Abstract
The decoy or deflection hypothesis, which states that conspicuous colouration is present in non-vital parts of the body to divert attacks from head and trunk, thus increasing survival probability, is a possible explanation for the presence of such colouration in juveniles of non-aposematic species. To test this hypothesis we made plasticine and plaster lizard models of two colour morphs, red or dark-and-light striped tails, based on the colouration of spiny-footed lizard (Acanthodactylus erythrurus) hatchlings, which naturally show a dark-and-light striped dorsal pattern and red tail. Lizard models were placed in the field and also presented to captive common kestrels (Falco tinnunculus), a common avian lizard predator. The number of attacks and the body part attacked (tail or rest-of-body) were recorded, as well as the latency to attack. Our results suggest that models of both colour morphs were recognized as prey and attacked at a similar rate, but in the field, red-tailed models were detected, and thus attacked, sooner than striped-tailed. Despite this increase in detection rate by predators, red-tailed models effectively diverted attacks to the tail from the more vulnerable body parts, thus supporting the decoy hypothesis. Greater fitness benefits of attack diversion to the tail compared to the costs of increased detection rate by predators would explain the evolution and maintenance of red tail colouration in lizards.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the function of conspicuous colouration in prey has attracted much interest because it may also increase detection by predators (Arnold 1984; Endler 1980; Haskell 1996). Conspicuous colouration may be a consequence of sexual selection in adults (Darwin 1871; Kemp and Rutowski 2011; Stuart-Fox et al. 2003) because it may improve mating success. Because conspicuous colouration is sometimes also present in juveniles (Booth 1990), and sexual selection cannot be driving colour evolution in such cases, at least three alternative hypotheses have been proposed to explain its evolution. The inter-age-class signalling hypothesis proposes that conspicuous colouration in juveniles evolves to reduce aggression from conspecific adults (Clark and Hall 1970; Ochi and Awata 2009; VanderWerf and Freed 2003). Conspicuous colouration in juveniles may also advertise to predators prey toxicity or unpalatability (aposematism hypothesis), or may mimic an aposematic prey model (Booth 1990; Kraus and Allison 2009). Lastly, conspicuous colouration of non-vital body parts may divert attacks from vital parts (decoy or deflection hypothesis; Bateman et al. 2014; Johnson et al. 2008; Kodandaramaiah et al. 2013; Telemeco et al. 2011; Watson et al. 2012). This function as a lure can be reinforced by stereotyped movements of the conspicuously coloured body part (Telemeco et al. 2011).
The decoy hypothesis is more plausible in species where conspicuous colouration is present in expendable body parts. This is taken to the extreme in reptiles, where juveniles and/or adults of many lizard species show conspicuous colouration in the tail (Castilla et al. 1999; Clark and Hall 1970; Hawlena et al. 2006; Watson et al. 2012), which can be easily detached from the rest of the body, thus facilitating the escape from predators, although its loss might eventually lead to increased risk of mortality (Arnold 1988; Fox and McCoy 2000; Wilson 1992; review in Bateman and Fleming 2009). Although conspicuous colouration very often increases detection by predators (Arnold 1984; Bateman et al. 2014; Endler 1980; Haskell 1996; but see Castilla et al. 1999; Watson et al. 2012), this signal may still be selected if the benefits of escaping an attack exceed the costs of increased attack rate (Cooper and Vitt 1991). The first studies addressing the decoy hypothesis in lizards were conducted in captivity (Clark and Hall 1970; Cooper and Vitt 1985; Vitt and Cooper 1986), but the use of plasticine (Mochida 2011; Valkonen et al. 2011; Watson et al. 2012) or plaster (Baylis et al. 2012; Ruiz-Rodríguez et al. 2013) models has allowed experiments in the field with different vertebrate species, obtaining information on predator attacks either by video recording or by inferring the attacks by the marks left on the models.
