Abstract
The Japanese bunching onion, Allium fistulosum L., is an important vegetable in East Asia. However, the stone leek leafminer, Liriomyza chinensis (Kato), is a serious insect pest that invades the Allium species. As the feeding punctures on the leaf surface caused by the female adults as well as the larval mining inside the unifacial leaves reduce the commercial value of A. fistulosum, it is essential to control L. chinensis during its growth. The accession ‘Beicong’ has shown resistance to L. chinensis due to its egg-killing defense, but the degree of resistance varies within the ‘Beicong’ population. Therefore, in the present study, we selected highly resistant selfed lines from the ‘Beicong’ population by artificially inoculating L. chinensis eggs into the leaves to breed A. fistulosum with a resistance to L. chinensis. A highly resistant line was obtained by continuously self-pollinating the resistant ‘Beicong’ individual among the 191 individuals, which was inherited in the F1 hybrid of the resistant line and the susceptible variety. However, the F1 hybrid’s degree of resistance was an intermediate of the two parents’. It was also revealed that the resistance of ‘Beicong’ was due to both egg- and larval-killing defense mechanisms by artificially inoculating the eggs and larvae into the leaves. Hence, developing a highly resistant line could contribute to integrated pest management in A. fistulosum cultivation by decreasing the population of L. chinensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Japanese bunching onion, Allium fistulosum L., is one of the most economically important crops among the Allium species in East Asia, especially in Japan, Korea, and China (Kumazawa and Katsumata 1965; Ford-Lloyd and Armstrong 1993). In Japan, the 2018 production volume of A. fistulosum was approximately 146.6 billion Japanese Yen and is ranked the fourth highest among the vegetables (MAFF 2018). Both leaf parts, including the blade and sheath, are edible, so it is important to cultivate them by controlling infections and pests during their growth. The stone leek leafminer, Liriomyza chinensis (Kato) (Diptera: Agromyzidae), is a harmful insect pest that invades A. fistulosum in Japan, Korea, China, and other Asian countries (Chen et al. 2003; Choi et al. 2003; Tokumaru 2016; Tran and Takagi 2005). Furthermore, L. chinensis feeds on Allium plants and has been expanding its habitat in Eastern Europe (Martinez 1982; Martynov et al. 2016; Papp and Cerny 2017). Female adults puncture the epidermis by the ovipositor and cause feeding punctures. In addition, they insert the ovipositor in the leaves for oviposition, where the hatched larvae in the unifacial leaves feed on the inner parts of the leaves. In essence, the feeding punctures generated by the female adults and the larval mining reduce the production value of A. fistulosum. The number of next-generation L. chinensis individuals produced by one female adult was the largest at 25 °C (Tokumaru 2016), and the L. chinensis epidemic in Japan appears between spring and autumn. Therefore, there is concern regarding the extended occurrence period caused by global warming, which leads to an average temperature rise in Japan (+ 1.49 °C/100 years from March to May and + 1.23 °C/100 years from September to November; Japan Meteorological Agency 2020). Furthermore, since a different biotype of L.chinensis (biotype B) was identified in Japan from 2016 and the larval mining is more severe than that of the conventional L. chinensis (biotype A), it is necessary to control L. chinensis more precisely (Tokumaru and Uesugi 2019).
The insect pest control methods of L. chinensis in A. fistulosum were performed with a risk of producing pesticide-resistant individuals. To control the pest more sustainably, integrated pest management (IPM) practices should be encouraged. Oida and Kawana (2017) reported that hymenopterous parasitoids of Agromyzid leafminers (Diptera: Agromyzidae) on the leaves and flowers of Phacelia tanacetifolia acted as natural enemies of L. chinensis, whereas P. tanacetifolia can potentially act as a banker or insectary plant for the parasitoids to control L. chinensis in the A. fistulosum fields. In terms of cultural control, solarization using plastic mulching in summer effectively kills L. chinensis pupae in the soil (Kai and Morita 2001). To promote IPM when cultivating A. fistulosum, it is effective and necessary to develop A. fistulosum varieties resistant to the pest and introduce natural enemies. For instance, ‘Beicong’, an A. fistulosum accession, showed resistance to L. chinensis in terms of the adult’s non-preference (Sueyoshi et al. 2006) as well as egg-killing, which was revealed in our previous study when the pupation rates were evaluated by artificially inoculating the eggs (Takeda et al. 2020). However, the degree of resistance varies across the ‘Beicong’ population as ‘Beicong’ was propagated via an outcrossing approach to avoid any inbreeding depression. In addition, no other factor affecting the pupation rates, except for egg-killing, has been revealed to date.
