Abstract
The aim of this study was to assess morphological variation in wild Rosa L. germplasm from the Western Himalayan Region that includes the geographical area of two Union territories (Jammu and Kashmir [J&K] and Ladakh) and one state (Himachal Pradesh [HP]) of India. Field trips in different locations of J&K, Ladakh, and HP from 2014 to 2018 were undertaken in different seasons to collect accessions of wild Rosa species. A total of 59 accessions belonging to six wild Rosa species (Rosa canina L., R. foetida var. persiana [Lem.] Rehder, R. macrophylla Lindl., R. moschata Herrm., R. multiflora Thunb. and R. webbiana Wall ex. Royle) were collected. Fifty-five vegetative and reproductive characters (39 qualitative and 16 quantitative characters) were recorded for all of the accessions to determine the most significant morphological differences among the six species and among accessions of the same species. Phenotypic variability among the studied accessions was evaluated using descriptive statistics, principal component analysis (PCA), and cluster analysis. Among the quantitative characters analyzed, the coefficient of variation (CV) was highest for the number of rose hips per inflorescence and lowest for petal length; for the qualitative characters, the CV was highest for rose hip shape and lowest for sepal prickles. Results of the PCA indicated that the first six components accounted for 65.44% of the total variation. Leaflet length displayed a significant positive correlation with leaflet width, petiole length and pedicel length. Petal length, petal breadth, and flower diameter were positively correlated with each other. Hip length showed a positive correlation with hip width and a negative correlation with pedicel length, and hip color. Hip number per inflorescence showed a positive correlation with leaflet length, leaflet breadth, petiole length, pedicel length, and a strong negative correlation with prickle length. Clustering analysis categorized the Rosa accessions into two clusters and several groups and subgroups, indicating a high level of morphological variation in the germplasm. The key traits among the species identified to have a high discriminating value were leaflet length, leaflet breadth, prickle shape and size, stipule margin, style nature, sepal margin, flower color, and size and shape of hips. These results indicate that there is a high potential for obtaining desirable trait combinations from wild Rosa germplasm of the Western Himalaya Region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Rosa L. belongs to the family Rosaceae and comprises about 200 species (Wissemann 2003) distributed throughout the temperate and sub-tropical habitats of the northern hemisphere (Gu and Robertson 2003). The majority of wild rose species are found in Asia, which is one of the major gene centers of these species (Broertjes and van Harten 1978). Various species of Rosa have adapted to the conditions found between 500 and 4700 m a.s.l. in the Indian Himalayan Region (Hooker 1879; Duthie 1971; Bamber 1976; Ambasta 1986; Pal 1991; Tejaswini and Prakash 2005). During the adaption process, these species are likely to have evolved into distinct taxa with the potential to develop into varieties and potential strains that can play a key role in the development of future roses.
A noteworthy feature of rose diversity is the availability of variation in almost all visible morphological characters. This wealth of indigenous germplasm is an important source of many important commercially valued traits, such as perpetual flowering, winter hardiness, fragrance, color, thornlessness, among others. The extremely large phenotypic versatility in subgenus Rosa L. has often represented a challenge to botanists, and many species of Rosa are remarkably polymorphic and possess different geographical races and ecotypes that are not yet understood. Indeed, species delimitation is so difficult in this subgenus that can become almost impossible to differentiate even between different morphotypes of the same species and the hybrids. This awkward identification of hybrids is caused by the mixed presence of parental, intermediate, transgressive, and newly developed characteristics (Rieseberg and Ellstrand 1993; Werlemark and Nybom 2001). The enormous phenotypic, genotypic, and ecological variability and plasticity in this genus may be due to one or more evolutionary processes, such as hybridization and introgression or others (De Cock 2008).
Roses have been popularized and habitualized throughout the world for their important medicinal and ornamental value (Hummer and Janick 2009). It is one of the more important ornamental plants due to it being a garden plant, a cut flower and a source of essential oil. During recent years, wild roses have become a major focus of research, not only in genetic studies but in other fields. Rose hips, the pseudo-fruits of the rose plant, are utilized in food products in many European countries (Gao et al. 2005) as well as for medicinal purposes (Warholm et al. 2003; Rein et al. 2004; Boskabady et al. 2006). Various phytochemicals reported in rose hips have medicinal properties, including vitamin C (Demir and Ozcan 2001; Roman et al. 2013), quercetin and ellagic acid (Tumbaset et al. 2011), β-carotene and lycopene (Hodisanet et al. 1997). Recently, anti-inflammatory and chondroprotective (Schwager et al. 2014; Marstrand and Campbell-Tofte 2016), anti-ulcerogenic (Gurbuz et al. 2003), anti-oxidant (Gao et al. 2005; Roman et al. 2013), anti-arthritic (Winther 2014; Marstrand and Campbell-Tofte 2016), anti-mutagenic (Karakaya and Kavas 1999) and anti-obese (Nagatomo et al. 2015) properties have also been demonstrated in extracts from various Rosa L. species. Wild roses are important source of valuable germplasm for creating variability in and improving cultivated roses, with the potential to satisfy future needs (Dhyani and Singh 2014).
