Abstract
Emmer wheat (Triticum. turgidum ssp. dicoccum) as an ancestor of bread and durum wheats, can be a potential resource to restore genetic diversity in modern durum wheats. In order to estimate the combining ability, the type of gene action, heritability, and other genetic parameters of agronomic traits, a full diallel cross (12 × 12) was made between eight durum cultivars and four emmer wheat accessions. The F1 hybrids (132 combinations) and their parents were evaluated for important traits that account for productivity during two cropping seasons. Considerable genetic diversity among the parents and the hybrids was evident, with most of the hybrids showing higher grain yields than their respective durum parent. High general combining ability for all of the measured traits, and higher Baker ratios for most of the traits indicated that additive gene action was involved. Based on the Griffing diallel method, the specific combining ability was significant for most of the measured traits. The Hayman analysis revealed the presence of partial dominance gene action for traits such as the number of tillers per plant (NT), grain weight per spike (GWS), harvest index (HI), days to heading (DH), and number of kernels per spike (NKS). However, plant height (PH), days to maturity (DM), peduncle length (PL), and grain yield (GY) were under the influence of the over-dominance gene action. The narrow-sense heritability for GWS, NKS, kernel diameter (KD), and HI was relatively high and these four were positively correlated with grain yield. Therefore, selection for these four traits in early generations may indirectly improve yield. The results indicate that Iranian emmer wheats are a good source of wild type alleles and valuable QTLs to improve the elite durum wheat cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the rise of human civilizations, wheat (Triticum spp.) has been one of the most important cultivated food crop worldwide (Asseng et al. 2020). During domestication, and later exacerbated by modern plant breeding, genetic diversity of the cultivated wheat germplasm has been significantly reduced (Fu and Somers 2009). This poses a potential threat of serious crop vulnerability to many adverse events, including global environmental and climate changes; Hence, as a prerequisite for sustainable future production, maintaining sufficient diversity in breeding stocks is crucial (Budak et al. 2013; Henkrar et al. 2016; Bassi and Nachit 2019; Xynias et al. 2020).
Durum wheat [Triticum turgidum ssp. durum (Desf.) Husn] derived from domesticated emmer wheat [T. turgidum ssp. dicoccum (Schrank ex Schübl.) Thell] about 10,000 years ago but its cultivation as a prominent crop traces back over the last 2300 years (Faris 2014). It is used mainly for the production of pasta and other semolina-based staples (Maccaferri et al. 2019). Emmer is a hulled wheat with strong glumes (husks) that enclose the grains, it has a semi-brittle rachis. It is considered as the primary gene pool for durum wheat and it harbors a rich allelic repertoire, including those conferring various climate-resilience traits (Lucas et al. 2017). Hybrids between durum and emmer wheats are quite consistent (Sheibanirad et al. 2014), and although the wild type traits such as strong glumes and brittle rachis are not entirely eliminated, they provide sufficient genetic diversity for many desirable traits (Zaharieva et al. 2010; Lucas et al. 2017; Maccaferri et al. 2019). Employing a mix of natural and artificial selection, traditional farmers have developed a vast array of local wheat cultivars or landraces with higher yield stability and better adaption to climate change relative to modern cultivars (Lopes et al. 2015; Mwadzingeni et al. 2017). This includes local landraces of Iranian Emmer wheat traditionally grown in the Zagros region (Sheibanirad et al. 2014) and are known to carry valuable traits, especially those linked to tolerance to biotic and abiotic stresses, and these traits would be beneficial for the improvement of durum wheat in general (Vaghar and Ehsanzadeh 2018; Abdehpour and Ehsanzadeh 2019; Fatholahi et al. 2020). However, the genetic potential of these local stocks is largely unexplored.
Reliable estimates of genetic parameters such as heritability, combining ability, and gene effects are necessary to decide on appropriate breeding strategies and selection schemes to create new cultivars with improved specific morpho-physiological traits. One of the most reliable and commonly used methods for estimating such parameters are the diallel crosses (Mather and Jinks 1982). In diallel analyses based on the Hayman method (Hayman 1954), a combination of genetic information is gathered with respect to allelic distribution, the average degree of dominance, the presence or absence of epistasis, the number of gene groups, broad and narrow sense heritability and the direction of dominance. Furthermore, using the Griffing method (Griffing 1956), appropriate statistical models such as, the general and specific combining ability (GCA and SCA, respectively) are introduced into the estimates and thus the types of gene effects (additive or non-additive) are further determined.
Reif et al. (2007), in a theoretical study, have shown that the inter-population improvement is more effective through more divergent heterotic groups rather than genetically similar heterotic groups. Therefore, the ratio of dominance to additive variance decreases with the increase in genetic divergence between two populations, which in turn leads to an increase in the GCA variance. As a result, the performance of the top hybrids can be predicted based on the effects of the GCA. In another study, Kulkarni et al. (2008) created a functional diversity for two traits: the thousand-grain weight parameter and number of days to maturity, in a population derived from a cross between durum and emmer wheats. This compared well with other studies where no reciprocal effects were observed in F2 populations derived from crosses between durum and emmer wheats for grain weight, and the dominance effect was observed for grain weight (Millet et al. 1984). In a study conducted on a population of recombinant inbred lines (RILs) derived from durum and wild emmer wheat hybrids, a positive correlation was found between the number of fertile tillers, grain yield, and grain number per spike, but these traits were negatively correlated with grain weight (Golan et al. 2019). In another study conducted on a durum × emmer RILs population, a positive correlation was observed between the harvest index and grain yield, while a negative correlation was observed between these traits and days to heading (Peleg et al. 2009). In an experiment to investigate the combining ability of tetraploid wheat using line × tester crosses of durum by emmer, the most significant effect of SCA was observed for the thousand-grain weight, grain yield and harvest index in hybrids of emmer with durum wheats (Lohithaswa et al. 2014).
