. Oxford University Press, Oxford
Degnan PH, Moran NA (2008) Diverse phage-encoded toxins in a protective insect endosymbiont. Appl Environ Microbiol 74:6782–6791
Derocles SAP, Plantegenest M, Rasplus JY et al (2016) Are generalist Aphidiinae (Hym. Braconidae) mostly cryptic species complexes? Syst Entomol 41:379–391
Dion E, Polin SE, Simon J-C, Outreman Y (2011a) Symbiont infection affects aphid defensive behaviours. Biol Lett 7:743–746
Dion E, ZéLé F, Simon J-C, Outreman Y (2011b) Rapid evolution of parasitoids when faced with the symbiont-mediated resistance of their hosts: symbiont effects on host-parasitoid evolution. J Evol Biol 24:741–750
Douglas AE (2009) The microbial dimension in insect nutritional ecology. Funct Ecol 23:38–47
Engelstädter J, Hurst GDD (2009) The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Evol Syst 40:127–149
Feldhaar H (2011) Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36:533–543
Ferrari J, Darby AC, Daniell TJ et al (2004) Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol Entomol 29:60–65
Ferrari J, West JA, Via S, Godfray HCJ (2012) Population genetic structure and secondary symbionts in host-associated populations of the pea aphid complex: population genetics and symbionts in the pea aphid. Evolution 66:375–390
Frago E, Dicke M, Godfray HCJ (2012) Insect symbionts as hidden players in insect–plant interactions. Trends Ecol Evol 27:705–711
Frantz A, Calcagno V, Mieuzet L et al (2009) Complex trait differentiation between host-populations of the pea aphid Acyrthosiphon pisum (Harris): implications for the evolution of ecological specialisation. Biol J Linn Soc 97:718–727
Friesen ML, Porter SS, Stark SC et al (2011) Microbially mediated plant functional traits. Annu Rev Ecol Evol Syst 42:23–46
Gauthier J-P, Outreman Y, Mieuzet L, Simon J-C (2015) Bacterial communities associated with host-adapted populations of pea aphids revealed by deep sequencing of 16S ribosomal DNA. PLoS ONE 10:e0120664
Guay J-F, Boudreault S, Michaud D, Cloutier C (2009) Impact of environmental stress on aphid clonal resistance to parasitoids: role of Hamiltonella defensa bacterial symbiosis in association with a new facultative symbiont of the pea aphid. J Insect Physiol 55:919–926
Haine ER (2008) Symbiont-mediated protection. Proc R Soc B Biol Sci 275:353–361
Henry LM, Peccoud J, Simon J-C et al (2013) Horizontally transmitted symbionts and host colonization of ecological niches. Curr Biol 23:1713–1717
Henry LM, Maiden MCJ, Ferrari J, Godfray HCJ (2015) Insect life history and the evolution of bacterial mutualism. Ecol Lett 18:516–525
Hilgenboecker K, Hammerstein P, Schlattmann P et al (2008) How many species are infected with Wolbachia?—a statistical analysis of current data: Wolbachia infection rates. FEMS Microbiol Lett 281:215–220
Hufbauer RA, Via S (1999) Evolution of an aphid-parasitoid interaction: variation in resistance to parasitism among aphid populations specialized on different plants. Evolution 53:1435–1445
Jukes TH, Cantor CR (1969) Evolution of protein molecules. Mamm Protein Metab 3:132
Kearse M, Moir R, Wilson A et al (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Kwiatkowski M, Vorburger C (2012) Modeling the ecology of symbiont-mediated protection against parasites. Am Nat 179:595–605
Lamelas A, Gosalbes MJ, Manzano-Marín A et al (2011) Serratia symbiotica from the aphid Cinara cedri: a missing link from facultative to obligate insect endosymbiont. PLoS Genet 7(e1002357):7
Leclair M, Polin S, Jousseaume T et al (2016) Consequences of co-infection with protective symbionts on the host phenotype and symbiont titres in the pea aphid system. Insect Sci. doi:10.1111/1744-7917.12380
Legendre P, Legendre LF (2012) Numerical ecology. Elsevier, Amsterdam
Martinez AJ, Ritter SG, Doremus MR et al (2014) Aphid-encoded variability in susceptibility to a parasitoid. BMC Evol Biol 14:1
McBrien H, Mackauer M (1990) Heterospecific larval competition and host discrimination in two species of aphid parasitoids: Aphidius ervi and Aphidius smithi. Entomol Exp Appl 56:145–153
McLean AHC, Godfray HCJ (2015) Evidence for specificity in symbiont-conferred protection against parasitoids. Proc R Soc B Biol Sci 282:20150977
Moran NA, Degnan PH, Santos SR et al (2005) The players in a mutualistic symbiosis: insects, bacteria, viruses, and virulence genes. Proc Natl Acad Sci USA 102:16919–16926
Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190
Mouton L, Dedeine F, Henri H et al (2004) Virulence, multiple infections and regulation of symbiotic population in the Wolbachia-Asobara tabida symbiosis. Genetics 168:181–189
Oliver KM, Martinez AJ (2014) How resident microbes modulate ecologically-important traits of insects. Curr Opin Insect Sci 4:1–7
Oliver KM, Russell JA, Moran NA, Hunter MS (2003) Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Natl Acad Sci 100:1803–1807
Oliver KM, Moran NA, Hunter MS (2005) Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc Natl Acad Sci USA 102:12795–12800
Oliver KM, Moran NA, Hunter MS (2006) Costs and benefits of a superinfection of facultative symbionts in aphids. Proc R Soc B Biol Sci 273:1273–1280