The aim of this study was to assess morphological variation in wild Rosa L. germplasm from the Western Himalayan Region that includes the geographical area of two Union territories (Jammu and Kashmir [J&K] and Ladakh) and one state (Himachal Pradesh [HP]) of India. Evaluation of important morphological characters was also conducted to identify wild Rosa accessions with good potential for use in rose breeding programs.
Materials and methods
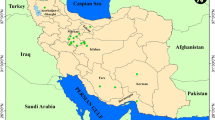
The study area comprised the Western Himalayan Region and included the two Indian Union territories of J&K and Ladakh and the Indian state of HP. The region shares a boundary with China to the east and Pakistan to the west (Fig. 1) and has a total surface area of 3,31,392 km2 ranging between latitudes 28° 43′ and 37° 05′ N and longitudes 72° 31′ and 81° 02′ E. Owing to its multiplicity of habitats, wide range in altitude, conglomerate topography and unique geographic location, this region is home to a rich diversity of plants.
During the study period, field visits were carried out in different parts of the study area in different seasons between 2014 and 2018 to collect accessions of wild Rosa species. Ultimately, a total of 59 accessions belonging to six wild Rosa species were collected from the different parts of the study area (Table 1; Fig. 2). To identify the specimens, we consulted various monographs (Andrews 1805; Lewis 1957, 1958; Baker 1869), regional Floras (Singh and Kachroo 1976; Kachroo et al. 1977; Sharma and Kachroo 1981; Chowdhery and Wadhwa 1984), electronic Floras (eFloras 2019), eFlora of India (eFI 2018) and regional herbaria (Janaki Ammal Herbarium [RRLH] of CSIR–Indian Institute of Integrative Medicine [IIIM], Jammu, J&K, India; Herbarium of University of Jammu, J&K). Duly identified specimens were submitted to the internationally recognized RRLH at CSIR–IIIM.
In total, we examined 55 morphological characters (39 qualitative and 16 quantitative traits) in all specimens, both in their natural state and in the laboratory (Tables 2, 3). For each of the six wild species of Rosa identified, a due effort was made to collect as many accessions as possible. Most traits selected for measurement in this study were chosen based on traits documented in previous studies on rose (Khatamsaz 1992; Olsson et al. 2000; Werlemark and Nybom 2001; Koobaz et al. 2009; Cheikh-Affeneet et al. 2015), with modifications. Rose descriptors developed by the National Bureau of Plant Genetic Resources (NBPGR; Regional Station, Phagli, Shimla, HP, India; Rathore and Srivastava 1992) were used with modification for assigning descriptor nodes for the documentation of morphological traits. For the morphological studies, we randomly selected four plants from each accession for study. The methodology of De Cock (2008) was followed for leaflet measurements. Flower traits were analyzed at the flowering stage. Hips (fruits) were collected for measurements at the ripened stage. All measurements were made with a Vernier caliper with an accuracy of 0.1 mm.
Statistical analysis
Data were analyzed for variance, and comparison of means were performed with Tukey’s post hoc test using Minitab 17 (Minitab, LLC, State College, PA, USA). Coefficients of variation (CV) were determined as indicators of variability. A correlation analysis was ascertained using the Pearson correlation coefficient for the assessment of relationships between some economically important morphological traits (both quantitative and qualitative) in the studied Rosa species. For the grouping of the wild Rosa accessions based on similarities in their morphological traits, we performed principal component analysis (PCA) and cluster analysis with the unweighted pair group method with arithmetic mean (UPGMA) using the PAST 3.22 software program (Hammer et al. 2001).