The efficiency of blue and green lizard tails in diverting attacks has already been tested (Castilla et al. 1999; Cooper and Vitt 1985; Watson et al. 2012), but there are a number of species from several lizard families (e.g., Scincidae, Gymnophthalmidae, Lacertidae) that have red tails. Avian predators are known to discriminate colours (Bowmaker 2008), and different colours might cause different responses in bird predators, either because of differences in conspicuousness (e.g., Schaefer et al. 2006) or because of a sensory bias for certain colour (e.g., Møller and Erritzøe 2010). Consequently, results obtained when testing the decoy hypothesis in blue and green tails might not apply to red tails. The effectiveness of red tails as decoys has not yet been addressed. In the present study, our aim was to answer two specific questions: (1) do red tails make lizards more detectable to avian predators? and (2) are red tails effective decoys for diverting bird attacks from vital body parts? To answer these questions we used plasticine and plaster models based on the morphology of spiny-footed lizards (Acanthodactylus erythrurus, Schinz 1833), a species in which hatchlings have red tails and dark-and-light striped bodies. We checked whether birds attacked red-tailed models (striped body and red tail) more and/or sooner than those with striped tails (striped body and tail), and whether red-tailed models received more attacks on the tail than striped-tailed. Models were placed in the field and also presented to captive common kestrels (Falco tinnunculus, Linnaeus 1758), one of the main avian predators of A. erythrurus (Martín and López 1990).
Materials and methods
Study species
Acanthodactylus erythrurus is a medium-sized lizard [snout-vent length (SVL) and total length up to around 80 and 230 mm, respectively, although size can vary among the populations (Carretero and Llorente 1993; Seva Román 1982)] present in southern and central Iberian Peninsula and northern Africa (Belliure 2006). In populations in central Spain, new-born lizards appear in mid-August and are active until November. At the beginning of April the following spring, these lizards become active again, and they do not reach sexual maturity until their second spring (Castilla et al. 1992; Pollo and Pérez-Mellado 1990). Therefore, all individuals can be classified in three age classes, hereafter referred to as hatchlings (from hatching until the first winter), juveniles (from first to second winter) and adults (from the second winter onwards).
Colouration in this species undergoes ontogenetic changes. Hatchlings show a dorsal pattern of strongly contrasting dark and light bands that run the length of their bodies. This pattern starts reticulating in juveniles, and becomes dark and light patches in adults (Seva Román 1982). The ventrolateral parts of hatchling and juvenile tails are red (Carretero and Llorente 1993; Seva Román 1982), and this colour is clearly visible from above in hatchlings and small juveniles (personal observation). The rear part of the hind limbs of juveniles is also red. Juvenile males lose their red colouring when they are around 1 year old, whereas juvenile females retain it through adulthood until they become gravid (Cuervo and Belliure 2013; Seva Román 1982).
Lizard models
We made some lizard models with plasticine and some with plaster, because both types of models have been widely used in similar studies in various taxa (Castilla et al. 1999; Castilla and Labra 1998; Mochida 2011; Ruiz-Rodríguez et al. 2013; Valkonen et al. 2011; Vervust et al. 2007; Watson et al. 2012). Plaster models were made using plastic lizard toys which we covered with a thin layer of plaster (Aquaplast Standard, Beissier S.A., Guipúzcoa, Spain). These models were larger than real hatchlings (plastic lizard toys: mean SVL ± SD = 56.6 ± 7.9 mm, mean total length ± SD = 131.8 ± 31.4 mm, N = 10; hatchlings: mean SVL ± SD = 38.3 ± 2.6 mm, mean total length ± SD = 107.7 ± 10.7 mm, N = 23; t Student test, t 31 ≥ 3.32, P ≤ 0.002 in the two tests), but both juveniles and adults are common prey of avian predators (Belliure 2006), so we think these models could still be easily recognized as prey. Plasticine models were made by hand from black oil-based modelling clay (Plastilina Jovi, Barcelona, Spain) and attempted to mimic the size of real hatchlings (random sample of models: mean SVL ± SD = 38.4 ± 2.8 mm, mean total length ± SD = 107.7 ± 7.9 mm, N = 15; comparison with hatchlings: t Student test, 0.00 ≤ t 36 ≤ 0.09, P ≥ 0.928 in the two tests).
Model colouring was based on real A. erythrurus hatchlings. We used non-toxic paints (Satin Paint, La Pajarita, Manises, Spain) to create two colour morphs that differed in tail colouration: red-tailed models and striped-tailed models (Fig. 1). In both colour morphs, the back of the body consisted of a contrasting pattern of dark and light bands (Fig. 1). Neither the paints used in the experiment nor real lizard dorsal colouration reflect UV light (B. Fresnillo, J. Belliure and J. J. Cuervo, unpublished data), so differences in colour discrimination by birds and humans was probably not an issue in this study.