In this study, four local accessions of emmer were crossed with eight durum cultivars in a full diallel pattern, and genetic mechanisms controlling several important agronomics traits were investigated. In addition, different aspects of the combing ability between the two species were addressed. Through identification and reintroduction of rare but valuable alleles from wild relatives of wheat, we hope to provide some insight into the potential for an improvement of current durum wheat cultivars.
Materials and methods
Breeding material and experimental method
A total of 12 tetraploid wheat genotypes including eight durum cultivars and four emmer accessions were used in a full diallel cross to produce 132 hybrids [p × (p – 1) = 12 × 11 = 132; P = number of parents]. The eight durum cultivars were selected from among a wide range of cultivated varieties showing reasonable diversity. The four emmer accessions were landraces (local varieties) collected from different villages in central Zagros region of Iran. The accession names correspond exactly to the village from which each accession was collected (Sheibanirad et al. 2014). Plant materials used in this study are kept at the Isfahan University of Technology (IUT) seed bank and can be requested through communication with the corresponding author. Information on the parental genotypes are presented in Table 1. The 132 F1 hybrids along with their 12 parents (144 entries) were studied at the IUT experimental field (32°32′ N and 51°23′ E, with 1630 m altitude) for two years during autumn to spring seasons (2016–2017 and 2017–2018) using a simple lattice design (12 × 12) with two replications. For each experimental year, the F1 seeds were independently obtained by crossing the parental genotypes in the previous cropping season. The seeds from each entry were planted in a plot with two rows, each consisting of 150 cm of length and an individual plant distance of 10 cm, with 20 cm of space between the rows. Field operations such as irrigation, fertilization and weeding were performed uniformly for all entries. The experimental site has a clay loam soil (pH 7.5), an average annual precipitation of 140 mm, and an average temperature of 15 °C (Fig. 1). Irrigation was done using a pump station and polyethylene pipes based on the evapotranspiration rate (Allen et al. 1998). About 500 m3/ha of water was delivered in each of the eight irrigation cycles.
Data collection
For each entry (parents and F1s), five samples were randomly selected from each replicate, and the following data were recorded: kernel length (KL), kernel diameter (KD), plant height (PH), number of tillers (NT), number of fertile tillers (NFT), number of sterile tillers (NST), peduncle length (PL), peduncle extrusion (PE), number of kernels per spike (NKS), grain weight per spike (GWS), number of spikelets per spike (NSLS), number of kernels per spikelet (NKSL), spike length (SL), flag leaf length (FLL), flag leaf width (FLW), flag leaf area (FLA), days to heading (DH), days to anthesis (DA), days to maturity (DM), grain yield per plant (GY), biological yield per plant (BY), and the harvest index (HI).
Genetic and statistical analyses
The recorded data were initially assessed for statistical normality (residuals) and homogeneity of variance (Kozak and Piepho 2018). The ANOVA and diallel analyses were performed using the average of five randomly selected samples from each replicate. The principal component analysis (PCA) was performed to determine the relationship between traits and genotypes. The PCA was based on a correlation matrix obtained from mean data, and two principal components were extracted using eigenvalues (Malik and Piepho 2018). Efficiency of the randomized complete block design (RCBD) relative to the simple lattice design was checked and for most of the studied traits the RCBD was found to be as efficient as the lattice design. Therefore, all analyses were performed using RCBD. Combined data from the two experimental years were analyzed using SAS program version 9.4 (SAS Institute 2014). Genotypes were considered as fixed and years were regarded as random variables in the statistical model. The mean square of genotypes was found to be significant, and was subdivided into three sections for parents, hybrids, and parents versus hybrids. Further calculations were performed by diallel analysis.
The relative mid-parent Heterosis (MPH) and heterobeltiosis or relative better parent Heterosis (BPH) were estimated for F1, using the following equations suggested by Mather and Jinks (1982):
In these relations F1 is mean value of the progeny obtained by crossing parents, MP = mean value of the two parents or mid-parent value, and BP = mean value of the better parent.
As the selection of parents were from a fixed germplasm set, Griffing diallel was performed based on Method Ι (parents, F1s and their reciprocals) of Model Ι (fixed model) using the following statistical model (Griffing 1956):
where u is the population mean, gi and gj are the GCA effects for the ith and jth parents, sij is the SCA effect for the cross between the ith and jth parents such that sij = sji, rij is the reciprocal effect involving the reciprocal crosses between the ith and jth parents such that rij = −r ji, eijkl is the environmental error effect associated with the ijklth individual observation, and p, b, and c are the number of parents, blocks and sampled plants, respectively.
The Griffing diallel was analyzed using the DIALLEL-SAS05 program (Zhang et al. 2005). The diallel analysis method was also performed according to the Hayman model to estimate genetic parameters, the effects of genes, the average degree of dominance, non-allelic interactions, allele distribution in parents, and to determine heritability (Hayman 1960). Based on the assumptions of the additive-dominance model, genetic parameters and statistical indices were estimated using the SASHAYDIALL-SAS program developed by Makumbi et al. (2018). The linear model used for the Hayman diallel in the SASHAYDIALL program is shown in the following equations (Makumbi et al. 2018; Hayman 1954):
where yrs is the entry in the rth row (female parents) and sth column (male parents), m is the grand mean of the diallel table, jr is the mean deviation from the grand mean due to the rth parents, lr is further dominance deviation due to the rth parent, lrs is the remaining discrepancy in the rsth reciprocal sum, kr is the average maternal effect of each parental line, and krs is the variation in the rsth reciprocal differences (Hayman 1954). The parameters in the second model measure different sources of variation whereby jr = a is the variation due to additive genes, l = b1 is the mean dominance deviation, lr = b2 is further dominance deviation due to the rth parental line, lrs = b3 is the residual dominance variation, kr = c is the average maternal effects of each parental line, and krs = d is variation in the reciprocal difference not due to c (Hayman 1954).
