Abstract
Reciprocal F1, F2 and F3 populations of lentil (Lens culinaris Medik.) were developed by crossing between lines, with a chloroplastic psbA gene mutation conferring tolerance to the photosystem II inhibitor herbicide metribuzin, and sensitive parent PBA Flash, to understand the genetic control of the herbicide tolerance. Additionally, reciprocal BC1F2 populations were developed to identify any fitness penalty associated with the metribuzin tolerance. Phenotyping and genotyping results of the F1, F2, and F3 populations identified a predominantly maternal inheritance pattern, but with a level of paternal leakage. Paternal leakage occurred in approximately 20% of F1 phenotypes, when including lines showing heteroplasmy (the existence of maternal and paternal chloroplasts within an individual). Field experiments with BC1F2 and BC1F3 lines confirmed this biparental inheritance pattern. Grain yield was reduced by 20–40% in metribuzin tolerant backcrossed lines compared with sensitive lines. Net assimilation rate at the onset of anthesis and plant dry weight at mid anthesis and maturity were also reduced in the tolerant lines suggesting reduced photosynthetic efficiency associated with the metribuzin tolerance results in lower dry weight and grain yield in the tolerant lines. The mode of inheritance and associated yield penalty of the tolerance trait will complicate its introgression in lentil breeding programs. However, the high level of tolerance and unique weed control benefits of this trait suggest that this form of metribuzin tolerance in lentil, which is similar to triazine tolerant canola (Brassica napus L.), could be adopted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lentil (Lens culinaris) is an important pulse crop worldwide, providing an excellent food source high in protein and amino acids but relatively free of anti-nutritional factors (Bhatty 1988). World lentil production increased from 2.8 Mt in 1997 to more than 7.5 Mt in 2017 with over 55% from the developed countries of Canada, Australia and the United States (FAOSTAT 2019). Weed competition is a major limitation to lentil production with grain yield (GY) losses as high as 84% reported (Mohamed et al. 1997; McDonald et al. 2007). In countries where large scale mechanised production of lentil occurs, the main means of weed control is by application of herbicides (Yenish et al. 2009). However, due to a lack of safe and effective herbicide options, variable levels of weed control and yield loss from crop phytotoxicity often occur (Brand et al. 2007). Improvement in tolerance to existing herbicides and/or tolerance to novel herbicides is required in lentil to improve weed control in these systems.
Photosystem II (PSII) inhibitor herbicides such as the triazines, triazinones and uracils, are commonly used for broadleaf weed control in Australian lentil production due to their broad-spectrum weed activity (Preston 2002). However, their use in lentil is limited to a pre-emergent application due to a lack of selectivity between the crop and weeds (White 2015). This lack of selectivity can also limit the suitability and effectiveness of these herbicides due to numerous application restrictions dependent on soil and weather conditions around the time of application. Lentil cultivars with improved tolerance to imidazolinone (IMI: acetohydroxyacid synthase [AHAS] inhibitors) herbicides are commercially available in Australia (Materne et al. 2011). However, PSII inhibitors are still widely used due to a broader range of broadleaf weeds controlled, and the increasing spread of IMI resistant weeds, caused by an over reliance on IMI herbicides (Boutsalis et al. 2016). Lentil lines with tolerance to the PSII inhibitor herbicide metribuzin have recently been developed (McMurray et al. 2019a). Metribuzin tolerance is attributed to an Ala251Thr substitution in the D1 protein encoded by the chloroplast psbA gene and is being incorporated into Australian lentil breeding programs (McMurray et al. 2019b). However, the genetic factors controlling this tolerance have not been reported.
Although the development of other crop species with target site tolerance to metribuzin are not reported, there are numerous examples of weeds with evolved target site resistance, including annual bluegrass (Poa annua L.) (Mengistu et al. 2000), kochia (Kochia scoparia L.) (Mengistu et al. 2005), common groundsel (Senecio vulgaris L.) (Park and Mallory-Smith 2006), shepherd’s purse (Capsella bursa-pastoris L.) (Perez-Jones et al. 2009) and lamb’s quarters (Chenopodium album L.) (Thiel and Varrelmann 2013). Furthermore, triazine tolerant (TT) canola (Brassica napus L.) with tolerance to the PSII inhibitor herbicide atrazine was developed through a back-crossing program with canola and a triazine-tolerant biotype of birdsrape mustard (B. rapa L.) (Beversdorf and Kott 1987). A Ser264Gly amino acid substitution in the chloroplast D1 protein of B. rapa was identified as being responsible for the triazine tolerance (Reith and Straus 1987) and is inherited maternally.
PSII inhibiting herbicides disrupt photosynthesis by displacing the native plastoquinone at the QB binding site of the D1 protein in the PSII complex of the chloroplast (Fuerst and Norman 1991). In reported weed species and TT canola, the Ser264Gly mutation removes a hydrogen bond that prevents triazine binding. It also reduces plastoquinone binding, thereby compromising photosynthesis and leading to a fitness cost in tolerant plants (Powles and Yu 2010). Less is known about fitness costs associated with other psbA mutations in higher plants. Analysis of mutants of Chlamydomonas reinhardtii found that the substitutions Ala251Val, Gly256Asp and Ser264Gly/Ala greatly slowed electron transfer from QA to QB, but substitutions at Val219Ile, Phe255Tyr and Leu275Phe had no effect on electron transfer rates (Rochaix and Erikson 1988). The Ala251Thr substitution attributed to the metribuzin tolerance in lentil is unique in higher plants except for its identification as part of double or triple mutations in Chenopodium rubrum cell cultures (Schwenger-Erger et al. 1993). The determination of the presence and extent of any fitness penalty associated with the Ala251Thr induced metribuzin tolerance is required by lentil plant breeders incorporating this trait into their programs.
Therefore, the aims of this research in lentil were to: (1) identify the genetic controls of metribuzin tolerance, and (2) confirm the presence or absence of a fitness cost associated with the chloroplastic psbA gene conferring metribuzin tolerance.
Material and methods
Plant materials
The genetic controls of metribuzin tolerance and any associated fitness cost were investigated in two mutagenized lentil genotypes (PBA Flash-EMS10-11SK-12PAHM009 (M009)) and (PBA Flash-EMS10-11SVHM043 (M043)), both with an Ala251Thr psbA mutation (McMurray et al. 2019b), in comparison to the parent line PBA Flash. Two separate streams of plant material were created. The first was developed at the University of Saskatchewan, Saskatoon, SK, Canada and the second at the Waite Research Precinct, Urrbrae, SA, Australia. The latter was created primarily to develop reciprocal BC1F2 derived lines for the fitness cost experiments; however, it was also used to confirm the findings from the experiments conducted in Canada.