In the present study, we selected A. fistulosum lines from the ‘Beicong’ progeny that showed a high antibiosis resistance to L. chinensis (biotype A) as described by Takeda et al. (2020). Furthermore, we investigated the inheritance of resistance in the progeny to develop breeding materials. In addition, we attempted to elucidate the insecticidal ability at the larval stage to identify resistant factors besides egg-killing.
Materials and methods
Plant material
‘Chouetsu’, ‘Kujou futo’, ‘Kokusen natsuyo’, and ‘Fuyuwarabe’ are commercial varieties of A. fistulosum in Japan, where ‘Beicong’ is an accession of A. fistulosum that is preserved in the NARO Genebank. ‘Beicong’ and ‘Kokusen natsuyo’ were observed to show resistance to L. chinensis based on the pupation rate of the eggs (Takeda et al. 2020). Thus, ‘Beicong’ and ‘Kokusen natsuyo’ as well as their progenies were used to evaluate their resistance to L. chinensis (Table 1). The plants were grown in greenhouses at the Institute of Vegetable and Floriculture Science, NARO, Japan (N34°61′, E136°25′). To prepare the plants, seeds of A. fistulosum were sown in 200-cell plug trays, while the seedlings were grown by regularly spraying the bioinsecticide (Beauveria bassiana) BotaniGard® ES (Arysta LifeScience, Tokyo, Japan) without any synthetic pesticides. The bioinsecticide was sprayed three times or once a week from May to September or from October to April, respectively, to protect from the onion thrips (Thrips tabaci). Approximately three months after germination, the seedlings were individually transplanted into a pipe container (φ22 mm, 80 mm high) and placed on the tray with water, as described by Takeda et al. (2020). To avoid spraying before the experiment, the test plants were transplanted into the pipe container 2 days before the experiment.
Insect material
Liriomyza chinensis (biotype A; Tokumaru and Uesugi 2019) was collected from a colony that was established on several varieties of A. fistulosum plants at the National Institute of Vegetable and Tea Science (present name: Institute of Vegetable and Floriculture Science, NARO) in October 2011, which were reared with an A. fistulosum variety ‘Kujou futo’ in a vinyl chloride cage (300 × 150 × 200 mm high). The rearing cage was placed in a growth chamber (TAM131AM-SV; Toshiba Carrier, Co., Ltd., Japan) that was set to 22 °C and a photoperiod of 14 h/10 h light/dark (14L10D), following our previous study (Takeda et al. 2020). Mass-rearing of L. chinensis was performed according to Urairi et al. (2020); for oviposition, A. fistulosum plants (six plastic pots with six plants per pot) were introduced in the rearing cage with two bottles containing a solution of 20 % hydromel and hundreds of emerged adults. The exchange with a new set of plants was carried out three times a week.
Evaluating A. fistulosum resistance to L. chinensis
Evaluating the resistance of A. fistulosum to L. chinensis was conducted by artificially inoculating the eggs into the newest leaf, as described by Takeda et al. (2020). To collect thousands of fresh eggs, 200 adult flies in two plastic pots, with six plants of ‘Kujou futo’ per pot, were introduced into a vinyl chloride cage (300 × 150 × 200 mm) at approximately 9 AM. Female flies were left to lay eggs in the leaves for 24 h (24 °C, 14L10D); it is necessary to let adult females oviposit for 24 h to successfully collect thousands of fresh eggs. Thereafter, at approximately 9 AM the next day, the eggs were collected from the inner parts of the unifacial leaves and placed in a petri dish filled with water. Five eggs were inoculated with a plunger microsyringe (MS-GAN050 0.5 ml volume, Ito Co., Ltd., Japan) into four points, respectively, resulting in 20 eggs inoculated into the newest leaf (leaf length was longer than 20 cm) of each A. fistulosum sample, as shown in Fig. 1. Immediately after inoculation, each tested plant was placed in a testing pipe cage, covered individually with a nylon mesh (φ22 mm, 320 mm high), firmly sealed with adhesive tape, and then placed in a growth chamber set to 24 °C and 14L10D. Here, the temperature was set to 24 °C for mass-rearing and not 22 °C because of the shortened experimental period. After 14 days of inoculation, the soil pupae were placed in a tea strainer containing water, and the floated pupae were collected and counted.