Two analyses were performed to test the hypothesis of Hayman. The t2 test was done to examine the uniformity of variance–covariance of arrays (Wr-Vr). In this respect, if t2 was significant, the hypothesis was not confirmed. The second test was the regression analysis (Wr-Vr), according to Mather and Jinks (1982), in which the data will be valid for genetic interpretation if the regression coefficient value departs significantly from zero (b = 0), but not from unity (b = 1). A significant difference from unity suggests epistasis in the genetic control of the traits. Graphical analysis was performed based on the regression of the offspring parent covariance (Wr) on parental array variance (Vr) (Hayman 1954). Mather and Jinks (1982) in Vr-Wr graphical analysis described that if the regression line cuts off the Wr-axis below or above the point of origin, it reveals over-dominance or an additive type of gene action, respectively. Also, genotypes possess maximum dominant or recessive genes when they are closest to or farthest from the origin, respectively.
Results
Association of traits, trait selection and analysis of variance
Results of principal component analysis (PCA) showed that the first two principal components justified 75.3% of the data variation. The specified traits were divided into two groups based on the correlation matrix and the cosine of the angles between vectors (Supplementary Fig. S1a and Table S2). The first group included NKSL, NKS, FLW, FLA, GWS, KD, HI, and GY traits which were positively associated with each other and resembled the durum wheat parents. The second group comprised of NT, NPT, NST, FLL, NSLS, SL, KL, PH, PL, PE, and BY traits which were also positively associated with each other and resembled the emmer wheat parents. Results also showed that the NKSL, NKS, FLW, FLA, GWS, KD, and HI had negative associations with NT, NPT, NST, FLL, NSLS, SL, KL, PH, DH, and DA traits. Therefore, according to traits’ correlations and different plant architecture between emmer and durum wheat, the more important and influential traits including PH, PL, NT, NPT, DH, DM, SL, NKS, GWS, KL, KD, HI, and GY were selected for further genetic analysis (Fig. 2).
Biplot based on principal component analysis of the selective traits in 12 tetraploid wheat genotypes and 132 their hybrids: (a) First year, (b) Second year. Abbreviations: PH plant height (cm), PL peduncle length (cm), NT number of tillers per plant, NPT number of productive tillers per plant, DH day to heading, DM day to maturity, SL spike length (cm), NKS number of kernels per spike, GWS grain weight per spike (g), KL kernel length (mm), KD kernel diameter (mm), GY grain yield per plant (g), HI harvest index (%)
The two cropping seasons (experimental years) differed in rainfall distribution, relative humidity, and temperature during the grain filling period (Fig. 1). As a result the grain yield and its components were affected. The results of the combined analysis of variance showed significant differences (P < 0.01) due to the interaction effect of year × genotypes for all studied traits except for KL (Supplementary Table S1). Also a significant difference (P < 0.01) was observed among the genotypes revealing the existence of variability for all measured traits in both experimental years. Mean squares showed that the twelve parents differed considerably and a significant variability (P < 0.01) was observed among the hybrids for all the measured traits. Taken together, the results revealed that there was sufficient genetic variability in the set of plant materials for diallel analysis. The distribution of parents and their hybrids in the scatter plot divided the genotypes into three distinct groups. Parental emmer, parental durum, and their hybrid progenies were found to be in groups one, two, and three respectively (Fig. 2 and supplementary Fig. S1b).
Heterosis for grain yield
The estimated values for the two categories of heterosis, best-parent heterosis (BPH) and mid-parent heterosis (MPH), differed in magnitude (Table 2). Overall, the best estimates for MPH were related to grain yield. In the first experimental year, approximately 97.0 and 91.0% of the hybrids showed positive values for MPH and BPH respectively. Also, in the second year, about 95.0 and 88.0% of the hybrids showed positive values for MPH and BPH, respectively. The hybrids with the best and positive MPH and BPH values were Karkheh × Singerd, Khoyghan × Karkheh, Singerd × Yavaros, Yavaros × Behrang, and Shabrang × Zarneh. In contrast, the three hybrids of Ariya × Saji, Ariya × Shabrang, and Dena × Yavaros, had negative values in this respect in both years.
Griffing analysis
Griffing analysis of variance was used to evaluate the effects of general and specific combining ability (GCA and SCA) and the reciprocals (Table 3). The mean square of GCA was significant for all traits (P < 0.05) in both years. SCA effects were significantly different between hybrids for all the measured traits except for NKS and KD in the first year and NT in the second year of the study. Moreover, the reciprocal effects were significantly different for PL, NT, NPT, DM, SL, NKS, GWS, KL, GY, and HI and not significant for PH, DH, and KD in the first year. In the second year, the reciprocal effects were significant for PH, PL, NPT, DH, DM, NKS, GWS, and HI, while non-significant for NT, SL, KL, KD, and GY. In general, maternal effects were non-significant for traits affecting yield such as NT, DM, SL, GWS, HI, and GY in both experimental years. This was also evident in the PCA results (Fig. 2). The Baker’s ratio was closer to unity for all the measured traits except for DM, PL, NPT, and GY in both years.