The Canadian materials used seed sourced from a M4 field multiplication near Riverton, SA, during winter, 2013. The two mutant lines were crossed with PBA Flash, including reciprocal crosses, to produce F1 hybrid seed for phenotyping metribuzin response and for producing F2 seed. All seeds were produced in a controlled environment room (CER) and grown in potting mix comprised of equal parts of Sunshine Mix #3 and #4 (Sun Grow, Seba Beach, AB, Canada).
In Australia, parental seeds were obtained from plants recovered from an M4 metribuzin progeny screen (McMurray et al. 2019a), and all generations grown in a potting mix of 90% composted pine bark and 10% sand. Parental, F1 and BC1F1 plants were grown in a CER both for phenotyping metribuzin response and producing F2 seed. Ten random F2 seeds from the two most productive F1 plants within each cross, including reciprocals, were sown separately in an outdoor shade house facility for F3 seed production. All F2 plants were harvested and stored separately, and all BC1F3 seed was obtained from bulk harvested plot-rows of field grown BC1F2 plants in the fitness cost experiment.
Phenotyping of F1 and F2 plants in Canada
All plants were grown in pots (8 cm by 10 cm by 8 cm) filled with a custom soil mix of 60% Sunshine Mix #3 and 40% coarse sand in a CER under conditions of 14/10 h day/night, 21/15 °C day/night temperatures, light intensity of 540 μmol m−2 s−1 and relative humidity maintained at 40%. All seed coats were scarified with a scalpel and four seeds of each parent, F1 or F2 line were sown per pot.
The F1 experiment was arranged as a square array consisting of seven columns (one pot per column) by seven rows. Within the array three pots containing a total of 12 F1 seeds each of M043 × PBA Flash, reciprocals (PBA Flash × M043), M009 × PBA Flash and reciprocals (PBA Flash × M009) were randomly arranged along with nine or ten pots of each parent.
The F2 experiment was arranged as two rectangular arrays of pots, one array for all crosses involving M009 and the other for all crosses involving M043. The arrays consisted of seven columns as for the F1 experiment, by 25 rows (M009) and 24 rows (M043). Each pot within each row was sown to seed from the same individual F1 plant (4 seeds per pot, 28 seeds in total), except for four rows which were sown equally to each parent. In the M009 array, 11 individual M009 × PBA Flash and 10 individual PBA Flash × M009 F1 hybrid plants were represented. The two rows of each parent were randomly arranged within the array. The M043 array was sown as per the first array and contained F2 seed from 10 M043 × PBA Flash F1 plants and 10 reciprocals along with two rows of each parent. Pots received 100 ml of water after sowing and were then watered twice weekly with 100 ml of a nutrient solution consisting of Ca(NO3)2 (205 mg l−1), KNO3 (126 mg l−1), MgSO4·7H2O (123 mg l−1), KH2PO4 (34 mg l−1), Fe EDTA (10 mg l−1), ZnSO4·7H2O (0.055 mg l−1), CuSO4·5H20 (0.02 mg l−1), H3BO4 (0.715 mg l−1), MnCl2·4H2O (0.453 mg l−1) and Na2MoO4·2H2O (0.006 mg l−1). Metribuzin (Sencor®, 750 g a.i. kg−1, Bayer Crop Science, Calgary, AB, Canada) was applied using a laboratory track applicator with a single even flat spray nozzle 8001 EVS (Lechler, St Charles, IL, USA) delivering 108 L ha−1 at 240 kPa at a rate of 750 g a.i. ha−1, at 12 days after sowing (DAS). Plants were watered immediately before herbicide treatment, then 24 h post-treatment with water (50 ml), and thereafter twice weekly with 100 ml of nutrient solution. All plants were assessed individually for growth stage immediately before spraying, which occurred when the majority of plants were between the fourth and sixth-node above ground stage of development (V6–V8; Erskine et al. 1990). Seeds that had failed to emerge and seedlings with unopened cotyledons at the time of spraying were excluded from the statistical calculations. At 14 days after treatment (DAT) plants were assessed for herbicide injury. Plant injury was scored as the percentage of necrotic plant tissue.
Phenotyping of F1, F2 and F3 plants in Australia
The Australian phenotyping experiment followed the Canadian experiment, but with the addition of F3 seed and the following modifications. Pots were filled with a potting mix of 90% composted pine bark and 10% sand. Daylength in the CER was as for Canada, but other conditions were 20/10 °C day/night temperatures, light intensity of 1100 μmol m2 s−1 and relative humidity maintained at 90%. Each rectangular array of pots consisted of eight columns by variable numbers of rows per F1, F2 and F3 groupings.
The F1 array consisted of three rows containing a total of 10 F1 seeds (three pots) from M043 × PBA Flash and 17 reciprocals (five pots), and 16 seeds (four pots) from M009 × PBA Flash and 24 (six pots) reciprocals, randomly arranged with each parent. The F2 arrays were grouped by the two mutant parents and each array consisted of 36 rows. Each parent array contained F2 seed from six separate F1 plants, six separate reciprocal F1 plants and each parent. Seed of each F2 and reciprocal F2 line was sown into three consecutive rows containing 22 pots (88 seeds) along with two pots, each containing two seeds of each relevant parent (eight seeds). The F3 arrays were grouped by the two mutant parents and each parent array consisted of eight rows. Within each array two consecutive rows contained seed from each F3 family or reciprocal F3 family, consisting of three pots (12 seeds) from each of five separate F2 plants derived from the same F1 (totaling 15 pots) plus one pot containing two seeds of each relevant parent. Metribuzin (Mentor, 750 g a.i. kg−1, Farm Oz Pty Ltd, St Leonards, NSW, Australia) was applied to all plants 13 DAS using a laboratory track applicator with a twin nozzle (110° flat fan) moving boom situated 40 cm above the top of the plants, to generate an even measured spray distribution between the nozzles, and delivering 103 L ha−1 at 1 m s−1 and 250 kPa.
Genotyping
DNA was extracted from leaf tissue of all plants in the Canadian phenotyping experiment and from selected plants in the Australia phenotyping experiment. In Canada, a 2 mm2 piece of plant tissue was harvested with tweezers from the second youngest emerged leaf. Each leaf tissue sample was placed in an individual well of a PCR micro-plate with 40 µl of 0.25 M NaOH before shaking for 30 s followed by heating at 95 °C for 2 min. Samples were cooled at room temperature before the addition of 60 µl of 0.5 M Tris–HCl (pH 8.0), then shaken for 30 s, heated at 95 °C for 2 min and stored in a refrigerator. DNA was diluted ten-fold in sterile water and 2.0 µl of the resulting dilution used in a 10 µl KASP™ (LGC Biosearch Technologies, Middlesex, UK) reaction. The assay was completed using the forward primer allele 1 (sensitive) 5’—AAGGTGACCAAGTTCATGCTAGGAAGAAACCTACAATATTGTAGCTG—3’, forward primer allele 2 (tolerant) 5’—GAAGGTCGGAGTCAACGGATTGAGGAAGAAACC TACAATATTGTAGCTA—3’ and reverse primer 5’—GCGAGAATTGTTGAAACTAGCATAT TGGAA—3’ designed using LGC PrimerPicker software. The reaction was run on a StepOnePlus™ Real-Time PCR system with the following program: step 1, 21 °C for 2 min (fluorescence read); step 2, 95 °C for 15 min (hot start); step 3, 94 °C for 20 s (denaturing); step 4, 65 °C for 1 min (annealing and extension), repeat step 3–4, 10 × reducing the annealing and extension temperature 0.8° each cycle; step 5, 94 °C for 20 s (denaturing); step 6, 57 °C for 1 min (annealing and extension), repeat step 5–6, 48 × taking a fluorescence reading at 21 °C for 2 min after cycles 32, 36, 40, 44 and 48. Multiple end-point fluorescence readings were taken towards the end of the PCR to identify the optimal time-point for the segregation of genotypes.