Selecting highly resistant ‘Beicong’ lines and elucidating the resistance level of the F1 hybrid
To select the lines with a high resistance to L. chinensis, the resistance of the ‘Beicong’ individuals was evaluated in 2014. A total of 200 seeds of ‘Beicong’ were sown on a 200-cell tray (one seed per cell), while the seedlings were grown in a greenhouse. In winter, the greenhouse was heated such that the temperature was maintained at or above 15 °C. Approximately 3 months after germination, each seedling was transplanted into a pipe container, where the resistance to L. chinensis was individually evaluated by artificially inoculating the eggs into the newest leaf and investigating the pupation rate according to Takeda et al. (2020). After the evaluation, individuals showing high resistance (i.e., pupation rate: 0 %) were self-pollinated to investigate the inheritance of the resistance in the next progeny. Similarly, the evaluation of resistance to L. chinensis was conducted on 20 individuals of each self-pollinated progeny in 2015. Thus, the selected ‘Beicong’ plant with a high resistance to L. chinensis was self-pollinated several times (described as Sx in Table 1). To elucidate the inheritance of the resistance in the F1 hybrid between the resistant line and the susceptible variety, the D8s S2 progeny plant was crossed with ‘Chouetsu’. During the experiments conducted in 2016 and 2017, evaluating the resistance to L. chinensis of the obtained F1 hybrid was conducted by artificially inoculating the eggs, as described by Takeda et al. (2020).
Investigating the effect of D8s on larval growth
During the experiments in 2018, the hatched larvae of L. chinensis were inoculated into the leaves of A. fistulosum using a method described by Takeda et al. (2020) to elucidate the insecticidal ability at the larval stage of L. chinensis. The eggs were collected from the ‘Kujou futo’ inner leaf parts and placed in a petri dish filled with water. To hatch the collected eggs, the petri dish was placed in a growth chamber (24 °C, 14L10D) for 3 days. Within 24 h after hatching, the five newly hatched larvae were inoculated into each of the four parts of the tested A. fistulosum’s newest leaf using the plunger microsyringe (MS-GAN050 0.5 ml volume, Ito Co., Ltd., Japan) similar to the egg inoculation. After inoculation, each plant was placed in a testing pipe cage, individually covered with a nylon mesh (φ22 mm, 320 mm high), firmly sealed with adhesive tape (as shown in Fig. 1), and then placed in a growth chamber set to 24 °C and 14L10D. Here, the temperature was set to 24 °C for mass-rearing and not 22 °C because of the shortened experimental period. After 14 days of inoculation, the soil pupae were placed in a tea strainer containing water, and the floated pupae were collected and counted.
Statistical analysis
The pupation rates of the eggs or the larvae to pupae were compared among the tested A. fistulosum lines or varieties using the Steel-Dwass test. All statistical analyses were conducted using the JMP statistical package (SAS Institute 2020, USA).
Results
Selecting highly resistant lines from ‘Beicong’
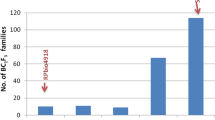
Out of the 200 ‘Beicong’ individuals, 191 were germinated normally, in which the resistance to L. chinensis of the 191 individuals was evaluated in 2014 to select the highly resistant plants, which included A1–50, B1 – 50, C1–50, and D1–41. As a result, 14 individuals (A13, A19, A20, A29, A30, A34, A36, A37, A38, A42, A43, C30, C33, and D8) showed high resistance to L. chinensis with a pupation rate of 0 % (Fig. 2). However, C33 was excluded due to its limited growth; therefore, the other 13 individuals were self-pollinated, where 20 S1 progeny plants per individual were used in the following experiment in 2015. As a result of evaluating the S1 progeny, the line which showed the lowest mean of pupation rate was selected as the highly resistant line (D8s; Fig. 3). Moreover, during the experiments conducted in 2016 and 2017, the resistance of the D8s progeny plants (S2 and S3) and the plant material were evaluated. As a result of these experiments, it was revealed that the D8s progeny plants (S2 and S3) showed significantly higher resistance to L. chinensis when compared to ‘Kujou futo’ and ‘Chouetsu’ (p < 0.05; Table 2). Finally, the pupation rate of ‘Kokusen natsuyo’ was found to be 6.5 %, reflecting no significant difference between the D8s progeny and ‘Kokusen natsuyo’.