GCA effects of parental genotypes
The results of the GCA estimates of parental genotypes showed both positive and negative directions for the measured traits (Table 4). For HI, KD, GWS, and NKS traits which had a positive correlation with grain yield (Supplementary Table S2), durum parents Yavaros, Dena, and Behrang in the first year and Yavaros, Dena, and Shwa in the second year had high positive GCA effects. However, for the PH, NT, DH, and DM traits which had a negative correlation with grain yield, durum parents Shwa, Yavaros, and Behrang in the first year and Shabrang, Behrang, and Ariya in the second year had high negative GCA effects. Also, for the PL and NPT traits which had positive correlations with grain yield as well as SL and KL traits, parents of emmer wheat Khoyghan, Ozonbelagh, Singerd, and Zarneh had high positive GCA effects in both years. Altogether, the emmer parents had high positive GCA effects for NT, NPT, PH, PL, SL, DH, DM, and KL traits and the durum ones for NKS, GWS, KD, GY and HI in both experimental years.
SCA effects of hybrids
The highest positive significant SCA effects for the GY and HI traits was observed in Shwa × Singerd in the first year, and in Karkheh × Singerd and Shabrang × Singerd in the second year (Supplementary Table S3 and S4). Dena × Karkheh, Behrang × Singerd, and Ariya × Saji had high positive significant SCA effects for NKS and GWS traits and considered to be good combiners. Also a high significant SCA effect in the positive direction was exhibited by the Dena × Singerd for SL trait in both experimental years. Ariya × Singerd and Shwa × Singerd in the first year and Yavaros × Singerd in the second year showed the highest positive significant SCA effects for KL and KD traits. For PH Khoyghan × Zarneh, Khoyghan × Ozonbelagh, and Ozonbelagh × Zarneh showed high significant SCA effects in the negative direction. Yavaros × Singerd, Karkheh × Singerd, and Shwa × Singerd showed high significant SCA effects in the positive direction for PL in both experimental years. The two hybrids of Saji × Khoyghan and Ariya × singerd in the first year, and the Yavaros × Zarneh in the second year showed a high negative significant SCA effect for NT, while Yavaros × Singerd exhibited the highest significant SCA effect in the positive direction for NPT. For DH and DM traits, Saji × Khoyghan in the first year and Yavaros × Zarneh in the second year showed significantly high SCA effects in the negative direction.
The reciprocal effect for most of the aforementioned hybrids was found to be non-significant and only hybrids Ozonbelagh × Zarneh, Dena × Karkheh, and Behrang × Singerd showed significant reciprocal effects. Similarly, the distribution of parents and their hybrids in the scatter plot indicated very little maternal and reciprocal effects. This showed that hybrids acted differently from their parents in the expression of traits (Fig. 2).
Hayman analysis
Test of Hayman diallel hypothesis
As required, the validity of several assumptions was tested before the Hayman analysis was conducted. Results indicated that the additive-dominance model was fully adequate for most of the examined traits including NT, NPT, DH, GWS, and GY in both experimental years, DM in the first year and NKS, KL, KD, and HI in the second year (Supplementary Table S5). The model was also partially adequate for PH, SL, KL, and HI in the first year, and PL and DM in the second year. However, for PL and NKS in the first year and PH and SL in the second year, the model assumptions were not confirmed, and the adequacy of the model was rejected.
Estimates of genetic components variance
Estimation of the genetic variance components and related parameters showed that the additive component (D) was highly significant for all measured traits except for PL in both experimental years (Table 5). The values of the dominant components (H1 and H2) were significant for the PH, PL, NPT, DH, SL, GWS, KL and GY traits in both experimental years. For NT, DM, and KD they were significant in the first year, and for NKS in the second year. The estimates of the average degree of dominance (√(H1/D)) were lower than unity for NT, DH, SL, NKS, GWS, KL, KD and HI traits in both experimental years (Table5). However, it was more than unity for PL, PH, NPT and GY in both years, and for DM in the first year, indicating the preponderance of the over dominance type of gene action.
The F component, which estimates an unequal frequency of dominant and recessive alleles in the parental genotypes, was highly significant and positive for the DH and HI traits in both studied years and for NT and DM in the first year (Table5). The proportion of genes in the parents with positive and negative effects (H2/4H1) was almost equal to its theoretical value (0.25) for PH, DH, SL, NKS, KL and GY traits in both experimental years. This suggested an even distribution of increasing (positive) and decreasing (negative) alleles in the parents. Also, the proportion of dominant and recessive genes in the parents ((4DH1)0.5 + F/(4DH1)0.5-F) was equal to unity for PH, NPT, SL, NKS, GWS, KL and GY traits in both years. For HI it was equal to unity in the first year and for PL, DH and KD in the second year. NT, DH, DM and KD traits revealed more dominant genes in the parents.
The E component which indicates environmental effects on the expression of genes was highly significant for all measured traits in both years (Table5). However, its value was much lower than that of the D or H components for all measured traits except KL in the first year, DM in the second year and NPT in both experimental years. The broad-sense heritability (h2b) values were moderately high for nearly all traits in both years. NPT, DM and GY showed a lower value of h2b in the second year. Narrow-sense heritability values (h2n) for the NT, DH, NKS, GWS, KL, KD and HI were moderately high in both years.
Graphical representation by Hayman analysis
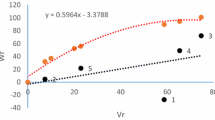
Since the regression line slope was significantly different from zero for all traits, Hayman graphical analysis was performed (Fig. 3 for harvest index, and Supplementary Fig. S2 and S3 for other traits). The Wr/Vr graphical presentation revealed that the regression line passes above the origin, cutting the Wr axis in the positive region for NT, DH, NKS, GWS, and HI in both experimental years, and KD in the second year. This suggested the presence of a partial dominance gene action for these traits. Results also indicated that PH, PL, DM, and GY in both experimental years, and NPT in the second year were under the control of over-dominance as the regression line cut the Wr axis below the point of origin. For NPT and KD traits in the first year, and for the SL and KL in both experimental years, the regression line almost passed the point of origin indicating the presence of complete dominance gene action.