A similar method was used in Australia with the following modifications. Fresh leaf tissue was harvested for genetic analysis from 87 parental, all F1 plants, 368 selected F2 and 237 selected F3 lines with the latter two providing a representation of all F2 and F3 crosses and reciprocals. Lyophilized leaf tissue was ground in 1.1 mL tubes with stainless steel ball bearings in a MM-300 Retsch-mill grinder for 1 min at 30 strokes s−1. Genomic DNA was extracted using a modified CTAB protocol (Doyle and Doyle 1987). Reaction was run using the LGC Genomics SNPline™ system with the following program: step 1, 94 °C for 15 min; step 2, 94 °C for 20 s; step 3, 61 °C (− 0.6 °C per cycle) for 60 s, repeat step 2–3, 10x; step 4, 94 °C for 20 s; step 5, 55 °C for 60 s, repeat step 4–5, 26x; step 6, fluorescence read at 37 °C; step 7, 94 °C for 20 s; step 8, 55 °C for 60 s; step 9, fluorescence read at 37 °C, repeat step 7–9, 3x. Fluorescence readings were taken using a BMG PHERAstar spectrophotometer and SNP results were analysed using Kraken™ software (LGC Biosearch Technologies, Middlesex, UK).
BC1F2 and BC1F3 fitness cost experiments
The BC1F2 and bulked BC1F3 populations were grown at field sites of Riverton and Turretfield, SA, over two successive years to ascertain if a fitness cost was associated with the chloroplastic psbA gene mutation that encodes the Ala251Thr substitution in lentil mutants M009 and M043. The sites represented favourable lentil growing areas in southern Australia with mean annual rainfall totals of 528 and 464 mm at Riverton and Turretfield, respectively. The soil type at Riverton was a calcareous clay loam over light clay with a pH (H20) ranging from 6.7 on the surface to 8.9 at a depth of 60 cm, and at Turretfield, an alkaline, calcareous clay loam with a pH (H20) ranging from 7.5 to 8.2. Annual rainfall totals were 439 and 361 mm in 2015 and 825 and 633 mm in 2016 at Riverton and Turretfield, respectively.
In 2015 three pairs of BC1F2s from the same F1 parents, but backcrossed to a different PBA Flash plant, derived from PBA Flash × (M009 × PBA Flash) and PBA Flash × (M043 × PBA Flash) and their reciprocals (Table 1), and all parents were sown in 1 m paired row plots in a randomised complete block design with three replicates. All lines were included once within each block except for PBA Flash (four times, representing each different PBA Flash plant used in the backcross) and the mutant parents (twice). Seed rows were formed by banding basal fertilizer at a depth of 8 cm and rate of 7 kg N ha−1, 15 kg P ha−1 and 1.8 kg Zn ha−1 using a small plot cone seeder with six narrow tynes on 0.225 m row spacing. An unsown row separated every paired row plot. All seed coats were scarified pre-sowing and 40 seeds were sown at 2.5 cm spacing into two adjacent 1 m rows at a depth of 5 cm on July 8 and 9 at Turretfield and Riverton, respectively. These sowing dates were approximately 4 weeks later than the optimal sowing time in these environments due to delayed seed availability from indoor seed multiplication. General insecticide and fungicide application were as best agronomic practice for lentil. Both sites were hand weeded to remove weed competition and the Turretfield site received two supplementary irrigation events of 56 mm in spring. Net assimilation rate (NAR) in g of whole plant dry weight (DW) per square meter of leaf surface area (LA) per day (d) was calculated as per Williams (1946):
Three plants of uniform size were sampled from each paired row-plot initially in mid-September (t1), for calculation of DW1 and LA1, and then 16 (Turretfield) and 17 (Riverton) days later (t2), for calculation of DW2 and LA2, with the second sampling occurring at the onset of anthesis in PBA Flash. Plant samples from each plot were bulked in paper bags and stored in a cool dark room for processing. Random leaflets from all parts of each plant were removed with tweezers and placed on white paper (210 mm by 297 mm) on a flatbed scanner for leaf area measurement. The remaining leaflets were removed from plants and placed in a second envelope and all remaining plant parts were placed in a third envelope. All envelopes were oven-dried at 80 °C for 48 h before weighing. The total leaf surface area for each sample was estimated from the total plant leaf weight using the weight of the known measured leaf area. At maturity all paired row plots were hand harvested separately into bags, dried and threshed for DW and GY determination. All grain samples were bagged separately and kept for use as seed in the 2016 BC1F3 experiments.
Methods used in the BC1F3 field experiments in 2016 were as for the BC1F2 experiments with some modifications. The experimental design consisted of four replicates at each site and all seed was sourced from the 2015 experiments. The seed was sown at a density of 120 seeds m−2 in 1.35 m by 5 m plots. Experiments were sown with a small plot cone seeder into retained straw residue at a depth of 5 cm at Riverton (June 3) and Turretfield (June 15). Basal fertilizer was banded below the seed and a trailing steel roller provided a level and uniform surface for herbicide application and machine harvesting. Each plot was divided into two sections and at the five node above ground stage of development (V7; Erskine et al. 1990) metribuzin (Stacato, 750 g a.i. kg−1, Sipcam Australia, Geelong, Vic, Australia) at 1000 g a.i. ha−1 (more than 3 × the maximum label rate for clay soil types in Australia) was applied to the first 1.5 m by 6 row section of each plot using a hand-held sprayer at 107 L ha−1 using four Air Mix 110-015 low-pressure nozzles on 0.5 m spacing at an operating pressure of 240 kPa. Plant survival was estimated by counting all plants in the 4 internal rows by 1 m within the sprayed section before spraying, and then counting surviving plants at 21 days after spray application from the same section. Plant DW was estimated from the unsprayed area at the anthesis stage in PBA Flash. Cuts of 0.5 m by the four internal rows were taken at two locations in each plot, and the two sub-samples were combined and oven-dried at 80 °C for 48 h. GY was estimated by harvesting the remaining unsprayed area with a small plot harvester at maturity.