Investigating the inheritance of resistance to L. chinensis
The resistance of the F1 hybrid was tested in 2017 that was generated from the highly resistant line, the D8s progeny (S2), and the susceptible variety, ‘Chouetsu’, to L. chinensis. The pupation rate of the F1 hybrid, ‘D8s-19s × Chouetsu’, was 12.0 %, which was significantly lower than that of ‘Kujou futo’ and ‘Chouetsu’ (p < 0.05). Although there was no significant difference between the F1 hybrid and the S3 progeny, the mean values of the F1 hybrid were higher than those of the S3 progeny (Table 2).
Effects of the D8s on larval growth after artificially inoculating the hatched larvae
The D8s progeny (S2) and the commercial varieties (‘Kujou futo’, ‘Fuyuwarabe’, and ‘Kokusen natsuyo’) were used in the experiment conducted in 2018. As a result of the artificial larval inoculation into the newest leaf, the pupation rate values (from larvae to pupae) of D8s-19s and D8s-20s were found to be 5.9 % and 1.3 %, respectively (Table 3). In addition, a significant difference was observed between the D8s progeny plants and both ‘Kujou futo’ and ‘Fuyuwarabe’ (p < 0.05) individually. Moreover, the pupation rate of ‘Kokusen natsuyo’ was found to be 9.6 %, where no significant difference was observed between the D8s progeny plants and ‘Kokusen natsuyo’.
Discussion
To develop breeding materials with a high resistance to L. chinensis, the individual numbered as D8, which showed a high resistance with a pupation rate of 0 %, was selected from the ‘Beicong’ population according to Takeda et al. (2020). In addition, the D8 selfed lines showed lower pupation rate values relative to the commercial varieties; thus, the resistance of D8 was stably inherited into the progeny. By continuously self-pollinating the D8 individual, the D8s line with a fixed high resistance to L. chinensis was developed. As ‘Kokusen natsuyo’ showed a resistance as high as that of ‘Beicong’ in our previous study (Takeda et al. 2020) and the present study, ‘Kokusen natsuyo’ may be also a candidate for breeding the Japanese bunching onion. Moreover, the F1 hybrid between the D8s line and the commercial variety ‘Chouetsu’ also showed high resistance relative to the parental variety ‘Chouetsu’, but the pupation rate was higher than of the D8s line; thus, the F1 hybrid resistance was an intermediate between the D8s line and the susceptible variety. Hence, these results demonstrate that the mode of inheriting resistance is an incomplete dominance. For this reason, the genome region relevant to resistance must be homozygous in the D8 allele to maintain the high resistance. Furthermore, to reveal the number of genes that are related to the resistance in detail, it is necessary to investigate the segregation ratio in the F2 population between the D8s line and the susceptible variety.
Takeda et al. (2020) reported that the hatchability of L. chinensis was significantly different between ‘Beicong’ and ‘Kujou futo’ when artificially inoculating the eggs. In the present study, the pupation rate from the larvae to the pupae of the D8s line was also investigated to reveal the effects of resistance on the hatched larvae. Moreover, 2 weeks after inoculating the newly developed leaves with the hatched larvae, the pupation rate of the D8s line was found to be significantly lower relative to the susceptible varieties, ‘Kujou futo’ and ‘Fuyuwarabe’ (p < 0.05). In addition, the varietal resistance to the larvae showed almost the same tendency as observed with regards to the eggs, suggesting that the D8s line has both egg- and larval-killing defense mechanisms.