Results
Phenotypic characterization of Rosa accessions
A wide range of phenotypic plasticity was observed in the studied morphological characters (both qualitative and quantitative traits) of the wild Rosa species collected in this study. The range in the variation found for these floral and vegetative morphological traits (qualitative) in these wild Rosa species is presented in Table 3. Among the qualitative traits, maximum variation was observed for hip shape followed by leaflet shape and leaflet number. Prickles were present in various forms, ranging from curved in R. moschata and R. multiflora, straight in R. webbiana and R. foetida var. persiana, straight to subulate in R. macrophylla and hooked in R. canina. The distribution pattern of prickles along the stem and branches also showed variation in the studied accessions. All accessions of R. moschata, R. canina, R. multiflora, and R. foetida var. persiana displayed the moderately dense distribution of prickles, while accessions of R. webbiana and R. macrophylla showed a mixed-type distribution pattern. This overall range in the distribution pattern of prickles and their different forms are in accordance with the results of studies carried out by Debener and Linde (2009). One prickleless accession (F-4) of R. macrophylla was found during the field study, which is in consonance with results documented in the literature. As prickles are considered to be an undesirable trait in commercial rose cultivation, particularly in terms of cultivation and harvesting, the search for thornless cultivars has been a key breeding objective of almost all rose breeding programs (Zlesak 2007; Kanli and Kazaz 2009). Large-sized sticky glandular hairs were found on the hip surface of R. macrophylla accession F-1 and F-6; these may be an adaptation against herbivory.
One new observation recorded was the presence of fine needle-shaped prickles on the sepal surface of accession F-6, which was collected from the Gurez Valley, Kashmir. Some leaflets of accessions F-1 and F-8 have a glandular abaxial surface, which is one of the characters of R. macrophylla var. glandulifera, suggesting that different Rosa species intermediates may occur naturally, possibly as the result of frequent and unrestricted crossing in wild Rosa. Wild Rosa species harbor different alleles that can be incorporated by introgression into various other species of Rosa as well as into other important crop plants for generating variability. Of all the species of Rosa studied here, the petals of R. moschata possess the strongest scent, thereby suggesting its potential use in the aroma industry. However, further studies are required to actually prove the economic potential of the oil of these Rosa species.
Prickle shape, leaflet shape, stipule and sepal attributes, filament color, nature of style, and shape of hips were found to be major discriminating qualitative traits in some of the studied species in terms of species delimitation. However, the contribution of other qualitative characters in affecting either species delimitation or accession discrimination cannot be ignored, as observed in our study.
The descriptive statistical results for each of the 16 quantitative characters studied are shown in Table 3. For the quantitative traits, as indicated by the value of the CV (Table 3), the lowest variation was documented for petal length (10.03%), and the highest was documented for hip number/inflorescence (96.83%). The results of the comparison of means of the studied quantitative characters using Tukey’s post hoc test is shown in Table 4. Mean values of quantitative traits with the standard error of each accession growing wild in the Western Himalayan Region are given in Electronic Supplementary Material (ESM) Table 2. The highest mean values for leaflet length and leaflet breadth were recorded for R. moschata accessions A-4, and A-5, and the lowest (minimum) values were recorded for accession R. webbiana accession B-1 (Table 4). In terms of petal and flower dimensions, the highest mean values for petal length and petal breadth were determined for R. webbiana accession B-2, and the lowest values were found for R. webbiana accession B-17 and R. moschata accession A-5 (Table 4). Mean values for hip length (HL) ranged from 5.6 mm in R. multiflora accession E-1 to 28.45 mm in R. macrophylla accession F-3, and those for hip width ranged from 4.45 mm in R. multiflora accession E-1 to 12.35 mm in R. macrophylla accession F-5. Hips did not develop in R. foetida accession D-1. HNI ranged from zero in R. foetida accession D-1 to 17.75 in R. moschata A-18. The highest number of achenes per hip were found in R. moschata accession B-10, and the lowest number was found in R. multiflora accession E-1 (Table 4). Flower size, fruit size, and achene number should probably be among the first characteristics to be considered by the rose breeders as goals in selection programs, with the aim to achieve more benefits from the presence of flowers, fruits, and achenes as these plant parts have the maximum aromatic potential. This aromatic potential is especially important in the perfume and cosmetics industry (De Groot and Schmit 2016) and the pharmaceutical industry (Larsen et al. 2003; Nagatomo et al. 2015).
Analysis of our collected samples of wild Rosa accessions revealed a wide variation in leaflet number, ranging from 3 to 13 leaflets. The leaflet number in R. moschata was found to range from three to nine, which corresponds with the earlier documented range of three to seven leaflets for this species (Singh et al. 2017). In R. canina, the leaflet number ranged from three to seven leaflets, which is not in agreement the earlier reported range of five to seven leaflets as documented in different floras. Similarly, in R. macrophylla, leaflet number ranged from 3 to 13 leaflets, which is not consonance with the earlier range of 5–13 leaflets recorded for this species in different floras.