Data analyses
Data from both the Canadian and Australian genotyping experiments were compared to their relevant phenotyping data to determine the reaction of all parents and progeny plants to metribuzin herbicide.
All data from the fitness cost field studies were analysed using linear mixed models. The fixed component of the model included site, variety and site by variety effects and the random component was block. Additional fixed or random effects were added to model potential sources of spatial variation (Gilmour et al. 1997). A separable autoregressive process of order 1 in the rows and columns was used to model the spatial correlation between plot errors at each site. All data were analysed using the ASReml-R software (Butler et al. 2017) in the R environment (R core team 2020). The empirical logistic transformation (McCullagh and Nelder 1989) was used to improve the normality of the residuals of the percentage survival data:
where p is the percentage of live plants and n is the total number of plants before herbicide application. The line BC1F2-2015-29 was removed from the analysis due to a severely retarded plant growth habit compared to all other lines, most likely due to a deleterious mutation not associated with the herbicide trait. To understand the factors associated with the differences in NAR, DW, GY and plant survival, the fixed component of the model was decomposed into several contrasts based on the parents, crossing structure and assumed maternal inheritance nature of the herbicide tolerant trait (Table 1). To quantify the level of fitness cost associated with the herbicide tolerance trait, a second classification of the 23 back-crossed lines was constructed based on the mutant parent used in the cross and the obtained plant survival data for each line from the 2016 experiments. Additional contrasts were set up to determine differences in NAR, GY and DW between tolerant and sensitive line groupings and the Wald test was used to test the significance of the contrasts.
Results
Response of parents to metribuzin
Lentil plant response to metribuzin was classified into three categories based on the level of whole plant necrosis in both the Canadian and Australian genetic studies. Plants were considered tolerant if there was no evidence of necrosis or very minor necrotic speckling on individual leaflets and less than 6% necrosis on a whole plant basis; intermediate when necrotic regions resulted in leaflet or leaf death and accounted for between 6 and 50% of total plant leaf area; and sensitive when leaf and stem necrotic regions was greater than 50% of total plant area and was generally associated with whole plant death. Of the 281 PBA Flash control plants phenotyped across both experiments, 280 were classified as sensitive with 276 of these showing severe levels of whole plant necrosis (greater than 90% leaf and stem area necrosis) regardless of the origin of the plant (Tables 2 and 3). Conversely, all of the 128 M009 and 142 M043 mutant parent plants were classified as tolerant with no necrotic regions observed on leaflets or stems of any plant.
Phenotype response of F1, F2 and F3 lines to metribuzin
Plant necrosis levels of individual F1 and F1 reciprocals from crosses between both mutant parents and PBA Flash varied from 0 to 100% in both studies (Tables 2 and 3). Ratios of tolerant, intermediate and sensitive F1 plants from M009 × PBA Flash and reciprocal were 5:1:0 and 0:1:9, respectively in the Canadian study (Table 2) compared with 15:0:1 and 1:1:4 for the same F1 cross and reciprocal, respectively, in the Australian study (Table 3). Similar ratios were observed in the F1 progeny of crosses and reciprocals of M043 x PBA Flash where ratios of tolerant, intermediate and sensitive plants were 3:1:0 and 1:1:8 in the Canadian experiment and 7:1:0 and 1:1:5 in the Australian studies, respectively. The results suggested that metribuzin tolerance in both lentil mutants is predominantly maternally inherited with the occasional paternal transmission (paternal leakage, McCauley 2013) resulting in either paternal or more commonly biparental inheritance in some individuals.
Individual F2 and reciprocal F2 lines were treated separately due to paternal leakage observed in the F1 phenotypes. Results of the F2 segregation patterns are presented in Tables 2 and 3 and support a maternal inheritance with a level of paternal leakage across both experiments and both mutant genotypes. In the Canadian study 50% of F2 lines from M009 × PBA Flash, 40% of the reciprocals, 50% of M043 × PBA Flash and 70% of the reciprocals exhibited uniparental maternal inheritance. Only two lines (18%) from the M009 × PBA Flash cross and one line (10%) from the M043 × PBA Flash reciprocal showed uniparental paternal inheritance. The remaining lines of all crosses exhibited a mixed phenotypic response with the level of paternal leakage detected ranging from 4 to 85% based on the percentage of F2 progeny exhibiting the paternal phenotype. The Australian study produced similar results with 33% of F2 lines from M009 × PBA Flash and reciprocals along with 50% of M043 × PBA Flash and 67% of the reciprocals exhibiting uniparental maternal inheritance. Only 1 line (17%) from M009 × PBA Flash had uniparental paternal inheritance. The remaining 12 lines from all crosses of both mutants showed a mixed phenotype with the level of paternal leakage ranging from 1 to 97%.
Results of the Australian F2 phenotyping of the M009 × PBA Flash cross showed that one of the two F1 plants used for F3 seed production had a mixed tolerance response with 97% of the F2 progeny (F2C5F1-1) showing a paternal phenotype. The other F1 line showed uniparental paternal inheritance in the F2 phenotyping (F2C5F1-2). One reciprocal line (F2C5RF1-3) showed maternal inheritance and the other had a mixed response in the F2 screening with 30% of the F2 progeny (F2C5RF1-2) exhibiting a paternal phenotype. Phenotyping of the F3 progeny from the same F1 plants supported the F2 phenotyping results, although all F3 plants from F2 lines with paternal leakage detected were classified as sensitive, despite only 70% being identified as sensitive in the F2.
In the M043 × PBA Flash cross F2 phenotyping, one line (F2C4F1-1) derived from the two F1 plants used for F3 phenotyping exhibited uniparental maternal inheritance and the other (F2C4F1-2) had a mixed tolerance response with 82% of the F2 progeny (F2C5F1-1) showing a paternal phenotype. Both reciprocal lines (F2C4RF1-1 and F2C4RF1-2) showed maternal inheritance in the F2. Phenotyping of the F3 progeny from these plants supported the F2 phenotyping results although, again, all F3 plants from the F2 line with paternal leakage were classified as sensitive.
Genotype response of F1, F2 and F3 lines to metribuzin
The KASP marker effectively separated mutant parents and PBA Flash into discrete clusters in the Canadian experiment (Table 2, Fig. 1a). A similar result occurred in the Australian experiment (Table 3) although two of 24 M009 mutant parents were genotyped as sensitive, a result that disagreed with the phenotyping results and is unexplained.
