Abstract
Wild abortive (WA)-type cytoplasmic male sterility (CMS) has been exclusively used for breeding three-line hybrid indica rice, but it has not been applied for generating japonica hybrids because of the difficulties related to breeding japonica restorer lines. Determining whether the major restorer-of-fertility (Rf) gene used for indica hybrids can efficiently restore the fertility of WA-type japonica CMS lines may be useful for breeding WA-type japonica restorer lines. In this study, japonica restorer lines for Chinsurah Boro II (BT)-type CMS exhibited varying abilities to restore the fertility of ‘WA-LiuqianxinA’, which is a WA-type japonica CMS line. Additionally, Rf genes for WA-type CMS were identified in the BT-type japonica restorers. Meanwhile, ‘C9083’, which is a BT-type japonica restorer, exhibited a limited ability to restore the fertility of WA-type japonica CMS lines, and a genetic analysis revealed that the fertility restoration was controlled by one locus. The Rf gene was mapped to an approximately 370-kb physical region and was identified as Rf4. Furthermore, Rf gene dosage effects and the temperature influenced the fertility restoration of WA-type japonica CMS lines. This study is the first to confirm that Rf4 has only minor effects on the fertility restoration of WA-type japonica CMS lines. These results may be relevant for the development of WA-type japonica hybrids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is a staple food for more than half of the global population, and the breeding of high-yielding rice varieties is essential for ensuring the food demands of an increasing worldwide population continue to be satisfied. Over the last few decades, the cultivation of hybrid rice varieties has resulted in increased grain yields (Fujimura et al. 1996; Yuan 1994). The development of hybrid rice varieties, including three-line and two-line hybrids, has involved cytoplasmic male sterility (CMS) and thermosensitive genic male sterility, respectively (Cheng et al. 2007; Huang et al. 2014). To develop three-line hybrids, a CMS line, a maintainer line, and a restorer line, which carries the restorer-of-fertility (Rf) gene, are required. Three representative CMS types [i.e., wild abortive (WA), Honglian (HL), and Chinsurah Boro II (BT)] have been used for developing three-line hybrid rice (Chen and Liu 2014; Huang et al. 2014; Li et al. 2007). Additionally, several Rf loci applied for the breeding of restorer lines have been identified and mapped. For example, Rf3 and Rf4, which are two major fertility restorer genes for WA-CMS, have been mapped on chromosomes 1 and 10, respectively (Ahmadikhah and Karlov 2006; Tang et al. 2014; Zhang et al. 1997), while Rf5 and Rf6, which are two major fertility restorer genes for HL-CMS, have been mapped on chromosomes 10 and 8, respectively (Hu et al. 2012; Huang et al. 2000, 2012, 2015). Meanwhile, Rf1a and Rf1b at the Rf1 locus for BT-CMS have been mapped on chromosome 10 (Akagi et al. 1996; Komori et al. 2004; Wang et al. 2006). Additionally, Rf17 on chromosome 4 for ‘Chinese wild rice’ CMS (Fujii and Toriyama 2009) and Rf2 on chromosome 2 for ‘Lead Rice’ CMS (Itabashi et al. 2011) have been identified. With the exception of Rf3, these Rf genes have been cloned. Furthermore, Rf5 and Rf1a represent the same gene, and Rf1a (Rf5) and Rf1b are closely linked to Rf4 on chromosome 10.
To date, three-line indica hybrids have been produced mainly via WA-type and HL-type CMS, while japonica hybrids have been developed primarily through BT-type CMS (Chen and Liu 2014; Huang et al. 2014; Li et al. 2007; Yuan 1994). Because of the successful application of WA-type CMS for generating indica hybrids, breeders in China have attempted to use this CMS type to produce japonica hybrids. Wild abortive-type japonica CMS lines can be obtained from BT-type maintainers by backcrossing, and they exhibit stable sterility with typical abortive pollen grains. However, the japonica restorers for WA-type japonica CMS lines are difficult to breed using the pre-existing BT-type japonica restorers and even the WA-type indica restorers (Tang et al. 2008; Yang 1994). Thus, WA-type japonica hybrids have not been generated. Moreover, most BT-type japonica restorers carry the Rf1 locus, and Rf1 in BT-type japonica restorers used in China was transferred from ‘IR8’, which was the Rf gene donor for ‘IR24’ (i.e., a known indica restorer for WA-type indica CMS lines) (Akagi et al. 1996; Chen and Liu 2014; Huang et al. 2014; Komori et al. 2004; Wang et al. 2006; Yang et al. 2016). Because Rf1 is closely linked to Rf4 in WA-type indica restorers, most BT-type japonica restorers should theoretically carry Rf4 and be able to restore the fertility of WA-type japonica CMS lines. However, the testcrossed F1 plants of hybridizations between WA-type japonica CMS lines and BT-type japonica restorers are usually sterile or exhibit low fertility levels. Unfortunately, the genetic basis for the inability of BT-type japonica restorers to efficiently restore the fertility of WA-type japonica CMS lines has not been characterized. Thus, determining whether Rf4 exists in BT-type japonica restorers and is involved in restoring the fertility of WA-type japonica CMS lines may be relevant for analyzing the inefficiency of the fertility restoration by BT-type japonica restorers and for breeding WA-type japonica restorers.