Furthermore, De Jong and Rademaker (1991) reported varietal differences in the susceptibility of chrysanthemum to L. trifoli (Burgess) (Diptera: Agromyzidae) after a no-choice oviposition, in which the varietal differences were considered to involve the larval-killing defense mechanism on the leaves. This study, however, has not reported the mechanism or the inheritance of resistance. Moreover, Sueyoshi et al. (2006) reported that resistance to L. chinensis on ‘Beicong’ was not caused by a physical defense mechanism, such as the hardness of leaves, but by other factors. Since the inoculated eggs and larvae were unaffected by the physical factor when using the artificial inoculation methods, the egg- and larval-killing defense mechanisms could be caused by chemical factors, such as chemical compounds found in the leaves. However, it remains unclear whether the same factors in antibiosis resistance were also associated with egg- and larval-killing. Comparing the chemical compositions among the D8s line and other A. fistulosum varieties and conducting the bioassay with L. chinensis eggs and larvae will aid identification of the antibiotic chemical components.
In addition to the antibiotic effects of ‘Beicong’, it was also reported that the number of feeding punctures generated by the female adults on ‘Beicong’ was lower than that of other varieties of A. fistulosum in the choice tests (Sueyoshi et al. 2006). Moreover, the D8s line would also reduce the feeding puncture damage generated by female adults. Thus, it was assumed that the D8s line decreased the feeding behavior of adults and suppressed the generation cycles of L. chinensis by killing the eggs and larvae, and finally, preventing large outbreaks.
It is known that other leafminer flies such as South American leafminer (Liriomyza huidobrensis) and garden pea leafminer (Chromatomyia horticola) also attack Allium spp. (Schrameyer 2001; Scheffer 2000). Furthermore, a new genotype of L. chinensis (biotype B) was recently identified in Japan, where the number of larvae in the leaves and the larval mining surface area of the unifacial leaves were mostly larger than those of the conventional strain (biotype A) (Tokumaru and Uesugi 2019). However, in the present study, the ovicidal and larvicidal effect of D8s on the biotype A eggs and larvae were elucidated. To develop the breeding materials with a high resistance to both biotypes A and B and other leafminer species attacking Allium spp., it is necessary to reveal whether the D8s line shows the same resistance to L. chinensis biotype B and those other leafminer species attacking Allium spp. such as L. chinensis biotype A by artificially inoculating the eggs or larvae in future studies. In addition, field resistance to these leafminer flies should be confirmed by conducting field experiments. We believe that by developing the resistant varieties to both biotypes and these other leafminer species could contribute to IPM by decreasing the population of L. chinensis and using less amount of pesticides.
Data availability
Not applicable.
Code availability
Not applicable.
References
Chen XJ, Lang F, Xu Z, Jun-hua HE, Ma Y (2003) The occurrence of leafminers and their parasitoids on vegetables and weeds in Hangzhou area, Southeast China. Biocontrol 48:515–527. https://doi.org/10.1023/A:1025726813462
Choi IH, Kim JW, Kim GH, Kim CW (2003) Injury aspects of the stone leek leafminer, Liriomyza chinensis Kato (Diptera: Agromyzidae) on Welsh onion. Korean J Appl Entomol 42:335–343 (in Korean with English abstract)
De Jong J, Rademaker W (1991) Life history studies of the leaf miner Liriomyza trifolii on susceptible and resistent cultivars of Dendranthema grandiflori. Euphytica 56:47–53. https://doi.org/10.1007/BF00041743
Ford-Lloyd BV, Armstrong SJ (1993) Welsh onion Allium fistulosum L. In: G. and Bergh BO (eds) “Genetic Improvement of Vegetable Crops” Kalloo. Pergamon Press, London, pp 51–58