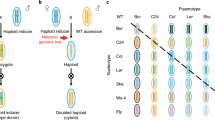
Allelic discrimination plot of KASP SNP genotyping of populations from F2 M043xPBAFlash (M002a-4 (a), M002a-26 (b), M002a-24 (c)) and reciprocal PBAFlash/M043 (M002b-31 (a), M002b-5 (b), M002b-26 (c)) and parents M043 and PBA Flash from the Canadian genetic study. The three populations within the F2 and F2 reciprocal represent an example of no apparent paternal leakage (a), majority apparent paternal leakage (b) and partial apparent paternal leakage (c) according to phenotyping results (Table 2)
Using the parental clustering of fluorescence data, individual plants of the F1, F2 and F3 generations were classed tolerant, as for M043 or M009, or sensitive like PBA Flash. Lines with a fluorescence reading located between the two parental clusters were classified as ambiguous, and likely possess some level of both tolerant and sensitive chloroplasts (Fig. 1b, c). Generally, genotyping results of the F1 hybrids matched phenotyping data however, there were a low number of discrepancies that in the majority of instances were when an intermediate phenotype was observed. Correlations between F1 genotype and phenotype data were R2 = 0.85 in the Canadian experiment and R2 = 0.88 in the Australian experiment.
Stronger correlations between genotype and phenotype data occurred for F2 lines (R2 = 0.99 (Canada) and R2 = 0.97 (Australia)). Again, a lack of a complete correlation between all genotypes and phenotypes occurred and was due to lines with a mixed phenotype response to metribuzin and/or ambiguous genotype results. Discrepancies between genotyping and phenotyping were likely due to variable numbers of tolerant and sensitive chloroplasts being present in individual plants. Genotyping of F3 plants accurately predicted the herbicide response, except for a few plants where an intermediate phenotype was matched with a sensitive genotype. In all lines a maternal genotype was observed regardless of whether the F3 line had originated from a F2 line exhibiting a level of paternal inheritance or not.
BC1F2 and BC1F3 fitness cost experiments
The analyses performed identified significant genotype by site interactions for GY, 2015DW and plant survival, but not for NAR or 2016DW (data not shown). The Wald test showed that the higher level interactions, incorporating the crossing structure, parent type and assumed maternal inheritance nature of the tolerance were significant for all variates except for Parents:Sex:Mutant:ReciprocalRep:Dup for NAR and that they did not interact with site except for Parents:Sex:Mutant:ReciprocalRep for plant survival (Table 4). These results suggest that differences occurred between some backcrossed lines and reciprocals regardless of how close genetically they were related to each other, and these differences were similar across sites. Therefore, data is presented at the individual genotype level and across sites in Fig. 2. Furthermore, the back-transformed means of plant survival were highly correlated between sites and are also presented across sites.
Mean response of BC1F2 (a and d) and BC1F3 (b and c) lines and reciprocals derived from M009/PBAFlash//PBAFlash (lines − 2 to − 6, reciprocals − 1 to − 5) and M043/PBAFlash//PBAFlash (lines − 20 to − 23, reciprocals − 19 to − 24) compared to their parents for a GY 2015, b GY 2016, c plant survival following post-emergent metribuzin application (1000 g a.i. ha−1) 2016, d net assimilation rate 2015 at field sites Riverton and Turretfield
Mean site GY was 3.6 (2015) and 2.1 (2016) t ha−1 at Riverton compared with 2.8 (2015) and 4.0 (2016) t ha−1 at Turretfield. In 2015 more favourable growing conditions occurred at Riverton compared with Turretfield, with the latter requiring two irrigation events. Growing conditions were very favourable in 2016. However, the Riverton site was located on a relatively poorly structured heavy soil type, which led to waterlogging and reduced yields compared to Turretfield.
The two mutant parents had similar GY in both years, but were 44 and 35% lower than that of PBA Flash at Riverton and Turretfield, respectively, in 2015 and 46% lower at both sites in 2016 (Fig. 2a, b). The contrast term sex was highly significant for GY and DW. In 2015 GY was 3.53 t ha−1 when the mutant line was used as a male in the backcross compared with 2.91 t ha−1 when used as the female (contrast estimate (CE) = 0.62, SE = ± 0.06). In 2016 there was an interaction between site and sex for GY. Grain yield at Riverton was 2.35 t ha−1 when the mutant was used as male in the backcross compared with 1.92 t ha−1 when used as the female, and 4.37 t ha−1 (male) compared to 3.75 t ha−1 (female) (CE = 0.19, SE = ± 0.1) at Turretfield. The GY reduction associated with using the mutant as a female compared to as a male in the backcross ranged from 14 to 18%.
The relative performance of individual BC1F2 lines in 2015 were similar to their corresponding BC1F3 yields in 2016 (R2 = 0.6, P < 0.001) (Fig. 2a, b). The higher order contrast interactions of Parents:Sex:Mutant:ReciprocalRep and Parents:Sex:Mutant:ReciprocalRep:Dup were significant for GY and DW in both the BC1F2 (2015) and the BC1F3 (2016) experiments. This suggests that the difference in using the mutant lines as a female or male is not the same across the different PBA Flash parents and that there could be differences between the duplicate pairs of the same cross. For example, genetically similar paired lines of BC1F2-2015-20 and − 32 were significantly different in GY in both 2015 and 2016, however, the paired lines from the same cross, BC1F2-2015-11 and − 23 had similar yield in both years. Furthermore, the highest and lowest yielding lines in both years came from different combinations of the sex, mutant, reciprocalrep and dup contrasts suggesting that not all lines within a specific cross were exhibiting the same level of GY reduction or fitness cost (Fig. 2a, b). The patterns of DW at maturity in 2015 and at the onset of anthesis in 2016 (data not presented) were similar to the relative line GY performance with linear relationships of R2 = 0.98, P < 0.001 (2015) and R2 = 0.77, P < 0.001 (2016).
The two mutant parents had similar NAR values, but averaged slightly lower than PBA Flash. The NAR of PBA Flash, M009 and M043 was 4.41, 4.11 and 3.74 g DW m−2 LA d−1 respectively (CE = 0.48, SE = ± 0.25). Again, the parent sex term was significant for NAR (P < 0.05). The mean NAR of all lines using the mutant parent as a female in the backcross was lower than that of lines where the mutant was used as a male, 4.01 compared to 4.36 g DW m−2 LA d−1 (CE = 0.35, SE = ± 0.15), respectively. The parent:sex term interacted with mutant and reciprocalrep (P < 0.01) for NAR suggesting that the differences in NAR from using the mutant parents as female varied depending upon mutant parent and PBA Flash back cross parent (reciprocalrep).