Types of models used in the study: a red-tailed plaster model (tail completely red), b striped-tailed plaster model (dark and light banded tail pattern), c red-tailed plasticine model (red tail with a light band on the back), and d striped-tailed plasticine model (dark and light banded tail pattern). (Color figure online)
In both plaster and plasticine models, it was possible to identify the type of predator by the marks left on the model based on previous studies (Baylis et al. 2012; Brodie 1993; Valkonen et al. 2011; see Fig. 2). As plaster is harder than plasticine, we are aware that some avian attacks might not have left any mark on the plaster models. We only recorded avian attacks in this study because birds are mainly visually-directed predators, whereas mammals and reptiles also rely on chemical cues (Greene 1988). Materials used to make the models are not odourless and certainly do not smell like lizards, which might have affected the predatory behaviour of mammals and reptiles.
Types of marks left on the models by predators: a V-shaped mark left by a bird on a plasticine model, b U-shaped mark left by a bird on a plasticine model, c puncture mark left by a bird on a plaster model, d V-shaped mark with tooth marks left by lizards on a plasticine model, e tooth marks left by rodents on a plasticine model, f tooth marks left by rodents on a plaster model, and g tracks left on the ground after an avian attack when the model disappeared. (Color figure online)
Field study
We distributed lizard models when hatchlings were active (August–November) in two different years (2010 and 2011) and in two localities in central Spain where A. erythrurus is common. One study area was located in Chapinería, in south-western Region of Madrid (N40°22′; W4°13′), a Mediterranean oak forest with a meadow structure, where oaks (Quercus ilex L.) and lavender (Lavandula stoechas Lam.) dominated vegetation patches surrounded by open areas. The other study area was located in Aranjuez, in southern Region of Madrid (N40°1′; W3°33′), a steppe dominated by esparto grass (Stipa tenacissima L.) where trees were scarce and the space surrounding plants was mainly bare soil. The study areas were 67 km apart in a straight line, and were selected because vegetation cover differed dramatically (many fewer trees in Aranjuez), which may have influenced prey detectability by visually-directed predators (Cuadrado et al. 2001; Vásquez et al. 2002). Moreover, both study areas might also have different predator communities. The study was replicated in two different habitats because the effect of model colouration on avian predation rate might depend on the habitat (a stronger effect was expected in more open habitats or where the community of avian predators was more abundant).
In both localities and years, models were placed in open areas near bushes (as if they were basking lizards), at least 100 m apart from one another, and alternating both colour morphs. When both plaster and plasticine models were present in the same study area, locations of the two model types were randomized. In 2010, we worked only in Chapinería and only with plaster models. We distributed an equal number of models of both colour morphs and inspected them every 4–5 days to record the number of models attacked by birds and the body parts attacked (tail and/or rest-of-body). A total of ten models were always present in the field, as a new model was added in a new location when any model was removed due to an avian attack or after four visits without avian attacks. This makes a maximum of 20 days of exposition in the field for these models. In 2011, we worked in both localities, using plasticine and plaster models in Chapinería but only plasticine models in Aranjuez. Both study areas were visited every 3 days, and 10–16 models were left in each area, always evenly distributed between both colour morphs. The protocol for recording attacks and replacing models was the same as in 2010, so models were exposed to predators a maximum of 12 days in both localities.
A total of 189 lizard models were placed in the field, but 85 of them (two plaster and 83 plasticine models) disappeared. We considered these models as attacked (in both body parts) if there were bird tracks (Fig. 2g) on the ground (11 models), or as “disappeared” if there were no clear bird tracks (74 models). Lizard models considered as disappeared were excluded from statistical analyses. Most plasticine models that disappeared were very probably eaten by ants.
Captivity study
Falco tinnunculus is a bird of prey in the Falconidae family that is widely distributed across Eurasia and Africa (BirdLife International 2014). It commonly preys on lizards, at least in some areas (Carrillo and González-Dávila 2009; Carrillo et al. 1994; Vanzyl 1994), and is a typical predator of A. erythrurus in Spain (Martín and López 1990), what makes this species a good model organism for studying predatory interactions with our lizard models.
For this study, we used captive F. tinnunculus from the Santa Faz Wildlife Rehabilitation Centre (Alicante, Spain) from August to October in three consecutive years (2011, 2012 and 2013). Only birds that were able to fly were involved in the experiment, although some of them had sustained wing injuries. We conducted a total of 31 trials in which an F. tinnunculus (which had not been fed for 24 h before the trial) was presented with a plasticine lizard model that was either red-tailed (N = 15) or striped-tailed (N = 16). Each bird was used only once. Trials were conducted in individual cages (4.80 × 1.65 × 3.20 m). The lizard model was placed in the centre of the concrete cage floor at the same time that the bird was placed on a perch about 3 m from the lizard model and 1.3 m off the floor. Models were checked for attacks from outside the cage two or three times per day. The observer only entered the cage when an attack on the model was suspected. The trial ended when the model was attacked or after 4 days without attacks. If the model was attacked, the body part attacked, i.e., with marks (tail or rest-of-body), and the latency to attack in days were recorded.