Regression of Wr/Vr and dispersion of parents around origin for harvest index (HI) in two years: (a) the first year (b) the second year, the points of Wr/Vr intercepts refer to Shabrang (1), Dena (2), Ariya (3), Behrang (4), Yavaros (5), Shwa (6), Karkheh (7), Saji (8), Khoyghan (9), Ozonbelagh (10), Zarneh (11), Singerd (12) arrays
Discussion
Existence of genetic variation
Emmer wheat is part of primary gene pool for durum wheat and has many valuable traits such as tolerance to environmental stresses, resistance to pests and diseases, and beneficial quality traits (Zaharieva et al. 2010). Our results showed that there was considerable variation among the studied parental genotypes, particularly between the two species of emmer and durum. This variation was better manifested in the hybrids, as they were significantly different for all measured traits. This suggest the potential of emmer as a rich pool of genetic diversity from which to obtain genes for desirable traits and improvement of durum wheat cultivars (Faris et al. 2014). Also the created inter- and intra-specific variation provided the condition for genetic analysis and selection processes.
The interaction between genotype and experimental years were significant for all traits except KL, indicating different responses of hybrids to environmental variations in two years (Daugüstü 2008). The two cropping seasons (2016–2017 and 2017–2018) were significantly different in terms of precipitation patterns, air humidity, and air temperature especially during the grain filling stage. This significantly affected the mean values of studied traits. Drought and high temperatures are the leading causes of stress during the post-flowering period, which shortened the grain-filling period and decreased grain weight (Mohammadi 2019).
Genetic potential of durum and emmer wheat crosses
Grain yield is a function of its components that are directly involved in yield fluctuations (Mwadzingeni et al. 2018). Grain yield was significantly and positively correlated with KD, NPT, PL, NKS, GWS, and HI. These traits individually or in combination may be used for indirect selection to improve the grain yield in early generations of durum × emmer hybrids (Fischer and Rebetzke 2018). Golan et al. (2019), investigated a population of RILs derived from crossing durum with wild emmer wheats and reported a positive correlation between grain yield and NPT and NKS. In another durum × emmer RILs population, a positive correlation was found between HI and grain yield, while both were negatively correlated with DH (Peleg et al. 2009). Others have also reported a significant positive correlation between NKS and GWS in a durum × emmer wheat population (Faris et al. 2014). The peduncle length (PL) was also positively correlated with grain yield (Fig. 2 and Table S2). Given that in wheat, the peduncle has the highest stored soluble carbohydrates, such as fructan and starch, some of these carbohydrates are transported back to the kernel during the grain-filling period (Wardlaw and Willenbrink 1994). The wheat peduncle due to its attachment to the flag leaf, its high photosynthetic activity, and the proximity to the spike plays an important role in transporting photosynthate during the grain filling period (Ataei et al. 2017). Peduncle plays a key role in increasing wheat's final yield in well-watered conditions (about 10%) and even more so (about 40%) in drought and heat stress conditions (Davidson and Chevalier 1992).
Some studies have reported QTLs with additive effects for grain yield and its components, showing that traits can be improved through combinations of superior parents followed by selection (Mwadzingeni et al. 2016; Zhang et al. 2010). Peng et al. (2003), in a T. durum × T. dicoccoides mapping population, identified 18 QTLs in wild emmer wheat related to higher yield, higher kernel number, higher spike number and weight, and early flowering. Likewise, in the present study, more than 87% of the hybrids showed positive values for the relative best-parent heterosis (BPH) in respect to grain yield in both experimental years. This indicates that the emmer parents carried additive genes that positively affected grain yield and its components. However, it is unlikely to have parents with completely desirable GCA effects for all traits of interest. Therefore, to achieve and stabilize superior recombinants, several cycles of hybridization and selection may be needed for gene pyramiding (Joshi and Nayak 2010; Mwadzingeni et al. 2016). Terzi et al. (2007), in an assessment of genetic diversity of emmer × durum derived lines and their parents, discovered that six advanced breeding lines had yield values equal to or greater than the durum parent. Moreover, all six lines showed significantly different plant height and earliness compared to the emmer parent and were closer to the durum parent. Given that hybrid breeding in wheat is not currently commercially viable, an important goal in wheat hybridization is to find transgressive segregates or superior recombinants for specific purposes and particular traits (Mwadzingeni et al. 2018). The high positive SCA effects for grain yield and its components in most of the hybrids indicated that emmer and durum wheats are good combiners. Successive bottlenecks due to domestication followed by breeding practices have reduced the diversity in elite wheat cultivars in a way that many loci possess similar alleles (Haudry et al. 2007). This may suggest that durum × durum hybrids may not create new variations and additive effects at QTLs. However, crossing emmer × durum may provide the desired genetic diversity (Holtz et al. 2017), and as a result, gain from selection can be more prominent.
Heritability of traits and indirect selection
High narrow-sense heritability (h2n) estimates for several traits including NT, DH, NKS, GWS, KL, KD, and HI in both experimental years suggest that a few major genes are involved in controlling the inheritance of a particular trait. This indicates a positive response to selection during early segregating generations and successful genetic advances (Kearsey and Pooni 1998). Grain yield is a highly polygenic characteristic with low heritability due to genetic, environmental, and management factors (e.g., G × E × M) (Golan et al. 2019). Also as grain yield is a function of its components (such as KD, GWS, NKS, and HI), a high positive correlation between grain yield and these traits coupled with high heritability assures a positive response to indirect selection for yield in early-generations (Fischer and Rebetzke 2018).