Plant survival percentage of PBA Flash treated with metribuzin in the BC1F3 experiments at both sites was 0% compared with 99 and 93% in M043 and M009, respectively. Percentage survival of the BC1F3 lines varied from 0 to 100% and as found for GY and DW results varied across the dup contrast (Fig. 2c). A plant survival rating of > 90% in the BC1F3 lines was considered as tolerant. Conversely a plant survival rate of < 10% was considered sensitive, while an intermediate classification was given for plant survival rates ≥ 10% and ≤ 90%. Sensitive and tolerant genotypes were present in each group derived from the crosses PBAFlash//M043/PBAFlash, M043/PBAFlash//PBAFlash and M009/PBAFlash//PBAFlash (Fig. 2c). In the group of lines derived from PBAFlash//PBAFlash/M009 three of the five lines showed an intermediate response for plant survival to metribuzin and the other two a sensitive reaction. These findings provide further evidence that a strict uniparental maternal inheritance of the tolerance trait is not occurring in lentil. GY decreased linearly with plant survival in both years, R2 = 0.64, P < 0.001 in 2015 and R2 = 0.83, P < 0.001 in 2016. A relatively weak linear decrease in the NAR of all lines occurred with plant survival (R2 = 0.38, P < 0.001). However, eight of the 10 lines with the highest NAR values were all classed as sensitive and the other two as intermediate for plant survival to metribuzin (Fig. 2c, d). This is further evidence to support a fitness cost is associated with the metribuzin tolerance trait in lentil.
Fitness cost assessment
The extent of the apparent fitness cost associated with the metribuzin tolerance gene in lentil was quantified as the mean NAR, DW and GY for backcrossed lines rated as tolerant, according to the BC1F3 plant survival data, compared with the mean of all lines rated as sensitive within each mutant group (Table 5). Lines rated as intermediate in tolerance were omitted from this comparison, as they were likely to possess variable levels of associated fitness costs. The interaction of site and genotype for NAR was not significant. The NAR for the tolerant M009 line group was reduced by 16% compared to the sensitive M009 line group, with a 13% reduction for the same comparison occurring in the M043 lines. The site by genotype interaction was significant for GY and DW at maturity in 2015, but not for DW at anthesis in 2016. In all comparisons, the tolerant groups had significantly lower means than the sensitive groups, with the amount of the reduction varying from 18 to 41% across sites and measurements (Table 5). GY and DW reductions were approximately 25% at both sites in 2015 for tolerant compared to sensitive M043 derived groups, but varied from approximately 40% at Turretfield to approximately 25% at Riverton for the M009 derived group. In 2016, DW reductions at anthesis between tolerant and sensitive groups were similar across sites, averaging 18% in M009 derived groups and 28% in the M043 derived groups. However, GY reductions between the two groups in 2016 varied across sites. For both groups, the lower yielding Riverton site had greater reductions, approximately 10% more than those recorded at Turretfield.
Discussion
The metribuzin tolerant lentil populations used in separate genetic experiments in this research were created, phenotyped and genotyped in different countries but using highly comparable methods. Phenotyping results from F1 and F2 progeny screening with metribuzin in both experiments indicated that the chloroplast psbA mutation responsible for metribuzin tolerance in lentil had a maternal inheritance pattern with a level of paternal leakage. Genotype data from both studies along with field collected phenotype data from independently created BC1F3 lines confirmed this inheritance pattern.
Although uniparental maternal inheritance of chloroplast genes is the usual form of transmission in angiosperms, biparental plastid inheritance has been reported (Corriveau and Coleman 1988; Zhang et al. 2003). Cytological evidence of plastid DNA in the generative and/or sperm cells of pollen was found in 43 of 235 angiosperm species investigated, however, it was not identified in lentil (Corriveau and Coleman 1988). Restriction fragment length polymorphism analysis has confirmed biparental chloroplast inheritance in medic (Medicago sativa L). (Lee et al. 1988; Schuman and Hancock 1989; Masoud et al. 1990) and tobacco (Nicotiana tabacum L.) (Horlow et al. 1990). In more recent studies, where a larger number of progenies were assessed, low-level frequencies (< 5%) of paternal chloroplast inheritance have been reported in sunflower (Helianthus verticillatus Small.) (Ellis et al. 2008) and canola (Schneider et al. 2015). Despite a negative response for lentil in cytological studies, RFLP analysis of progeny from interspecific crosses of lentil identified paternal chloroplasts in 1 of 10 F1 progeny (Rajora and Mahon 1995). This latter finding suggests paternal leakage could occur in lentil.
Based on the F1 herbicide phenotyping results, we detected paternal chloroplast leakage in 14 and 23% of progeny from M009 crosses, and in 23 and 24% of progeny from M043 crosses, when including lines showing potential heteroplasmy (the existence of maternal and paternal chloroplasts within an individual (Birky 2001)). The whole plant phenotyping assay used in this study may not have accurately detected low levels of paternal chloroplast leakage, as evidenced by a heteroplasmic genotype but a uniparental phenotype in the same F1, and therefore the actual frequency of paternal leakage inheritance in lentil could be higher. The paternal leakage transmission rates in this research are far higher than those identified in canola and sunflower and also higher than the 1 in 10 ratio identified previously in lentil (Rajora and Mahon 1995). Very high paternal plastid transmission rates were reported in medic where only 5 of 212 progeny showed no evidence of paternal plastid fragments (Masoud et al. 1990). This finding provides some evidence that the relative high frequencies we observed in lentil could occur more commonly in angiosperms.
The identification of chloroplast paternal leakage in lentil is likely to complicate the incorporation of the metribuzin herbicide tolerance trait into plant breeding programs. The major discrepancies between genotype and phenotype data occurred in the F1 generation and were linked to plants showing a heteroplasmic phenotype. This relatively high frequency of heteroplasmy, may reduce the value of a rapid marker platform, such as KASP in the introgression of this trait by plant breeders. Assay tissue samples taken from one plant part may misrepresent the level of chloroplast DNA at the whole plant level in F1 plants displaying biparental inheritance of chloroplasts, necessitating a different sampling or marker approach (Weihe et al. 2009). The accuracy of the KASP marker when compared with the phenotyping data did improve across generations (F1–F2–F3) with almost 100% correlation in the F3 generation. This finding is consistent with the understanding that the distribution of organelle DNA to daughter cells during mitosis is a random process and resolution of chloroplast heteroplasmy typically will occur in the following rounds of cell division, with sorting-out of plastids completed before flower formation (Greiner et al. 2015). In addition, selection with metribuzin herbicide of F2 and subsequent F3 families, derived from F1 plants showing evidence of paternal leakage, could eliminate the sensitive chloroplast as observed for common groundsel with resistance to atrazine (Frey 1998). Breeding programs incorporating the metribuzin herbicide trait in lentil could spray early generation plants with this herbicide to eliminate lines with a low level of paternally inherited sensitive chloroplasts.