Correlations among morphological traits (quantitative and qualitative) in the studied wild Rosa accessions of the Western Himalayan Region, India are shown in ESM Table 1. Given the economic importance of the leaves, flower hips (fruits) and achenes of wild Rosa species, we determined the correlations among various morphological quantitative traits (Table 5). According to the results of our study, leaflet length showed a significant positive correlation with leaflet width (r = 0.922), petiole length (r = 0.830), pedicel length (r = 0.670), prickle length (r = 0.840), hip number per inflorescence (r = 0.862) and petal color (r = 0.572) and a negative correlation with flower fragrance (r = − 0.840). Petal length, petal breadth and flower diameter had a strong positive correlation with each other (r = 0.896 and 0.635, respectively). Flower diameter showed a negative correlation with pedicel length (r = − 0.100). Hip length showed a strong positive correlation with hip width (r = 0.732), and hip width displayed a strong negative correlation with pedicel length (r = − 0.605) and hip color (r = − 0.690). Hip number per inflorescence showed a positive correlation with leaflet length (r = 0.862), leaflet breadth (r = 0.749), petiole length (r = 0.655) and pedicel length (r = 0.642) and a strong negative correlation with prickle length (r = − 0.794). No comparable morphometric studies on wild Rosa species have been conducted to date that can be used for comparison with the pattern observed in the present study.
Principal component analysis
Principal component analysis has been previously used to study the morphometric variations in wild rose populations from Tunisia (Cheikh-Affene et al. 2015). In our study, the results of PCA on the morphological traits indicated that the first six principal components (PCs) accounted for 65.44% of the total variation (Table 6). According to Reim et al. (2012), such results indicate a very high level of morphological variation, symbolizing a high genetic diversity between the accessions and suggesting that in terms of full characterization of the accession, evaluation of the different morphological traits is still necessary. The first two PCs explained about 41.70% of the total observed variability (Fig. 3), with PC1 presenting 30.36% of the variation and featuring maximum values for a number of characters, such as leaflet length, leaflet breadth, petiole length, pedicel length, hip length, hip width, prickle length, number of hips per inflorescence, prickle shape, leaf hairs on adaxial surface, leaflet shape, leaflet apex and base, bract number, bract shape, sepal edge, sepal permanency, sepal apex, petal apex, petal color, flower fragrance, style nature, anther color, hairs on hips, hip color, and hip shape. PC2 explained 11.34% of the total variance and was related to stipule length, stipule breadth, sepal length, sepal breadth, and prickle shape.
Cluster analysis
The dendrogram obtained with the UPGMA method exhibits diverse hierarchical levels of wild Rosa accessions and is separated into two main clusters (Fig. 4), namely Cluster A and Cluster B, on the basis of the long pedicel length, absence of hips, leaflet number, petal color and flower fragrance possessed by Rosa foetida var. persiana of cluster B. Cluster A can be seen to encompass accessions of five species of wild Rosa (R. moschata, R. multiflora, R. canina, R. macrophylla, R. webbiana) and is divided into two groups A1 and A2 on the basis of the shorter prickle length, hip number per inflorescence, ovate-to-lanceolate leaflet shape, connate style, roundish hip shape and reddish-orange hip color of R. moschata of group A1. Group A2 segregates into subgroup A2a and A2b on the basis of the shorter hip length and width, smaller number of achenes, presence of only two pinna along the sepal margins, and connate and glabrous style possessed by R. multiflora of subgroup A2a. Subgroup A2b forms two more subgroups, A2ba and A2bb, based on the shorter leaflet length and breadth, stipule breadth, and stipule length of R. webbiana. R. canina accessions of subgroup A2ba differ from R. macrophylla accessions in having hooked prickles, an absence of glands on hips and pedicel, bract number (1), deciduous sepals and urceolate-to-ovoid hip shape, which was also justified by their position in the dendrogram.
Dendrogram of grouping for the 59 accessions belonging to the six wild Rosa species collected from the Western Himalaya Region, India using the PAST 3.22 software program (Hammer et al. 2001)
All other accessions of different species were resolved into relatively homogenous groups, which suggested that morphological distinction and species delimitation in wild Rosa species is extremely difficult, possibly due to their morphological similarity together with extensive hybridization in the wild (Guoliang 2003).