Oliver KM, Campos J, Moran NA, Hunter MS (2008) Population dynamics of defensive symbionts in aphids. Proc R Soc B Biol Sci 275:293–299
Oliver KM, Degnan PH, Hunter MS, Moran NA (2009) Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325:992–994
Oliver KM, Smith AH, Russell JA (2014) Defensive symbiosis in the real world—advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct Ecol 28:341–355
Peccoud J, Figueroa CC, Silva AX et al (2008) Host range expansion of an introduced insect pest through multiple colonizations of specialized clones. Mol Ecol 17:4608–4618
Peccoud J, Ollivier A, Plantegenest M, Simon J-C (2009) A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc Natl Acad Sci 106:7495–7500
Peccoud J, Bonhomme J, Mahéo F et al (2014) Inheritance patterns of secondary symbionts during sexual reproduction of pea aphid biotypes: sex and inheritance of aphid symbionts. Insect Sci 21:291–300
Peccoud J, Mahéo F, de la Huerta M et al (2015) Genetic characterisation of new host-specialised biotypes and novel associations with bacterial symbionts in the pea aphid complex. Insect Conserv Divers 8:484–492
Polin S, Simon J-C, Outreman Y (2014) An ecological cost associated with protective symbionts of aphids. Ecol Evol 4:836–840
Polin S, Le Gallic J-F, Simon J-C et al (2015) Conditional reduction of predation risk associated with a facultative symbiont in an insect. PLoS ONE 10:e0143728
Rao Q, Rollat-Farnier P-A, Zhu D-T et al (2015) Genome reduction and potential metabolic complementation of the dual endosymbionts in the whitefly Bemisia tabaci. BMC Genomics 16:226
Rouchet R, Vorburger C (2014) Experimental evolution of parasitoid infectivity on symbiont-protected hosts leads to the emergence of genotype specificity: experimental evolution of parasitoid infectivity. Evolution 68:1607–1616
Sachs JL, Skophammer RG, Regus JU (2011) Evolutionary transitions in bacterial symbiosis. Proc Natl Acad Sci 108:10800–10807
Simon J-C, Carre S, Boutin M et al (2003) Host-based divergence in populations of the pea aphid: insights from nuclear markers and the prevalence of facultative symbionts. Proc R Soc Lond B Biol Sci 270:1703–1712
Simon J-C, Boutin S, Tsuchida T et al (2011) Facultative symbiont infections affect aphid reproduction. PLoS ONE 6:e21831
Smith AH, Łukasik P, O’Connor MP et al (2015) Patterns, causes and consequences of defensive microbiome dynamics across multiple scales. Mol Ecol 24:1135–1149
Su Q, Oliver KM, Pan H et al (2013) Facultative symbiont Hamiltonella confers benefits to Bemisia tabaci (Hemiptera: Aleyrodidae), an invasive agricultural pest worldwide. Environ Entomol 42:1265–1271
Su Q, Oliver KM, Xie W et al (2015) The whitefly-associated facultative symbiont Hamiltonella defensa suppresses induced plant defences in tomato. Funct Ecol 29:1007–1018
Team RC (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0
Vorburger C, Gouskov A (2011) Only helpful when required: a longevity cost of harbouring defensive symbionts: defensive symbionts reduce host longevity. J Evol Biol 24:1611–1617
Vorburger C, Ganesanandamoorthy P, Kwiatkowski M (2013) Comparing constitutive and induced costs of symbiont-conferred resistance to parasitoids in aphids. Ecol Evol 3:706–713
Werren JH, Windsor DM (2000) Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proc R Soc B Biol Sci 267:1277–1285
Werren JH, Zhang W, Guo LR (1995) Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc R Soc Lond B Biol Sci 261:55–63
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751
Zepeda-Paulo F, Lavandero B, Mahéo F, Dion E, Outreman Y, Simon JC, Figueroa CC (2016) Signatures of genetic bottleneck and differentiation after the introduction of an exotic parasitoid for classical biological control. Biol Inv 18:565–581
Zug R, Hammerstein P (2015) Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts: Wolbachia mutualisms in arthropods. Biol Rev 90:89–111
Acknowledgments
We are very grateful to Jean Peccoud for his description of Genista biotypes and stimulating discussions on the pea aphid system, to all people who helped in sampling aphid lineages in natural populations, to Gaëtan Denis for help with aphid cultures, to Jean-François Le Gallic and Grégory Toussain for helping in fitness measurements, to Jean-Pierre Gauthier for phylogenetic analyses and to Bernard Chaubet for the aphid pictures. Two anonymous referees are also acknowledged for their constructive comments on an earlier draft. This work was supported by French ‘Ministère de l’Enseignement Supérieur et de la Recherche’ and by a grant from INRA Santé des Plantes et Environnement department.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mélanie Leclair and Inès Pons have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Leclair, M., Pons, I., Mahéo, F. et al. Diversity in symbiont consortia in the pea aphid complex is associated with large phenotypic variation in the insect host. Evol Ecol 30, 925–941 (2016). https://doi.org/10.1007/s10682-016-9856-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-016-9856-1