Genetic control of traits
To design an effective strategy for a breeding program, it is essential to gain knowledge on the genetic control of traits. The genetic effects obtained from the Hayman and Griffing analyses showed that for the expression of DH, NT, NKS, GWS, SL, KL, KD, and HI traits, the contribution of additive effects were higher than the dominance component in both experimental years (Table6). In addition, due to the high Baker's ratios, which were close to unity for these traits, they may be used for selection in early generations with greater confidence and increase the selection efficiency in breeding programs (Baker 1978). Furthermore, in the Hayman graphical analysis, the regression line for the NT, DH, NKS, GWS, KD, and HI traits intercepted above the point of origin (in both experimental years) indicating the presence of additive gene action. This suggests that pedigree selection can be employed for genetic improvement of these traits when emmer is used in crosses with durum. Other durum wheat researches have shown that in the genetic control of traits such as DH (Hannachi et al. 2013), KL (Topal et al. 2004), NKS (Gowda et al. 2010; Hannachi et al. 2013), and HI (Solomon and Labuschagne 2004; Hannachi et al. 2013; Malchikov and Myasnikova 2016) the additive effects were more important than the non-additive ones.
Our results in graphical analysis showed that for NPT, PL, PH, DM, and GY traits, the contribution of the dominance effect was higher than the additive component. In addition, the average degree of dominance was more than unity for these traits. In other durum wheat studies the role of the dominance effect was more than the additive effect for several traits including NPT, GY (Solomon and Labuschagne 2004; Gowda et al. 2010; Hannachi et al. 2013), and GWS (Gowda et al. 2010; Malchikov and Myasnikova 2016). Our results were not in complete agreement with the previously reported findings for GWS. Furthermore, it should be noted that grain yield is a function of its components such as number of tillers, number of kernels per spike and grain weight per spike (Mwadzingeni et al. 2018). Our results as well as other reports (Such as: Solomon and Labuschagne 2004; Gowda et al. 2010; Hannachi et al. 2013; Malchikov and Myasnikova 2016), show that these traits are controlled by the additive gene action. It seems that aggregation of additive effects in hybrids had increased grain yield (Peng et al. 2003; Zhang et al. 2010). Consequently, based on the diallel model results, the dominant effect in genetic control of grain yield was confirmed. In situations where the role of dominance is greater than additive effects for a trait, selection should be made in advanced generations after the lines have reached genetic purity. Due to a different distribution of allele frequencies among the parents, the amount of dominance variance against additive variance differ among hybrids (Reif et al. 2007; Longin et al. 2013). The distribution of parents around the regression line and also proximity to the origin indicated maximum dominant alleles for PH, PL, NPT, SL, NKS, and KL traits in the emmer genotypes. Conversely, the durum genotypes possessed maximum dominant alleles for the NT, DH, DM, GWS, KD, HI, and GY traits.
Combining ability of durum and emmer wheat crosses
The SCA and GCA show a non-additive and additive effects in controlling traits, respectively (Griffing 1956). For all measured traits except for PL and DM, the non-additive effect was lower than the additive one in both experimental years. This can be attributed to genetic divergence between the parents, further increasing the variance of GCA (Gowda et al. 2010; Reif et al. 2007). In general, the ratio of GCA to SCA increases with an increased genetic divergence between the two parental groups (Fischer et al. 2009) and leads to increased gain from selection and also the identification of promising hybrids based on the GCA predictions (Reif et al. 2007; Longin et al. 2012). Specifically, this genetic divergence can be clearly observed in the scatter plot of parents and their hybrids in the present study (Fig. 2 and Supplementary Fig S1b). Also, in the absence of epistasis, parents with higher genetic divergence for the target trait tend to have higher GCA variance rather than SCA variance (Reif et al. 2007). The results of the additive-dominance model confirmed the absence of epistasis for most of the studied traits. Therefore, the role of both additive and non-additive effects were important in their genetic expression (Gowda et al. 2010).
Among the hybrids, Shwa × Singerd and Yavaros × Singerd were superior in most of the measured traits. As they belong to two divergent heterotic groups (Reif et al. 2007) and also based on Hayman's graphical analysis, these three parents must contain the maximum dominant or recessive alleles which will result in transgressive segregation.
Conclusion
Hybrids obtained from crosses between durum and emmer wheats produced higher grain yields than the superior durum parents. The results showed that for most of the studied traits, additive genetic effects played a more important role compared to non-additive one in controlling traits. The adequacy test of the additive-dominance model showed that the epistatic effects in controlling these traits were low. The specific combining ability was significant for all of the measured traits, indicating the possibility of the durum wheat improvement by interspecific hybridization. A partial dominance gene action was observed for the NT, DH, NKS, GWS, and HI traits in both experimental years. However, the PH, DM, PL, and GY were controlled by the over-dominance gene action. A relatively high narrow-sense heritability for the grain yield components and their positive correlation with grain yield indicated chances for yield improvement by indirect selection in early generations. Our findings revealed that Iranian emmer wheat landraces are a valuable gene source and can be further exploited for the improvement of durum wheat.