Discussion
In this study we found wild roses to be distributed from the subtropical areas of Kathua, J&K (accession A-1, 792 m a.s.l.) to the higher arid regions of Nyoma, Leh, Ladakh (accession B-14, 4504 m a.s.l.), indicating the robustness and adaptability of these species to this wide range of climatic conditions. The altitudinal range of wild Rosa species in the Indian Himalaya Region has also been recorded in earlier studies (Hooker 1879; Gupta 1979; Collet 1984; Sharma and Jamwal 1988; Pal 1991).
Rosa species collected from the Western Himalayan Region, India, in this study portrayed a wide range of morphological variability in both quantitative and qualitative characters. Clustering analysis also proved that the morphological variability in the accessions of the different Rosa species is of a continuous nature and affirmed strong overlaps between the different traits. The similarity observed in the characters relating to flower, fruit, and leaflet morphology in some accessions of R. macrophylla and R. webbiana in our study hint towards the possibility that these two species frequently undergo hybridization in the wild, thus making their identification difficult, as encountered in this study. However, a few traits that can be used to discriminate these two species are reported in the literature. One example is the presence of a caudate sepal apex and long pedicel length in R. macrophylla; however, these characters are also found in the accessions of R. webbiana. A similar taxonomic problem was also reported at the diploid level by Lewis (1962) in R. blanda and R. woodsii, but the problem is much more complex at the polyploidy level as polyploidy is very common in wild rose (Fougere-Denezan et al. 2015). Therefore, molecular approaches are also required to achieve a proper resolution of species identification in this region.
The dendrogram obtained with the UPGMA method categorized accessions within a species into different groups and subgroups (Fig. 4), suggesting that there is genetic variation which might be the outcome of unrestricted crossing between wild relatives of different Rosa species. The intermediates that result from this genetic exchange could be exploited through direct selection to obtain higher yield of the particular trait being studied. Similar results have been recorded in apple and papaya by various researchers (Coart et al. 2003; Mratinic and Aksic 2012; Chavez-Pesqueria et al. 2014). According to Vanhala et al. (2004), wild accessions contain important variations in their genetic makeup that are vital for the development of highly improved modern cultivars characterized by domestication and narrow genetic backgrounds. For the patenting and registration of varieties, a description of the morphological character is the customary methodology accepted from a legal point of view (Badeness et al. 1998).
We determined that some of the wild Rosa species collected have many favourable traits and thus would be good sources of germplasm for use in breeding programs. The comparatively smaller leaflet size in R. webbiana accessions indicate an adaptive response to water stress and drought, and this trait can be manipulated in breeding programs aimed at developing drought-resistant varieties. Accessions F-5, F-3 and A-18 can be directly selected for the production of long hips, wide hips and a higher number of hips per inflorescence, respectively. Similarly, as the petals of roses have immense economic potential in the aroma sector, larger flower size can be obtained by the direct selection of R. webbiana accession B-2. Morphological markers have been used for germplasm characterization in earlier studies (Veasey et al. 2001; Rakonjac et al. 2010), but morphological traits alone may not be sufficient for determination of the relationship among species (Llyod et al. 1992). Molecular markers, such as randomly amplified polymorphic DNA (RAPD; Wen et al. 2004), amplified fragment length polymorphism (AFLP; Panwar et al. 2015), inter-simple sequence repeat (ISSR; Ogras et al. 2017) and start codon targeted markers (SCoT; Agarwal et al. 2019), have been used to study the genetic diversity in wild Rosa. Jabbarzadeh et al. (2013) used ISSR analysis to study R. canina and R. moschata and found > 53% genetic similarity between these two species. To date, no genetic diversity assessment has been conducted on R. webbiana and R. macrophylla. A genetic diversity assessment of our collection using molecular markers would provide valuable information for further characterizing the genetic background of the Western Himalayan wild Rosa species.
The results of our study demonstrate that the wild species of Rosa carry important agronomic traits, such as leaflet length, flower diameter, number of achenes, number of hips per inflorescence and length and width of hips, that can be used to further improve the quality of cultivated Rosa species by introgressive hybridization. Our findings on wild Rosa species prove the complex nature of this morphologically variable genus. It is therefore necessary to make due efforts for the continuous collection, conservation, characterization and evaluation of new accessions and new species from the wild, with a particular focus on averting the loss of wild Rosa germplasm. In addition, the creation of a Rosa germplasm repository is essential. Rosa germplasm from wild genotypes are needed to expand the diversity of this important genetic resource and to utilize the genetic potential of these genotypes for improvement of traits needed for adaptation to various conditions in near future.