Resistance to PSII inhibitors due to mutations in the D1 protein of the photosystem II complex reduces the binding affinity of the herbicide (Murphy and Tranel 2019), which can also result in reduced efficiency of electron transfer between plastoquinone QA and QB resulting in lower photosynthetic rates and a reduction in plant productivity (Devine and Shukla 2000). A fitness cost is associated with the Ser264Gly substitution in some weed species and triazine tolerant canola, but less is known about the fitness costs of other psbA mutations (Powles and Yu 2010). Our results confirmed that a fitness cost is associated with the Ala251Thr substitution conferring metribuzin tolerance in lentil. In the analysis comparing tolerant and sensitive reciprocal BC1F2 and BC1F3 lentil lines, GY was reduced in the tolerant progeny group by 20–40% and 26–37% for M009 and M043 derived lines, respectively. These results are similar to the fitness penalty observed in TT canola, which has a chloroplast psbA mutation for tolerance to triazine herbicides. Studies across environments and canola genotypes with near-isogenic or reciprocal hybrids typically found that yield penalties of 20–30% were associated with the TT trait (Beversdorf et al. 1988; Robertson et al. 2002). However, a GY loss of 39% was reported in an irrigated study (Robertson et al. 2002). Combinations of light and temperature are known to amplify, neutralize or even reverse the negative effect of the Ser264Gly mutation on photosynthesis and plant growth (Vila-Aiub et al. 2009). A similar response with the Ala251Thr mutation could explain the variation in reduction of DW and GY in lentil across environments seen in the present study. Further evaluation of the metribuzin tolerance trait across more environments is required to ascertain if the fitness cost identified in this study changes under low yield conditions.
NAR can provide a measure of the photosynthetic efficiency of plants (Watson 1952; Vernon and Allison 1963). The NAR values measured in PBA Flash were 4.41 g DW m−2 LA d−1 and generally comparable with that measured in cereals (Watson 1952; Cannell 1967) and medic (Tan and Tan 1981). The NAR of mutant parent M043 was 15% lower than PBA Flash. The NAR of the tolerant group of BC1F2 lines of M009 and M043 had similar values to their mutant parents and were reduced by 16 and 13% respectively, when compared with their respective sensitive groups. The reductions in NAR at the onset of flowering in 2015 corresponded into DW reductions at maturity of 27–41% and 25–26% in the M009 and M043 tolerant groups respectively. This suggests that inefficiency in electron transport contributes to the fitness penalty for the Ala251Thr mutants as it does for Ser264Gly mutants with resistant to triazine herbicides.
In summary, this study showed that the metribuzin tolerance trait in lentil has a maternal inheritance pattern, but with some paternal leakage. It is also associated with a fitness penalty reducing GY by 20–40%. This yield reduction appears similar to that found in triazine tolerant canola in Australia. Despite this yield penalty, triazine tolerant canola is the major canola type grown in Australia due to its unique and advantageous weed control spectrum, and improved economics of production compared to other canola options (Zhang et al. 2016; Duke 2005). Given that lentil is a significantly less competitive crop than canola and has fewer herbicide options available, a similar uptake of metribuzin tolerance may occur, providing plant breeders can effectively incorporate it into modern cultivars.
Availability of data and material
The translated amino acid sequence from M009, M043 and the susceptible parent PBA Flash are available in the GenBank database as MH681284, MH681285 and MH681286. All data from this research is deposited with the University of Adelaide data depository and can be made available upon request.
References
Beversdorf WD, Kott LS (1987) Development of triazine resistance in crops by classical plant breeding. Weed Sci 35:9–11
Beversdorf WD, Hume DJ, Donnelly-Vanderloo MJ (1988) Agronomic performance of triazine-resistant and susceptible reciprocal spring canola hybrids. Crop Sci 28:932–934
Bhatty RS (1988) Composition and quality of lentil (Lens culinaris Medik): a review. Can Ins Food Sci Technol J 11:144–160
Birky CW (2001) The inheritance of genes in mitochondria and chloroplasts: laws, mechanisms, and models. Annu Rev Gen 35:125–148
Boutsalis P, Gill G, Preston C (2016) Risk of addiction to IMIs (Group B imidazolinone herbicides). In: 2016 Grains Research and Development Corporation Grains Research Update, Adelaide, Informed decisions—driving change. GRDC, Adelaide, pp 241–243
Brand J, Yaduraju NT, Shivakumar BG, McMurray L (2007) Weed management. In: Yadav SS, McNeil DL, Stevenson PC (eds) Lentil an ancient crop for modern times. Springer, Dordrecht, pp 159–172
Butler DG, Cullis BR, Gilmour AR, Gogel BJ, Thompson R (2017) ASREML-R reference manual version 4. VSN International Ltd, Hemel Hempstead, Brisbane, p 176
Cannell RQ (1967) Net assimilation rate in barley, oats and wheat. J Agric Sci Camb 68:157–164
Corriveau JL, Coleman AW (1988) Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am J Bot 75:1443–1458
Devine MD, Shukla A (2000) Altered target sites as a mechanism of herbicide resistance. Crop Prot 19:881–889
Doyle JJ, Doyle JL (1987) CTAB DNA extraction in plants. Phytochem Bull 19:11–15
Duke SO (2005) Taking stock of herbicide resistant crops ten years after introduction. Pest Manag Sci 61:211–218
Ellis JR, Bentley KE, McCauley DE (2008) Detection of rare paternal chloroplast inheritance in controlled crosses of the endangered sunflower Helianthus verticillatus. Heredity 100:574–580
Erskine W, Muehlbauer FJ, Short RW (1990) Stages of development in lentil. Exp Agric 26:297–302
FAOSTAT (2019) Food and Agriculture Organization of the United Nations, Statistics Division, Rome, Italy Production crops. FAOSTAT statistics database. http://www.fao.org/faostat/en/#data/QC. Accessed 10 May 2019
Frey JE (1998) Genetic flexibility of plant chloroplasts. Nature 398:115–116
Fuerst EP, Norman MA (1991) Interaction of herbicides with photosynthetic electron transport. Weed Sci 39:458–464
Gilmour AR, Cullis BR, Verbyla AP (1997) Accounting for natural and extraneous variation in the analysis of field experiments. J Agric Biol Environ Stat 2:269–293
Greiner S, Sobanski J, Bock R (2015) Why are most organelle genomes transmitted maternally? BioEssays 37:80–94
Horlow C, Goujaud J, Lepingle A, Missonier C, Bourgin JP (1990) Transmission of paternal chloroplasts in tobacco (Nicotiana tabacum). Plant Cell Rep 9:249–252
Lee DJ, Blake TK, Smith SE (1988) Biparental inheritance of chloroplast DNA and the existence of heteroplasmic cells in alfalfa. Theor Appl Genet 76:545–549
Masoud SA, Johnson LB, Sorenson EL (1990) High transmission of paternal plastid DNA in alfalfa plants demonstrated by restriction fragment polymorphic analysis. Theor Appl Genet 79:49–55