References
Abdehpour Z, Ehsanzadeh P (2019) Concurrence of ionic homeostasis alteration and dry mass sustainment in emmer wheats exposed to saline water: implications for tackling irrigation water salinity. Plant Soil 440:427–441. https://doi.org/10.1007/s11104-019-04090-1
Allen RG, Pereira LS, Raes D et al (1998) Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. Fao Rome 300:D05109
Asseng S, Guarin JR, Raman M et al (2020) Wheat yield potential in controlled-environment vertical farms. Proc Natl Acad Sci U S A 117:19131–19135. https://doi.org/10.1073/pnas.2002655117
Ataei R, Gholamhoseini M, Kamalizadeh M (2017) Genetic analysis for quantitative traits in bread wheat exposed to irrigated and drought stress conditions. Phyton 86:228–235
Baker RJ (1978) Issues in diallel analysis. Crop Sci 18:533–536. https://doi.org/10.2135/cropsci1978.0011183x001800040001x
Bassi FM, Nachit MM (2019) Genetic gain for yield and allelic diversity over 35 years of durum wheat breeding at ICARDA. Crop Breed Genet Genomics 1:1–19
Budak H, Kantar M, Yucebilgili KK (2013) Drought tolerance in modern and wild wheat. Sci World J
Daugüstü N (2008) Genetic analysis of grain yield per spike and some agronomic traits in diallel crosses of bread wheat (Triticum aestivum L.). Turk J Agric For 32:249–258
Davidson DJ, Chevalier PM (1992) Storage and remobilization of water-soluble carbohydrates in stems of spring wheat. Crop Sci 32:186–190
Faris JD (2014) Wheat domestication: key to agricultural revolutions past and future. In: Genomics of plant genetic resources. Springer, pp 439–464
Faris JD, Zhang Q, Chao S et al (2014) Analysis of agronomic and domestication traits in a durum × cultivated emmer wheat population using a high-density single nucleotide polymorphism-based linkage map. Theor Appl Genet 127:2333–2348. https://doi.org/10.1007/s00122-014-2380-1
Fatholahi S, Ehsanzadeh P, Karimmojeni H (2020) Ancient and improved wheats are discrepant in nitrogen uptake, remobilization, and use efficiency yet comparable in nitrogen assimilating enzymes capabilities. F Crop Res 249:107761. https://doi.org/10.1016/j.fcr.2020.107761
Fischer RA, Rebetzke GJ (2018) Indirect selection for potential yield in early-generation, spaced plantings of wheat and other small-grain cereals: a review. Crop Pasture Sci 69:439–459. https://doi.org/10.1071/CP17409
Fischer S, Möhring J, Maurer HP et al (2009) Impact of genetic divergence on the ratio of variance due to specific vs. general combining ability in winter triticale. Crop Sci 49:2119–2122. https://doi.org/10.2135/cropsci2008.12.0689
Fu YB, Somers DJ (2009) Genome-wide reduction of genetic diversity in wheat breeding. Crop Sci 49:161–168. https://doi.org/10.2135/cropsci2008.03.0125
Golan G, Ayalon I, Perry A et al (2019) GNI-A1 mediates trade-off between grain number and grain weight in tetraploid wheat. Theor Appl Genet 132:2353–2365
Gowda M, Kling C, Würschum T et al (2010) Hybrid breeding in durum wheat: heterosis and combining ability. Crop Sci 50:2224–2230. https://doi.org/10.2135/cropsci2009.10.0637
Griffing B (1956) Concept of general and specific combining ability in relation to diallel crossing systems. Aust J Biol Sci 9:463–493
Hannachi A, Fellahi ZEA, Bouzerzour H, Boutakrabt A (2013) Diallel-cross analysis of grain yield and stress tolerance-related traits under semi-arid conditions in Durum wheat (Triticum durum Desf.). Electron J Plant Breed 4:1027–1033
Haudry A, Cenci A, Ravel C et al (2007) Grinding up wheat: a massive loss of nucleotide diversity since domestication. Mol Biol Evol 24:1506–1517
Hayman BI (1954) The analysis of variance of diallel tables. Biometrics 10:235–244
Hayman BI (1960) The theory and analysis of diallel crosses. III Genetics 45:155–172
Henkrar F, El-Haddoury J, Ouabbou H et al (2016) Genetic diversity reduction in improved durum wheat cultivars of Morocco as revealed by microsatellite markers. Sci Agric 73:134–141. https://doi.org/10.1590/0103-9016-2015-0054
Holtz Y, Bonnefoy M, Viader V et al (2017) Epistatic determinism of durum wheat resistance to the wheat spindle streak mosaic virus. Theor Appl Genet 130:1491–1505
Joshi RK, Nayak S (2010) Gene pyramiding-a broad spectrum technique for developing durable stress resistance in crops. Biotechnol Mol Biol Rev 5:51–60
Kearsey MJ, Pooni HS (1998) The genetical analysis of quantitative traits. Stanley Thornes (Publishers) Ltd
Kozak M, Piepho HP (2018) What’s normal anyway? Residual plots are more telling than significance tests when checking ANOVA assumptions. J Agron Crop Sci 204:86–98. https://doi.org/10.1111/jac.12220
Kulkarni P, Desai SA, Lohithaswa HC, Hanchinal RR (2008) Breeding for free threshability in emmer wheat [Triticum dicoccum (Schrank.) Schubl.] through induced mutagenesis
Lohithaswa HC, Desai SA, Hanchinal RR, et al (2014) Combining ability in tetraploid wheat for yield, yield attributing traits, quality and rust resistance over environments. Karnataka J Agric Sci 26
Longin CFH, Gowda M, Mühleisen J et al (2013) Hybrid wheat: Quantitative genetic parameters and consequences for the design of breeding programs. Theor Appl Genet 126:2791–2801. https://doi.org/10.1007/s00122-013-2172-z
Longin CFH, Mühleisen J, Maurer HP et al (2012) Hybrid breeding in autogamous cereals. Theor Appl Genet 125:1087–1096. https://doi.org/10.1007/s00122-012-1967-7
Lopes MS, El-Basyoni I, Baenziger PS et al (2015) Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J Exp Bot 66:3477–3486. https://doi.org/10.1093/jxb/erv122