Statistical analyses
In the field study, Logistic Regression Models (GLZs) with binomial error distribution were used to test for any effect of model colour morph (red or striped tail) on (1) the probability of being attacked and (2) the probability of being attacked on each body part (tail or rest-of-body). In the first analysis, all the models not counted as disappeared (N = 115) were included, although the effect of model colour morph was qualitatively identical if models disappearing without the presence of bird tracks were considered attacked and included in the analysis (N = 189). For the second analysis, we included only lizard models that had been attacked by birds on a single body part (N = 38), because when there were marks on both parts, it was impossible to know which part was attacked first. To test for the possible effect of model colour morph on the latency to attack, a Generalized Linear Model (GLM) with Poisson error distribution was used. Only models attacked by birds (N = 63) were included in this analysis. All analyses in the field study simultaneously tested for differences between study areas (Chapinería and Aranjuez) and types of models (plaster and plasticine), and also checked for any interaction between these two factors and model colour morph. Year was excluded from our statistical analyses because it was strongly associated with the type of model in all data subsets used here (Fisher’s exact test; P < 0.001 in the three tests), but when year was included instead of type of model, results were qualitatively identical (see “Results” section).
In the captivity study, GLZs with binomial error distribution were used to test for the possible effects of model colour morph (red or striped tail) on (1) the probability of being attacked, and (2) the probability of being attacked on each body part (tail or rest-of-body). All models (N = 31) were used in the first analysis, whereas in the second, only lizard models that had been attacked on a single body part were included (N = 16). To test for the possible effect of model colour morph on the latency to attack, a GLM with Poisson error distribution was used. Only attacked models (N = 22) were included in this analysis. All analyses in the captivity study simultaneously tested for differences in years (2011, 2012 and 2013) and checked the interaction between model colour morph and year.
In both studies, a backward stepwise procedure was used in all analyses, retaining only terms associated with P values below 0.10 in the final statistical models. If Akaike’s information criterion (Burnham and Anderson 2002) was used to select final models, results were qualitatively identical in all analyses (results not shown for brevity). All statistical analyses were carried out using R (R Development Core Team 2013) and lme4 package (Bates et al. 2012). All tests were two-tailed and the significance level was set to 0.05.
Results
Field study
Excluding models that were counted as disappeared, we recorded data for 115 models, 31 plaster (14 with red tails and 17 with striped tails) and 84 plasticine (48 with red tails and 36 with striped tails). A total of 63 models were attacked by birds. Of the attacked models, 38 were attacked on one body part, whereas the rest (25 models) were attacked in both body parts (tail and rest-of-body). Taking into account the total number of models placed in the field (not including models replacing those attacked by non-avian predators), 39.16 % of them were counted as disappeared, 33.33 % were attacked by birds, and 27.51 % were not attacked by birds.
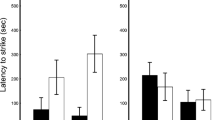
The probability of being attacked by birds was not significantly different for red and striped-tailed lizard models (Likelihood test; χ2 = 0.15, N = 115, P = 0.697; Table 2), types of models (plaster or plasticine), or study areas (Chapinería or Aranjuez), as none of these factors was retained in the final model. However, red-tailed models were attacked sooner than striped-tailed, and plasticine models were attacked sooner than plaster (Table 1; Fig. 3a). The interaction between model colour morph and type of model was statistically significant (Table 1), implying that the difference between colour morphs depended on the type of model. In separate analyses of plaster and plasticine models, we found that red-tailed plaster models were attacked sooner than striped-tailed (Likelihood test; χ2 = 36.51, N = 19, P < 0.001), but for plasticine models, the difference was not statistically significant (Likelihood test; χ2 = 2.44, N = 44, P = 0.118; Fig. 3a). When year was included in this analysis instead of type of model, red-tailed models were also attacked sooner than striped-tailed (Likelihood test; χ2 = 24.82, N = 63, P < 0.001). Models were attacked sooner in 2011 than in 2010 (Likelihood test; χ2 = 150.78, N = 63, P < 0.001), and the statistically significant interaction between model colour morph and year (Likelihood test; χ2 = 8.81, N = 63, P = 0.003) implied that the difference between colour morphs depended on the year. We analysed data for both years separately and found that red-tailed models were attacked sooner than striped-tailed in 2010 (Likelihood test; χ2 = 31.75, N = 16, P < 0.001), but the difference was not statistically significant in 2011 (Likelihood test; χ2 = 1.88, N = 47, P = 0.170).