Materne M, McMurray L, Brouwer J, Bretag T, Brand J, MacLean B, Hawthorne W (2011) Lentil in Australia. Grain Legumes 57:52–55
McCauley DE (2013) Paternal leakage, heteroplasmy, and the evolution of plant mitochondrial genomes. New Phytol 200:966–977
McCullagh P, Nelder J (1989) Generalized linear models, 2nd edn. Chapman & Hall/CRC, Boca Raton, pp 98–135
McDonald GK, Hollaway KL, McMurray L (2007) Increasing plant density improves weed competition in lentil (Lens culinaris). Aust J Exp Agric 47:48–56
McMurray LS, Preston C, Vandenberg A, Mao D, Oldach KH, Meier KS, Paull JG (2019a) Development of high levels of metribuzin tolerance in lentil. Weed Sci 67:83–90
McMurray LS, Preston C, Vandenberg A, Mao D, Bett KE, Paull JG (2019b) Induced novel psbA mutation (Ala251 to Thr) in higher plants confers resistance to PSII inhibitor metribuzin in Lens culinaris. Pest Manag Sci 75:1564–1570. https://doi.org/10.1002/ps.5328
Mengistu LW, Mueller-Warrant GW, Liston A, Barker R (2000) psbA mutation (valine219 to isoleucine) in Poa annua resistant to metribuzin and diuron. Pest Manag Sci 56:209–217
Mengistu LW, Christoffers MJ, Lym RG (2005) A psbA mutation in Kochia scoparia (L) Schrad from railroad rights-of-way with resistance to diuron, tebuthiuron and metribuzin. Pest Manag Sci 61:1035–1042
Mohamed ES, Nourai AH, Mohamed GF, MohamedSaxena MMMC (1997) Weeds and weed management in irrigated lentil in northern Sudan. Weed Res 37:211–218
Murphy BP, Tranel PJ (2019) Target-site mutations conferring herbicide resistance. Plants 8:382. https://doi.org/10.3390/plants8100382
Park KW, Mallory-Smith C (2006) psbA mutation (Asn266 to Thr) in Senecio vulgaris L. confers resistance to several PS II-inhibiting herbicides. Pest Manag Sci 62:880–885
Perez-Jones A, Intanon S, Mallory-Smith C (2009) Molecular analysis of hexazinone-resistant Shepherd’s-Purse (Capsella bursa-pastoris) reveals a novel psbA mutation. Weed Sci 57:574–578
Powles SB, Yu Q (2010) Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol 61:317–347
Preston C (2002) Managing an eternal pest—weeds. In: Brouwer JB (ed) Proceeding of lentil focus 2002. Pulse Austraila Ltd, Sydney
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rajora OR, Mahon JD (1995) Paternal plastid DNA can be inherited in lentil. Theor Appl Genet 90:607–610
Reith M, Straus NA (1987) Nucleotide sequence of the chloroplast gene responsible for triazine resistance in canola. Theor Appl Genet 73:357–363
Robertson MJ, Holland JF, Cawley S, Potter TD, Burton W, Walton GH, Thomas G (2002) Growth and yield differences between triazine-tolerant and non-triazine-tolerant cultivars of canola. Aust J Agric Res 53:643–651
Rochaix JD, Erickson J (1988) Function and assembly of photosystem II: genetic and molecular analysis. Trends Biochem Sci 13:56–60
Schneider A, Stelljes C, Adams C, Kirchner S, Burkhard G, Jarzombski S, Broer I, Horn P, Elsayed A, Hagl P, Leister D, Koop HU (2015) Low frequency paternal transmission of plastid genes in Brassicaceae. Transgenic Res 24:267–277
Schumann CM, Hancock JF (1989) Paternal inheritance of plastids in Medicago sativa. Theor Appl Genet 78:863–866
Schwenger-Erger C, Thiemann J, Barz W, Johanningmeier U, Naber D (1993) Metribuzin resistance in photoautotrophic Chenopodium rubrum cell cultures: characterization of double and triple mutations in the psbA gene. FEBS Lett 329:43–46
Tan GY, Tan WK (1981) Net assimilation rate and relative nitrogen assimilation rate in relation to the dry matter production of alfalfa cultivars. Plant Soil 59:185–192
Thiel H, Varrelmann M (2013) Identification of a new PSII target site psbA mutation leading to D1 amino acid Leu218Val exchange in the Chenopodium album D1 protein and comparison to cross-resistance profiles of known modifications at positions 251 and 264. Pest Manag Sci 70:278–285
Vernon AJ, Allison JCS (1963) A method of calculating net assimilation rate. Nature 200:814
Vila-Aiub MM, Neve P, Powles SB (2009) Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytol 184:751–767
Watson DG (1952) The physiological basis of variation in yield. Adv Agron 4:101–145
Weihe A, Apitz J, Pohlheim F, Salinas-Hartwig A, Borner T (2009) Biparental inheritance of plastidial and mitochondrial DNA and hybrid variegation in Pelargonium. Mol Genet Genom 282:587–593
White B (2015) Field crop herbicide guide, 9th edn. Kondinin Group, Leederville, pp 456–462
Williams RF (1946) The physiology of plant growth with special reference to the concept of net assimilation rate. Ann Botany 10:41–72
Yenish JP, Brand J, Pala M, Haddad A (2009) Weed management. In: Erskine W, Muehlbauer FJ, Sarker A, Sharma B (eds) The lentil: botany, production and uses. CABI International, Wallingford, pp 326–342
Zhang Q, Liu Y, Sodmergen (2003) Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant Cell Phys 44:941–951
Zhang H, Berger JD, Seymour M, Brill R, Herrmann C, Quinlan R, Knell G (2016) Relative yield and profit of Australian hybrid compared with open-pollinated canola is largely determined by growing-season rainfall. Crop Pasture Sci 67:323–331
Acknowledgements
The Grains Research Development Corporation (GRDC), the South Australian Research and Development Institute (SARDI), the University of Adelaide, the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Saskatchewan Pulse Growers (SPG) provided financial support for this study. The Plant Accelerator, Australian Plant Phenomics Facility, a research facility funded by the National Collaborative Research Infrastructure Strategy (NCRIS). Technical assistance and input were provided by Shi Ying Yang and Ruwan Lenorage of the University of Adelaide; Jamus Stonur, Kathy Fischer, Henk Venter, John Nairn, Phil Rundle, Brooke Schofield. Tim Jenkins, Parminder Sidhu of SARDI; and Robert Stonehouse, Vladimir Pajic, Kendra Meier, Brent Barlow, Devini De Silva, Chandra Bandara and Adam Harrison from the University of Saskatchewan. Dr Richard Jarret, University of Adelaide provided valuable contribution to the statistical analysis of the fitness cost field studies. No conflicts of interest have been declared.
Funding
The Grains Research Development Corporation (GRDC), the South Australian Research and Development Institute (SARDI), the University of Adelaide, the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Saskatchewan Pulse Growers (SPG) provided financial support for this study. The Plant Accelerator, Australian Plant Phenomics Facility, a research facility funded by the National Collaborative Research Infrastructure Strategy (NCRIS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McMurray, L.S., Preston, C., Vandenberg, A. et al. Paternal leakage inheritance and a fitness cost are associated with the chloroplastic psbA gene controlled metribuzin tolerance in lentil (Lens culinaris). Euphytica 217, 103 (2021). https://doi.org/10.1007/s10681-021-02841-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-021-02841-9