Japan Metrological Agency (2020) Seasonal mean temperature in Japan. Available from https://www.data.jma.go.jp/cpdinfo/temp (Accessed 15 November 2020) (in Japanese)
Kai S, Morita S (2001) Control by solar radiation against Liriomyza chinensis (Kato) on Welsh onion in the green house. Kyushu Pl Prot Res 47:108–111 (in Japanese)
Kumazawa S, Katsumata H (1965) Negi (Japanese bunching onion). In: “Sosai-engei kakuron (Vegetable crops)” Kumazawa. S. (ed.), Yokendo press, Tokyo, pp 280–289 (in Japanese)
Martinez M (1982) Contribution a l’etude des Agromyzidae de France (Dipt.). Liriomyza nietzkei Spencer et Liriomyza chinensis (Kato), deux especes d’importance economique presentes en France. Bulletin de la Societe entomologique de France 87:302–308 (in French)
Martynov VV, Nikulina TV, Gubin AI (2016) Range expansion of invasive stone leek leafminer Liriomyza chinensis (Kato, 1949) (Diptera: Agromyzidae) in Eastern Europe. Euroasian Entomol J 15:420–421
Oida H, Kawana T (2017) Hymenopterous parasitoids of Agromyzid leafminers (Diptera: Agromyzidae) on leaves and flowers of Phacelia tanacetifolia in Chiba Pref., Japan and potential of P. tanacetifolia as a banker plant or insectary plant to control Liriomyza chinensis (Diptera: Agromyzidae). Jpn J Appl Entomol Zool 61:233–241. https://doi.org/10.1303/jjaez.2017.233(in Japanese with English abstract)
Papp L, Cerny M (2017) Agromyzidae (Diptera) of Hungary, vol 3. Pars Ltd, Nagykovácsi
SAS Institute (2020) JMP, version 13.2.1. SAS Institute, Cary
Scheffer SJ (2000) Molecular evidence of cryptic species within the Liriomyza huidobrensis (Diptera: Agromyzidae). J Econ Entomol 93:1146–1151. https://doi.org/10.1603/0022-0493-93.4.1146
Schrameyer K (2001) Mining pests of Allium vegetable species. Gemüse (München) 37:17–20 (in German with English abstract)
Sueyoshi T, Shimomura K, Koga T, Yamamura Y, Takemoto H (2006) The varietal difference in resistance to stone leek leafminer in Welsh onions. Bull Fukuoka Agric Res Cent 25:37–41 (in Japanese with English abstract)
Takeda M, Kawai A, Mitsunaga T, Tsukazaki H, Yamashita K, Wako T (2020) A novel method for evaluating the egg killing defenses and varietal resistance of the bunching onion against Liriomyza chinensis (Diptera: Agromyzidae) via the artificial inoculation of eggs. Appl Entomol Zool 55:93–103. https://doi.org/10.1007/s13355-019-00657-7
Tokumaru S (2016) Effects of temperature and photoperiod on development and reproductive potential of Liriomyza chinensis (Diptera: Agromyzidae). Jpn J Appl Entomol Zool 60:189–196. https://doi.org/10.1303/jjaez.2016.189(in Japanese with English abstract)
Tokumaru S, Uesugi R (2019) Occurrence of genotype of Liriomyza chinensis Kato in Kyoto Prefecture. Shokubutsu-boueki. (Plant Protection) 73:581–583 (in Japanese)
Tran DH, Takagi M (2005) Developmental biology of Liriomyza chinensis (Diptera: Agromyzidae) on onion. J Facul Agric Kyushu Univ 50:375–382
Urairi C, Kawai A, Takeda M (2020) A method for mass-rearing Liriomyza chinensis (Diptera: Agromyzidae) Appl Entomol Zool 55:423–428. https://doi.org/10.1007/s13355-020-00697-4
Acknowledgements
We thank Ms. K. Nomura, Ms. K. Tanaka, Ms. M. Okamoto, Mr. M. Kitazumi, Mr. Y. Honda, Mr. T. Izumi, and Mr. T. Momiyama for breeding the plant material. We also thank Dr. T. Yamada for the useful discussions.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
Conceptualization, S. F., K. Y., T. W., and M. T.; Methodology, S. F., K. Y., T. W., and M. T.; Investigation, S. F., C. U., K. Y., and A. K.; Writing-Original Draft, S. F.; Writing-Review and Editing, S. F., C. U., K. Y., T. W., A. K., and M. T.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujito, S., Urairi, C., Yamashita, Ki. et al. Japanese bunching onion line with a high resistance to the stone leek leafminer, Liriomyza chinensis from the ‘Beicong’ population: evaluating the inheritance of resistance. Euphytica 217, 31 (2021). https://doi.org/10.1007/s10681-020-02764-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-02764-x