The proportion of attacks to each body part (tail or rest-of-body) was different for red and striped-tailed models, as the probability of being attacked on the tail was higher for red-tailed models (Likelihood test; χ2 = 3.91, N = 38, P = 0.048; Table 2). Neither type of model nor study area had a significant effect on the part of the body that was attacked, as none of these factors or their interactions was retained in the final model.
Captivity study
A total of 31 models were presented to the birds, and 22 of them were attacked. Of the attacked models, 16 were attacked on one body part, whereas the rest (6 models) were attacked on both body parts. The probability of being attacked by a captive F. tinnunculus was not significantly different for red or striped-tailed lizard models (Likelihood test; χ2 = 0.08, N = 31, P = 0.779; Table 2), and did not differ significantly among years either, as no factor was retained in the final model. Nor did we find significant differences in the latency to attack between model colour morphs (Likelihood test; χ2 = 0.17, N = 22, P = 0.680; Fig. 3b), or among years, as no factor was retained in the final model. The part of the body (tail or rest-of-body) that was attacked in red and striped-tailed lizard models did not differ significantly either (Likelihood test; χ2 = 2.08, N = 16, P = 0.150; Table 2).
Discussion
Our first goal was to find out whether red tails increase lizard detection rate by avian predators. The field study showed that red-tailed models were attacked sooner than striped-tailed, suggesting that the red colouring did indeed make models more easily detected by avian predators. Previous studies checking the decoy hypothesis with green (Castilla et al. 1999) and blue (Watson et al. 2012) tails concluded that they were not significantly more detected by predators than brown or black, respectively. However, recent studies found that lizard models with blue tails were attacked sooner and more often than models with brown tails (Bateman et al. 2014). Blue and green are relatively common colours among lizards (Arnold 1984; Pianka and Vitt 2006), possibly because they are inconspicuous at long distances against a background of vegetation, although they might serve as a lure at closer distances (Arnold 1984; Bateman et al. 2014). Red, however, is very conspicuous against a background of vegetation (e.g., Schaefer et al. 2006), which may explain why red-tailed models in our study were more easily detected, although other mechanisms such as a possible pre-existing sensory bias in avian predators towards red objects (e.g., Møller and Erritzøe 2010) could also explain the results. Determining the precise mechanism underlying the behaviour observed (avian predators attacking red-tailed models sooner) is beyond the scope of this study, but our results suggest that red colouration attracts bird attention and probably increase predatory pressure on red-tailed lizards. A significant interaction between colour morph and type of model was also found, but, unfortunately, our experimental design does not allow us to elucidate whether latency to attack differed between types of models or between years.
In free-ranging lizards, dorsal colour pattern is very important for crypsis (Calsbeek and Cox 2012; Lancaster et al. 2007), and we assumed that striped-tailed models matched background colour better than red-tailed. Therefore, a higher rate of attacks on red-tailed models was expected. Our results did not confirm this expectation, as models of both colour morphs were subject to a similar number of attacks in the field study. The fact that red-tailed models were attacked sooner but not more often than striped-tailed is counterintuitive, but the long periods between consecutive model inspections (3–5 days) might have contributed to the mismatch between timing and number of attacks. The similar attack rate for both colour morphs suggests that both were recognized as prey by avian predators. The captivity study showed similar attack rates and latency to attack for lizard models of both colour morphs, but this was expected due to the experimental setup (models of both colour morphs were probably very easily detected in relatively small cages with bare concrete floor).
Our second aim was to check whether red tails are effective decoys for diverting bird attacks from vital body parts. The field study corroborated this hypothesis, as red-tailed models received a larger proportion of attacks to the tail than striped-tailed models. Birds usually attack the head and body of their prey (Greene 1988; Shepard 2007; Vervust et al. 2011), so red tails seem to cause a change in avian predatory behaviour, thus increasing lizard probability of surviving an attack, particularly when the tail can easily be detached from the rest of the body (even though loss of the tail may imply later costs; see the Introduction). The benefits of having red tails might then outweigh the costs of increased detection by predators (Cooper and Vitt 1991), what might have driven the evolution and maintenance of this trait in A. erythrurus. In the captivity study, a similar pattern of a larger proportion of attacks on the tail was observed for red-tailed models, but the difference between both colour morphs was not statistically significant, maybe in part because of the small sample size.