References
Agarwal A, Gupta V, Haq S, Jatav PK, Kothari SL, Kachhwaha S (2019) Assessment of genetic diversity in 29 rose germplasm using SCoT marker. J King Saud University–Science 31(4):780–788.
Ambasta SP (1986) The useful plants of India. Publication and Information Directorate, CSIR, New Delhi
Andrews HC (1805) Roses, or, a monograph of the genus Rosa: containing colored figures of all known species and beautiful varieties. R Taylor and Co, London
Badeness ML, Martinez-Calvo J, Llacer G (1998) Analysis of apricot germplasm from the European ecogeographical group. Euphytica 102:93–99
Baker JG (1869) A monograph of the British roses. J Linn Soc Lond Bot 11:197–243
Bamber CJ (1976) Plants of the Punjab, North West Frontier Province and Kashmir. Bishen Singh Mahendra Pal Singh, Dehra Dun
Boskabady MH, Kiani S, Rakshandah H (2006) Relaxant effects of Rosa damascene on guinea pig tracheal chains and its possible mechanism(s). J Ethnopharmacol 106:377–382
Broertjes C, van Harten AM (1978) Application of mutation breeding methods in the improvement of vegetatively propagated crops. Elsevier, Amsterdam
Chavez-Pesqueira M, Suarez-Montes P, Castillo G, Nunez Farfan J (2014) Habitat fragmentation threatens wild populations of Carica papaya (Caricaeae) in a lowland rainforest. Am J Bot 101:1092–1101
Cheikh-Affene ZB, Haouala F, Harzallah-Skhiri F (2015) Morphometric variation and taxonomic identification of thirteen wild rose populations from Tunisia. Acta Bot Croat 74:1–17
Chowdhery HJ, Wadhwa BM (1984) Flora of Himachal Pradesh analysis, vol 1. B.S.I. Publication, Delhi
Coart E, Vekemanas X, Smudlers MJM, Wagner I, Huylenbroeck VJ, van Bockstaele E (2003) Genetic variation in endangered apple (Malus sylvestris (L) Mill.) in Belgium as revealed by amplified fragment length polymorphism and microsatellite markers. Mol Ecol 12:845–857
Collet H (1984) Flora simlensis. International Book Distributors, Dehra Dun
De Cock K (2008) Genetic diversity of wild roses (Rosa spp.) in Europe, with an in-depth morphological study of Flemish populations. PhD thesis. Faculty of Bioscience Engineering, Ghent University, Ghent
De Groot AC, Schmidt E (2016) Essential oils: contact allergy and chemical composition. CRC Press, Boca Raton
Debener T, Linde M (2009) Exploring complex ornamental genomes. The rose as a model plant. Crit Rev Plant Sci 28:267–280
Demir F, Ozcan M (2001) Chemical and technological properties of rose (Rosa canina L.) fruits grown wild in Turkey. J Food Eng 47:333–336
Dhyani D, Singh S (2014) Potential wild rose germplasm of Western Himalayas—conservation, evaluation and registration. Indian J Agr Sci 84:229–235
Duthie JF (1971) Flora of the Upper Gangetic Plain: and of the adjacent Shiwalik and Sub-Himalayan tracts, vol. 1. International Book Distributors, Uttarakhand
eFI (2018) Eflora of India. https://sites.google.com/site/efloraofindia. Accessed 23 Aug 2018
eFloras (2019) Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge. http://www.efloras.org Accessed 22 Feb 2019
Fougere-Denezan M, Joly S, Bruneau A, Gao XF, Zhang LB (2015) Phylogeny and biogeography of wild roses with specific attention to polyploids. Ann Bot 115:275–291
Gao X, Uggla M, Rumpunen K (2005) Antioxidant of dried and boiled rose hips. In: Nybom H, Rumpunen K (eds) Proc 1st Int Rose Hip Conf, 7–10. September 2004. International Society for Horticultural Science, pp 239–249
Gu C, Robertson KR (2003) Rosa L. In: FoCe Team (ed) Flora of China. Missouri Botanical Garden Press, St. Louis, pp 339–381
Guoliang W (2003) Ancient Chinese roses. In: Roberts A, Debener T, Gudin S (eds) Encyclopedia of rose science. Elsevier, Amsterdam, pp 387–395
Gupta BL (1979) Forest flora of the Chakrata, Dehra Dun and Saharanpur Forest Division, United Provinces. Jayyed Press, Delhi, pp 220–223
Gurbuz I, Ustun O, Yesilada E, Sezik E, Kutsal O (2003) Antiulcerogenic activity of some plants used as folk remedy in Turkey. J Ethnopharmacol 88:93–97