We herein report that the Rf1 locus in a BT-type japonica restorer was associated with the fertility restoration of WA-type japonica CMS lines and that Rf4 existed in the japonica restorer. Additionally, Rf4 was related to the fertility restoration of WA-type CMS japonica lines, but the restoration of male fertility by Rf4 was poor in the japonica nuclear background. Furthermore, the influence of temperature and the Rf4 dosage effects on the fertility restoration of WA-type japonica CMS lines was analyzed. These results may help elucidate the fertility restoration of WA-type CMS lines and aid in the breeding of WA-type japonica restorer lines for the development of WA-type japonica hybrids.
Materials and methods
Plant materials
A WA-type japonica CMS line bred from the BT-type maintainer ‘Liuqianxin’ [i.e., ‘WA-LiuqianxinA’ (WA-LqxA)] was crossed with 124 BT-type restorer accessions, resulting in a testcross population of 104 F1 hybrids. Because most BT-type restorer lines carry Rf4, ‘C9083’, which was unable to restore the fertility of WA-LqxA in the testcrossed F1 plants, was selected as the male line in a cross with the WA-LqxA/‘C9083’ F1 hybrids. This was followed by four backcrosses to ‘C9083’ from 2009 to 2012, resulting in the development of WA-C9083A. Some plants in each backcrossing population and the WA-C9083A plants produced normal anthers, stainable pollen grains, and self-crossing seeds, which confirmed the existence of Rf genes in ‘C9083’ for restoring the fertility of WA-type CMS plants. To identify the associated Rf locus, we constructed WA-LqxA/‘C9083’//‘C9083’ populations in 2013 and 2014, which were used for the genetic analysis of fertility restoration and gene mapping. We evaluated the ability of Rf genes to restore the fertility of WA-type CMS from 2013 to 2015 by crossing plants harboring the heterozygous alleles of Rf in the WA-LqxA/‘C9083’//‘C9083’ population with ‘C9083’, followed by three backcrosses to ‘C9083’. These materials were sown on May 12, and 30-day-old seedlings were transplanted into the field. To analyze the influence of the temperature on fertility levels, the BC5F1 plants were planted on May 12, 20, and 27, June 5, 15, and 25, and July 5, 15, and 25 in 2016. All of these materials were planted in the experimental field of Yangzhou University in Yangzhou, Jiangsu province, China.
Fertility scoring and genetic analysis
The pollen grain and natural spikelet fertility levels of five plants from the CMS lines, five plants from the testcrossed F1 population, and each plant from the WA-LqxA/‘C9083’//‘C9083’ population were analyzed. To assess pollen grain fertility, three mature anthers were harvested, and pollen grains were stained with a 1% I2–KI solution. Because most of the stained pollen grains of the testcrossed F1 plants were dark, it was difficult to distinguish the stained abortive pollen grains from the normal pollen grains. Thus, the exclusively dark-blue, clear (unstainable), and typical abortive pollen grains in each individual were counted using an optical microscope. The pollen grain fertility level was estimated using the stained-pollen-grain rate. Natural spikelet fertility levels were measured as the average seed-setting rates by counting the filled and unfilled grains of two panicles from one plant harvested 20 days after flowering. For the genetic analysis, the stained-pollen-grain rate was used as the main criterion for evaluating sterile and partially fertile plants in the WA-LqxA/‘C9083’//‘C9083’ populations. Plants with a stained-pollen-grain rate ≤ 5% were considered sterile, and those with a rate > 20% were regarded as partially fertile. A Chi square analysis was used to test the goodness-of-fit of the hypothesis.