The present study suggests that red tails in lizards are effective decoys for avian predators, a function that has also been suggested for green and blue tails (Bateman et al. 2014; Castilla et al. 1999; Watson et al. 2012). The tail colour developed in different lizard species does not seem to be strongly phylogenetically constrained, as the same colour may be present in species of different families (e.g., red tails are found in Morethia ruficauda, Scincidae family, Vanzosaura rubricauda, Gymnophthalmidae family, or A. erythrurus, Lacertidae family), whereas different colours may be present in species of the same genus (e.g., A. erythrurus and A. schreiberi juveniles have red tails, and A. boskianus and A. beershebensis juveniles have blue tails). Therefore, the specific tail colouration of each species might be more influenced by environmental variables [e.g., contrast with background (Håstad et al. 2005; Rosenblum 2006)], other lizard traits [e.g., contrast with the rest of the body (Arnold 1984)], or even the predator’s visual characteristics [e.g., different avian taxa can discriminate wavelengths at slightly different spectral ranges (Håstad et al. 2005)].
If conspicuously coloured lizard tails are an effective decoy for avian predators, we wonder why some lizard species lose this anti-predatory mechanism during the ontogenetic process (Booth 1990). A. erythrurus lizards, for example, lose the red colouration when they reach sexual maturity in the case of males, or when they become gravid in the case of females (Cuervo and Belliure 2013; Seva Román 1982). This ontogenetic colour change might be due to differences in behaviour between age classes in A. erythrurus (B. Fresnillo, J. Belliure, and J. J. Cuervo, unpublished data) and other lizard species (Hawlena 2009; Hawlena et al. 2006), as hatchlings and juveniles are usually more active than adults and, therefore, more vulnerable to predators (Jackson et al. 1976). For age classes with high activity rate, the development of an anti-predatory mechanism such as a lure to divert attacks to expendable body parts may be advantageous. However, adults might increase their survival rates by developing more cryptic colouration (Arnold 1984; Vitt and Cooper 1986).
In conclusion, this study suggests that lizards with conspicuous red tails are more easily detected by avian predators, but also that red tails effectively divert avian predator attacks from vital body parts to the expendable tail. If the benefits of diverting attacks to the tail exceed the costs of being more detectable by predators, red tails have a positive net effect on survival and, thus, on fitness, then favouring the evolution and maintenance of this conspicuous colouration. Therefore, our results support the decoy or deflection hypothesis as an explanation for conspicuous red colouration in juvenile lizards.
References
Arnold EN (1984) Evolutionary aspects of tail shedding in lizards and their relatives. J Nat Hist 18:127–169
Arnold EN (1988) Caudal autotomy as a defense. In: Gans C, Huey RB (eds) Biology of the reptilia, vol 16., Ecology B: defense and life historyAlan R. Liss, New York, pp 235–274
Bateman PW, Fleming PA (2009) To cut a long tail short: a review of lizard caudal autotomy studies carried out over the last 20 years. J Zool 277:1–14
Bateman PW, Fleming PA, Rolek B (2014) Bite me: blue tails as a ‘risky-decoy’ defense tactic for lizards. Curr Zool 60:333–337
Bates D, Maechler M, Bolker B (2012) lme4: linear mixed-effects models using S4 classes, R package version 0.999999-0 edn. http://CRAN.R-project.org/package=lme4
Baylis SM, Cassey P, Hauber ME (2012) Capsaicin as a deterrent against introduced mammalian nest predators. Wilson J Ornithol 124:518–524
Belliure J (2006) Lagartija colirroja—Acanthodactylus erythrurus. In: Carrascal LM, Salvador A (eds) Enciclopedia Virtual de los Vertebrados Españoles. Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org/
BirdLife International (2014) Species factsheet: Falco tinnunculus. http://www.birdlife.org. Accessed Jan 2014
Booth CL (1990) Evolutionary significance of ontogenic colour-cange in animals. Biol J Linn Soc 40:125–163