Lucas SJ, Salantur A, Yazar S, Budak H (2017) High-throughput SNP genotyping of modern and wild emmer wheat for yield and root morphology using a combined association and linkage analysis. Funct Integr Genomics 17:667–685
Maccaferri M, Harris NS, Twardziok SO et al (2019) Signatures and future improvement targets. Nat Genet 51:885–895
Makumbi D, Alvarado G, Crossa J, Burgueño J (2018) Sashaydiall: a SAS program for hayman’s diallel analysis. Crop Sci 58:1605–1615. https://doi.org/10.1038/s41588-019-0381-3
Malchikov PN, Myasnikova MG (2016) Formation of gene associations that code for general homeostasis and performance components of durum wheat (Triticum durum Desf.). Russ J Genet Appl Res 6:357–366. https://doi.org/10.1134/S2079059716040146
Malik WA, Piepho HP (2018) Biplots: do not stretch them! Crop Sci 58:1061–1069. https://doi.org/10.2135/cropsci2017.12.0747
Mather K, Jinks JL (1982) Introduction to Biometrical Genetics. 3rd editoion
Millet E, Levy AA, Avivi L et al (1984) Evidence for maternal effect in the inheritance of grain protein in crosses between cultivated and wild tetraploid wheats. Theor Appl Genet 67:521–524. https://doi.org/10.1007/BF00264897
Mohammadi R (2019) The use of a combination scoring index to improve durum productivity under drought stress. Exp Agric. https://doi.org/10.1017/S0014479719000231
Mwadzingeni L, Shimelis H, Tesfay S, Tsilo TJ (2016) Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front Plant Sci 7:1276
Mwadzingeni L, Figlan S, Shimelis H et al (2017) Genetic resources and breeding methodologies for improving drought tolerance in wheat. J Crop Improv 31:648–672. https://doi.org/10.1080/15427528.2017.1345816
Mwadzingeni L, Shimelis H, Tsilo TJ (2018) Combining ability and gene action controlling yield and yield components in bread wheat (Triticum aestivum L.) under drought-stressed and nonstressed conditions. Plant Breed 137:502–513. https://doi.org/10.1111/pbr.12609
Peleg Z, Fahima T, Krugman T et al (2009) Genomic dissection of drought resistance in durum wheat × wild emmer wheat recombinant inbreed line population. Plant, Cell Environ 32:758–779. https://doi.org/10.1111/j.1365-3040.2009.01956.x
Peng J, Ronin Y, Fahima T et al (2003) Domestication quantitative trait loci in Triticum dicoccoides, the progenitor of wheat. Proc Natl Acad Sci 100:2489–2494. https://doi.org/10.1073/pnas.252763199
Reif JC, Gumpert FM, Fischer S, Melchinger AE (2007) Impact of interpopulation divergence on additive and dominance variance in hybrid populations. Genetics 176:1931–1934. https://doi.org/10.1534/genetics.107.074146
SAS Institute (2014) SAS 9.4 Output delivery system: User’s guide. SAS Institute
Sheibanirad A, Mirlohi A, Mohammadi R et al (2014) Cytogenetic and crossability studies in hulled wheat collected from Central Zagros in Iran. Plant Syst Evol 300:1895–1901. https://doi.org/10.1007/s00606-014-1015-y
Solomon KF, Labuschagne MT (2004) Inheritance of evapotranspiration and transpiration efficiencies in diallel F 1 hybrids of durum wheat (Triticum turgidum L. var. durum). Euphytica 136:69–79
Topal A, Aydin C, Akgün N, Babaoglu M (2004) Diallel cross analysis in durum wheat (Triticum durum Desf.): Identification of best parents for some kernel physical features. F Crop Res 87:1–12. https://doi.org/10.1016/j.fcr.2003.08.015
Terzi V, Morcia C, Stanca AM et al (2007) Assessment of genetic diversity in emmer (Triticum dicoccon Schrank) x durum wheat (Triticum durum Desf.) derived lines and their parents using mapped and unmapped molecular markers. Genet Resour Crop Evol 54:1613–1621. https://doi.org/10.1007/s10722-006-9173-6
Vaghar M, Ehsanzadeh P (2018) Comparative photosynthetic attributes of emmer and modern wheats in response to water and nitrogen supply. Photosynthetica 56:1224–1234. https://doi.org/10.1007/s11099-018-0825-5
Wardlaw IF, Willenbrink J (1994) Carbohydrate storage and mobilisation by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Funct Plant Biol 21:255–271
Xynias IN, Mylonas I, Korpetis EG et al (2020) Durum wheat breeding in the Mediterranean region: current status and future prospects. Agronomy 10:1–27. https://doi.org/10.3390/agronomy10030432
Zaharieva M, Ayana NG, Al HA et al (2010) Cultivated emmer wheat (Triticum dicoccon Schrank), an old crop with promising future: a review. Genet Resour Crop Evol 57:937–962. https://doi.org/10.1007/s10722-010-9572-6
Zhang Y, Kang MS, Lamkey KR (2005) DIALLEL-SAS05: a comprehensive program for Griffing’s and Gardner-Eberhart analyses. Agron J 97:1097–1106. https://doi.org/10.2134/agronj2004.0260
Zhang L-Y, Liu D-C, Guo X-L et al (2010) Genomic distribution of quantitative trait loci for yield and yield-related traits in common wheat. J Integr Plant Biol 52:996–1007
Acknowledgements
The authors are grateful to Dr. Parviz Ehsanzadeh and Dr. Mohsen Esmaeilzadeh Moghaddam for providing some of the genetic material.
Author information
Authors and Affiliations
Contributions
M. Mohammadi developed the plant material and designed the experiment, recorded traits, analyzed the data, and wrote the manuscript; Prof A. Mirlohi and Prof M. Majidi was involved in designing and supervising the experiments, data interpretation, and critically revising the intellectual content of the manuscript, and critically reviewing the manuscript; E. Soleimani Kartalaei was involved in recording the traits.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi, M., Mirlohi, A., Majidi, M.M. et al. Emmer wheat as a source for trait improvement in durum wheat: a study of general and specific combining ability. Euphytica 217, 64 (2021). https://doi.org/10.1007/s10681-021-02796-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-021-02796-x