DNA extraction, PCR amplification, and sequencing
Total genomic DNA was extracted from freshly harvested leaves using a modified version of a published method involving cetyltrimethylammonium bromide (Rogers and Bendich 1985). Simple sequence repeat (SSR) markers were obtained from the Gramene database (http://www.gramene.org/). New insertion/deletion markers were identified based on the ‘Nipponbare’ (japonica) and ‘93-11’ (indica) sequences (http://www.ncbi.nlm.nih.gov/) using a BLAST algorithm-based online tool. Molecular markers were analyzed by a polymerase chain reaction (PCR) in a 20-µL volume containing 1 × PCR buffer, 0.1 mmol/L each dNTP, 1.0 U Taq polymerase, 0.2 µmol/L primers, and 20 ng template DNA. The PCR program was as follows: 94 °C for 4 min; 32 cycles at 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 50 s; 72 °C for 5 min. Amplicons were separated by electrophoresis in 3% agarose gels containing ethidium bromide and visualized with a Gel Doc 1000 system (Bio-Rad Company, Hercules, CA, USA).
The DNA fragments covering Rf4 from ‘C9083’ were amplified using the high-fidelity GXL-Taq polymerase (Takara, Dalian, China). The analyzed sequences contained the 206-bp 5′-upstream region and the 164-bp 3′-downstream region of the Rf4 gene. Amplicons were purified using a DNA gel extraction kit (Tiangen, Beijing, China) and then ligated into the pEASY-Blunt Zero vector. Five plasmids were sequenced by GENEWIZ (Suzhou, China) to confirm the accuracy of the sequences. The correct sequences were aligned with the BLAST algorithm-based online tools provided by the National Center for Biotechnology Information. The primers used for sequencing the Rf4 allele are listed in Supplemental Table 1.
RNA isolation and quantitative real-time PCR
Total RNA was extracted from young panicles using the Plant RNA Kit (Tiangen). First-strand cDNA was synthesized using the Perfect Real Time PrimeScript RT reagent (Takara) in a 20-µL reaction mixture containing 5 µg total RNA. A quantitative real-time PCR (qRT-PCR) assay was conducted using a CFX96 Real-Time PCR Detection System (Bio-Rad Company). The reactions were completed with three biological replicates. Data were analyzed according to the 2−ΔΔCt method (Livak and Schmittgen 2001). An Actin gene was used as an internal reference to assay the relative WA352 expression levels. The qRT-PCR primers are listed in Supplemental Table 1.
Construction of linkage maps and data analysis
The fertility of the materials and populations used in this study was analyzed with the analysis of variance procedure package in MATLAB (version 7.0). Molecular marker linkage maps for the target genes were constructed with MAPMAKER/EXP 3.0 (Lincoln et al. 1992).
Results
Ability of BT-type restorers to restore the fertility of WA-LqxA plants
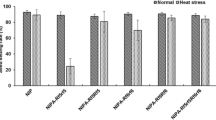
The pollen grain and spikelet fertility levels of WA-LqxA plants and the testcrossed F1 plants were analyzed in Yangzhou from 2007 to 2008. The WA-LqxA plants produced typical abortive pollen grains (i.e., shrunken pollen grains), with a natural spikelet fertility rate of 0. The stained-pollen-grain rates of 104 testcrossed F1 plants ranged from 0 to 98%, while the natural spikelet fertility rates ranged from 0 to 95% (Fig. 1). Spikelet fertility of the testcrossed F1 plants was significantly correlated with the stained-pollen-grain rates (P < 0.05), but the correlation coefficient (r = 0.2843) was low. Anther and pollen development was restored in most testcrossed F1 plants, but only the testcrossed F1 plants from four BT-type japonica restorers had natural spikelet fertility levels > 80% (Fig. 1). Thus, the ability of most of the BT-type japonica restorers to restore the fertility of WA-type japonica CMS lines was low. The testcrossed F1 hybrids with high stained-pollen-grain rates varied regarding natural spikelet fertility levels, ranging from sterile to fertile (Fig. 1). These results indicated that most of the stained pollen grains of the F1 plants with low spikelet fertility levels were abortive.