Hammer O, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hodisan T, Socaciu C, Ropan I, Neamtu G (1997) Carotenoid composition of Rosa canina fruits determined by thin-layer chromatography and high-performance liquid chromatography. J Pharm Biomed 16:521–528
Hooker JD (1879) Flora of British India, vol 2. L. Reev & Co Ltd, Kent
Hummer KE, Janick J (2009) Rosaceae: taxonomy, economic importance, genomics. In: Folta KM, Gardiner SE (eds) Plant genetics and genomics 6: genetics and genomics of Rosaceae. Springer, New York, pp 339–351
Jabbarzadeh Z, Khosh-khui M, Salehi H, Shahsavar AR, Saberivand A (2013) Assessment of genetic relatedness in roses by ISSR markers. World Appl Sci J 28:2085–2090
Kachroo P, Bansilal S, Dhar U (1977) Flora of Ladakh: an ecological and taxonomical appraisal. International Book Distributors, Dehradun
Kanli FA, Kazaz S (2009) Biotechnology of roses: progress and future prospects. Turk J For 1:167–183
Karakaya S, Kavas A (1999) Antimutagenic activities of some foods. J Sci Food Agric 79:237–242
Khatamsaz M (1992) Flora of Iran (Rosaceae) (Vol 6). Research Institute of Rangeland and forests, Tehran
Koobaz P, Kermani MJ, Hosseini ZS, Khatamsaz M (2009) Inter and Intraspecific morphological variation of four Iranian rose species. Global Science Books, Ikenobe, Kagawa
Larsen E, Kharazmi A, Christensen LP, Christensen SB (2003) An antiinflammatory galactolipid from rose hip (Rosa canina) that inhibits chemotaxis of human peripheral blood neutrophils in vitro. J Nat Prod 7:994–995
Lewis WH (1957) A monograph of the genus Rosa in North America east of the Rocky Mountains. PhD thesis. University of Virginia, Charlottesville
Lewis WH (1958) Minor forms of North American species of Rosa. Rhodora 60:237–243
Lewis WH (1962) A monograph of the genus Rosa in North America. R. dulcissima. Britonnia 14:65–71
Llyod A, Walbot V, Davis RW (1992) Arabidopsis and Nicotiana anthocyanin production activated by maize regulators R and Cl. Science 258:1773–1775
Marstrand K, Campbell-Tofte J (2016) The role of rose hip (Rosa canina L) powder in alleviating arthritis pain and inflammation–part II animal and human studies. Bot Targets Ther 6:59–73
Mratinic E, Aksic MF (2012) Phenotypic diversity of (Malus sp.) germplasm in south Serbia. Braz Arc Bio Tecnol 55:349–358
Nagatomo A, Nishida N, Fukuhara I (2015) Daily intake of rosehip extract decreases abdominal visceral fat in preobese subjects: a randomized, double-blind, placebo-controlled clinical trial. Diabetes Metab Syndr Obes 8:147–156
Ogras TL, Bastanlar EK, Metin OK, Kandemir I, Ozcelik H (2017) Assessment of genetic diversity of rose genotypes using ISSR markers. Turkish J Bot 41:347–355
Olsson A, Nybom H, Prentice HC (2000) Relationships between Nordic Dogroses (Rosa L. sect. Caninae, Rosaceae) assessed by RAPDs and elliptic Fourier analysis of leaflet shape. Syst Bot 25:511–521
Pal BP (1991) The rose in India. Publication and Information Division, ICAR, New Delhi
Panwar S, Singh KP, Sonah H, Deshmukh RK, Namita Prasad KV, Sharma TR (2015) Molecular fingerprinting and assessment of genetic diversity in rose (Rosa × hybrida). Indian J Biotechnol 14:518–524
Rakonjac V, Aksic MF, Nikolic D, Milatovic D (2010) Morphological characterization of ‘Oblacinska’ sour cherry by multivariate analysis. Sci Hortic 125:679–684
Rathore DS, Srivastava UC (1992) Rosa species (A bulletin). National Bureau of Plant Genetic Resources, Regional station, Phagli, Shimla
Reim S, Proft A, Heinz S, Hofer M (2012) Diversity of the European indigenous wild apple Malus sylvestris (L.) Mill. In the East Ore Mountains (Osterzgebirge), Germany: I morphological characterization. Genet Resour Crop Evol 59:1101–1114
Rein E, Kharazmi A, Winther K (2004) An herbal remedy, Hyben Vital (stand. powder of subspecies of Rosa canina fruits), reduces pain and improves general well being in patients with osteoarthritis. A double-blind, placebo-controlled, randomized trial. Phytomedicine 11:383–391