Bowmaker JK (2008) Evolution of vertebrate visual pigments. Vis Res 48:2022–2041
Brodie ED III (1993) Differential avoidance of coral snake banded patterns by free-ranging avian predators in Costa Rica. Evolution 47:227–235
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Calsbeek R, Cox RM (2012) An experimental test of the role of predators in the maintenance of a genetically based polymorphism. J Evol Biol 25:2091–2101
Carretero MA, Llorente GA (1993) Morphometry in a community of Mediterranean lacertid lizards, and its ecological relationships. Hist Anim 2:77–99
Carrillo J, González-Dávila E (2009) Latitudinal variation in breeding parameters of the common kestrel Falco tinnunculus. Ardeola 56:215–228
Carrillo J, Hernández EC, Nogales M, Delgado G, García R, Ramos T (1994) Geographic variation in the spring diet of Falco tinnunculus L. on the islands of Fuerteventura and El Hierro (Canary Islands). Bonn Zool Beitr 45:39–48
Castilla AM, Labra A (1998) Predation and spatial distribution of the lizard Podarcis hispanica atrata: an experimental approach. Acta Oecol 19:107–114
Castilla AM, Barbadillo LJ, Bauwens D (1992) Annual variation in reproductive traits in the lizard Acanthodactylus erythrurus. Can J Zool 70:395–402
Castilla AM, Gosá A, Galán P, Pérez-Mellado V (1999) Green tails in lizards of the genus Podarcis: do they influence the intensity of predation? Herpetologica 55:530–537
Clark DRJ, Hall RJ (1970) Function of the blue tail coloration of the five-lined skink Eumeces fasciatus. Herpetologica 26:271–274
Cooper WE Jr, Vitt LJ (1985) Blue tails and autotomy: enhancement of predation avoidance in juvenile skinks. Z Tierpsychol 70:265–276
Cooper WE Jr, Vitt LJ (1991) Influence of detectability and ability to escape on natural selection of conspicuous autotomous defenses. Can J Zool 69:757–764
Cuadrado M, Martín J, López P (2001) Camouflage and escape decisions in the common chameleon Chamaeleo chamaeleon. Biol J Linn Soc 72:547–554
Cuervo JJ, Belliure J (2013) Exploring the function of red colouration in female spiny-footed lizards (Acanthodactylus erythrurus): patterns of seasonal colour change. Amphib-Reptil 34:525–538
Darwin C (1871) The descent of man, and selection in relation to sex. D. Appleton and Company, New York
Endler JA (1980) Natural selection on color patterns in Poecilia reticulata. Evolution 34:76–91
Fox SF, McCoy JK (2000) The effects of tail loss on survival, growth, reproduction, and sex ratio of offspring in the lizard Uta stansburiana in the field. Oecologia 122:327–334
Greene H (1988) Antipredator mechanisms in reptiles. In: Gans C, Huey RB (eds) Biology of the reptilia, vol 16., Ecology B: defense and life historyAlan R. Liss, New York, pp 1–152
Haskell DG (1996) Do bright colors at nests incur a cost due to predation? Evol Ecol 10:285–288
Håstad O, Victorsson J, Ödeen A (2005) Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc Natl Acad Sci USA 102:6391–6394
Hawlena D (2009) Colorful tails fade when lizards adopt less risky behaviors. Behav Ecol Sociobiol 64:205–213
Hawlena D, Boochnik R, Abramsky Z, Bouskila A (2006) Blue tail and striped body: why do lizards change their infant costume when growing up? Behav Ecol 17:889–896
Jackson JF, Ingram W III, Campbell HW (1976) The dorsal pigmentation pattern of snakes as an antipredator strategy: a multivariate approach. Am Nat 110:1029–1053
Johnson J, Burt DB, DeWitt TJ (2008) Form, function, and fitness: pathways to survival. Evolution 62:1243–1251
Kemp DJ, Rutowski RL (2011) The role of coloration in mate choice and sexual interactions in butterflies. Adv Study Behav 43:55–92
Kodandaramaiah U, Lindenfors P, Tullberg BS (2013) Deflective and intimidating eyespots: a comparative study of eyespot size and position in butterflies. Ecol Evol 3:4518–4524
Kraus F, Allison A (2009) A remarkable ontogenetic change in color pattern in a new species of Oreophryne (Anura: Microhylidae) from Papua New Guinea. Copeia 2009:690–697
Lancaster LT, McAdam AG, Wingfield JC, Sinervo BR (2007) Adaptive social and maternal induction of antipredator dorsal patterns in a lizard with alternative social strategies. Ecol Lett 10:798–808
Martín J, López P (1990) Amphibians and reptiles as prey of birds in Southwestern Europe. Smithson Herpetol Inf Serv 82:1–43
Mochida K (2011) Combination of local selection pressures drives diversity in aposematic signals. Evol Ecol 25:1017–1028
Møller AP, Erritzøe J (2010) Why birds eat colourful grit: colour preferences revealed by the colour of gizzard stones. J Evol Biol 23:509–517
Ochi H, Awata S (2009) Resembling the juvenile colour of host cichlid facilitates access of the guest cichlid to host territory. Behaviour 146:741–756
Pianka ER, Vitt LJ (2006) Lizards: windows to the evolution of diversity. University of California Press, Berkeley
Pollo CJ, Pérez-Mellado V (1990) Biología reproductora de tres especies mediterráneas de Lacertidae. Mediterránea, Serie de Estudios Biológicos 12:149–160
R Development Core Team (2013) R: a language and environment for statistical computing. Version 2.15.3. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rosenblum EB (2006) Convergent evolution and divergent selection: lizards at the White Sands ecotone. Am Nat 167:1–15
Ruiz-Rodríguez M, Avilés JM, Cuervo JJ, Parejo D, Ruano F, Zamora-Muñoz C, Sergio F, López-Jiménez L, Tanferna A, Martín-Vivaldi M (2013) Does avian conspicuous colouration increase or reduce predation risk? Oecologia 173:83–93
Schaefer HM, Levey DJ, Schaefer V, Avery ML (2006) The role of chromatic and achromatic signals for fruit detection by birds. Behav Ecol 17:784–789
Seva Román E (1982) Taxocenosis de lacértidos en un arenal costero alicantino. Ph.D. Thesis, Universidad de Alicante, Alicante
Shepard DB (2007) Habitat but not body shape affects predator attack frequency on lizard models in the Brazilian Cerrado. Herpetologica 63:193–202
Stuart-Fox DM, Moussalli A, Marshall NJ, Owens IPF (2003) Conspicuous males suffer higher predation risk: visual modelling and experimental evidence from lizards. Anim Behav 66:541–550
Telemeco RS, Baird TA, Shine R (2011) Tail waving in a lizard (Bassiana duperreyi) functions to deflect attacks rather than as a pursuit-deterrent signal. Anim Behav 82:369–375
Valkonen J, Niskanen M, Björklund M, Mappes J (2011) Disruption or aposematism? Significance of dorsal zigzag pattern of European vipers. Evol Ecol 25:1047–1063
VanderWerf EA, Freed LA (2003) Elepaio subadult plumages reduce aggression through graded status-signaling, not mimicry. J Field Ornithol 74:406–415
Vanzyl AJ (1994) Comparison of the diet of the common kestrel Falco tinnunculus in South Africa and Europe. Bird Study 41:124–130
Vásquez RA, Ebensperger LA, Bozinovic F (2002) The influence of habitat on travel speed, intermittent locomotion, and vigilance in a diurnal rodent. Behav Ecol 13:182–187
Vervust B, Grbac I, Van Damme R (2007) Differences in morphology, performance and behaviour between recently diverged populations of Podarcis sicula mirror differences in predation pressure. Oikos 116:1343–1352
Vervust B, Van Loy H, Van Damme R (2011) Seeing through the lizard’s trick: do avian predators avoid autotomous tails? Cent Eur J Biol 6:293–299
Vitt LJ, Cooper WE Jr (1986) Tail loss, tail color, and predator escape in Eumeces (Lacertilia: Scincidae): age-specific differences in costs and benefits. Can J Zool 64:583–592
Watson CM, Roelke CE, Pasichnyk PN, Cox CL (2012) The fitness consequences of the autotomous blue tail in lizards: an empirical test of predator response using clay models. Zoology 115:339–344
Wilson BS (1992) Tail injuries increase the risk of mortality in free-living lizards (Uta stansburiana). Oecologia 92:145–152
Acknowledgments
We thank C. Esteban, J. Calatayud, M. Cruz, M. Almarcha and C. Zaragoza for their help in the field study, and the staff of the Santa Faz Wildlife Rehabilitation Centre (Alicante, Spain) for their help in the captivity study. Deborah Fuldauer revised English language usage. These studies were funded by the Spanish Ministry of Education and Science and the European Regional Development Fund (Grant CGL2008-00137/BOS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fresnillo, B., Belliure, J. & Cuervo, J.J. Red tails are effective decoys for avian predators. Evol Ecol 29, 123–135 (2015). https://doi.org/10.1007/s10682-014-9739-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-014-9739-2