Rieseberg LH, Ellstrand NC (1993) What can molecular and morphological markers tell us about plant hybridisation? Crit Rev Plant Sci 12:213–241
Roman I, Stanila A, Stanila S (2013) Bioactive compounds and antioxidant activity of Rosa canina L biotypes from spontaneous flora of Transylvania. Chem Cent J 7:73
Schwager J, Richard N, Schoop R, Wolfram S (2014) A novel rose hip preparation with enhanced anti-inflammatory and chondroprotective effects. Mediators of Inflammation 2014:105710
Sharma BM, Jamwal PS (1988) Flora of Upper Liddar Valley of Kashmir Himalayas, vol 1. Scientific Publisher, Jodhpur, pp 197–198
Sharma BM, Kachroo P (1981) Flora of Jammu and plants of neighborhood, vol 1. Bishen Singh Mahendra Pal Singh, Dehra Dun
Singh G, Kachroo P (1976) Forest flora of Srinagar and Plants of neighborhood. Bishen Singh Mahendra Pal Singh, Dehra Dun
Singh S, Dhyani D, Nag A, Sharma RK (2017) Morphological and molecular characterization revealed high species-level diversity among cultivated, introduced and wild roses (Rosa sp.) of western Himalayan region. Genet Resour Crop Evol 64:515–530
Tejaswini MS Prakash (2005) Utilization of wild rose species in India. Acta Hortic 690:91–96
Tumbas VT, Canadanovic-Brunet JM, Cetojevic-Simin DD, Cetkovic GS, Dilas SM, Gille L (2011) Effect of rose hip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J Sci Food Agric 92:1273–1281
Vanhala TK, van Rijn CPE, Buntjer J, Stam P, Nevo E, Poorter H, van Eeuwijk FA (2004) Environmental, phenotypic and genetic variation of wild barley (Hordeumspontaneum) from Israel. Euphytica 137:297–304
Veasey EA, Schammas EA, Vencovsky R, Martins PS, Bandel G (2001) Germplasm characterization of Sesbania accessions based on multivariate analysis. Genet Resour Crop Evol 48:79–90
Warholm O, Skaar S, Hedman E (2003) The effects of standardized herbal remedy made from subtype of Rosa canina in patients with osteoarthritis: a double-blind, randomized, placebo-controlled clinical trial. Curr Ther Res 64:21–31
Wen XP, Pang XM, Dang XY (2004) Characterization of genetic relationship of Rosa roxburghii Tratt. and its relatives using morphological traits RAPD and AFLP markers. J Hortic Sci Biotech 79:189–196
Werlemark G, Nybom H (2001) Skewed distribution of morphological character scores and molecular markers in three interspecific crosses in Rosa section Caninae. Hereditas 134:1–13
Winther K (2014) Low-dose seed and shell powder from rose-hip (Rosa canina) can alleviate symptoms of osteoarthritis and reduce C-reactive protein in patient suffering from osteoarthritis (abstract). Osteoarthr Cartilage 22:321–322
Wissemann V (2003) Conventional taxonomy wild (roses). In: Roberts AV, Debener T, Gudin S (eds) Encyclopedia of rose science. Elsevier, Amsterdam, pp 111–117
Zlesak DC (2007) Rose. In: Anderson NO (ed) Flower breeding and genetics: issues, challenges and opportunities for the 21st Century, Springer, Dordrecht, pp 695–737
Acknowledgements
The authors thank the director of IIIM Jammu for providing the necessary facilities to carry out the work. They are grateful to the Science and Engineering Research Board Department of Science and Technology (SERB DST), Government of India for financial assistance under Start Up Research Grant (Young Scientist), grant No YSS/2015/000442. KS acknowledges the financial assistance provided by Council for Scientific and Industrial Research (CSIR) in the form of JRF/SRF fellowship. CSIR-IIIM Publication No.- CSIR-IIIM/IPR/00139.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, K., Sharma, Y.P. & Gairola, S. Morphological characterization of wild Rosa L. germplasm from the Western Himalaya, India. Euphytica 216, 41 (2020). https://doi.org/10.1007/s10681-020-2567-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-2567-2