Ability of ‘C9083’ to restore the fertility of WA-LqxA plants
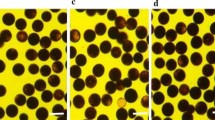
The sterility of WA-LqxA, WA-LqxA/‘C9083’ F1, and WA-C9083A plants at a relatively high temperature resulted in the production of late-generated panicles (Fig. 2a–c). In 2012, WA-LqxA plants flowered on August 22 and produced milky white, slender, and stunted anthers (i.e., degenerated anthers) and shrunken pollen grains on the panicles (Fig. 2d, e). Pollen abortion was consistently observed on late-generated WA-LqxA panicles, which flowered after September 19, resulting in a natural spikelet fertility level < 4%. The WA-LqxA/‘C9083’ F1 plants flowered on August 18, and the late-generated panicles flowered after September 20. The WA-LqxA/‘C9083’ F1 plants produced degenerated anthers as well as abundant shrunken pollen grains (> 97%) (Fig. 2f, g), but normal anther morphology and pollen stainability were recovered on the late-generated panicles (Fig. 2h, i). The WA-C9083A plants flowered on August 1 and produced morphologically normal pollen grains that could be thoroughly stained (Fig. 2j, k). However, the natural spikelet fertility levels for the panicles were < 3%. These results indicated that the Rf genes of ‘C9083’ alleviated the damage caused by the gene responsible for CMS, but could not restore pollen fertility. However, the late-generated panicles of WA-C9083A plants (flowered on September 7) had a high spikelet fertility rate (Fig. 2c). The temperature in August was much higher than in September. Therefore, the relatively low temperature likely facilitated the fertility restoration of WA-type CMS plants via the expression of ‘C9083’ Rf genes (Fig. S1).
Morphology of WA-LqxA, WA-LqxA/‘C9083’ F1, and WA-C9083A pollen grains, anthers, and plants. a, b, and c Morphology of WA-LqxA, WA-LqxA/‘C9083’ F1, and WA-C9083A plants. Arrows indicate the late-generated panicles. Scale bars = 10 mm. d and e Anthers and pollen grains on the panicles and late-generated panicles of WA-LqxA plants. f, g, h, and i Anthers and pollen grains on the panicles and late-generated panicles of WA-LqxA/‘C9083’ F1 plants. Arrows indicate the normal anthers in a floret. j and k Anthers and pollen grains on the panicles and late-generated panicles of WA-C9083A plants. Scale bars = 1 mm and 50 µm for anthers and pollen grains, respectively. The pollen grains were stained with 1% I2–KI solution
Genetic analysis of fertility restoration
The pollen grains and natural spikelet fertility levels of plants from the cross between WA-LqxA and ‘C9083’ were influenced by the air temperature on different flowering dates. Thus, the flowering dates of 170 and 1940 plants in the WA-LqxA/‘C9083’//‘C9083’ populations were observed during 2013 and 2014, respectively. The WA-LqxA/‘C9083’ F1 plants flowered between August 18 and 27 and produced degenerated anthers and shrunken pollen grains. Thus, only plants flowering before August 27 were selected from WA-LqxA/‘C9083’//‘C9083’ populations for further analyses. Of the 73,878 plants in 2013 and 2014, 33,453 plants produced degenerated anthers and shrunken pollen grains (sterile plants) and 40,425 plants produced normal anthers and stainable pollen grains. All of these early-flowering plants had low natural spikelet fertility levels (0–4.3%). The segregation ratio of sterile plants to partially fertile plants in the WA-LqxA/‘C9083’//‘C9083’ populations was 1:1 (χ2 = 0.65 and 0.89 in 2013 and 2014, respectively; < χ 20.05 = 3.84), indicating that a major Rf locus in ‘C9083’ was related to the restoration of normal pollen grains and anthers in WA-type japonica CMS lines. Additionally, 21,437 plants in the WA-LqxA/‘C9083’//‘C9083’ populations flowered after September 10. All of these plants produced normal anthers and stainable pollen grains, suggesting that the temperature affected the fertility restoration of WA-type CMS plants.
Molecular mapping of the major Rf locus
During 2013, over 400 SSR markers were used to detect polymorphisms between WA-LqxA and ‘C9083’ plants, and 57 markers were polymorphic. To map the major Rf locus, 23 individuals (i.e., 12 sterile and 11 partially fertile plants) were analyzed using these polymorphic markers. We hypothesized that the sterile plants carry a heterozygous genotype at a linkage marker locus, while the partially fertile plants carry a homozygous ‘C9083’ genotype. Among the 57 polymorphic markers, RM171 (i.e., SSR marker) supported this hypothesis, and was subsequently used for genotyping the other 21 sterile plants and 29 partially fertile plants. The sterile plants carried the heterozygous genotype, whereas the partially fertile plants carried the homozygous ‘C9083’ genotype, indicating that RM171 was linked to the Rf locus involved in the fertility restoration of WA-LqxA plants. Moreover, RM171 was located on chromosome 10 and linked to Rf1, which is associated with BT-type CMS (Akagi et al. 1996; Komori et al. 2004; Wang et al. 2006). Thus, Rf1, or its nearby locus in ‘C9083’, was most likely involved in restoring the fertility of WA-LqxA plants.
To clarify the precise position of Rf genes, 75 markers were developed for the region containing RM171, and 12 polymorphic markers were obtained for Rf mapping (Supplemental Table 1). One marker, STSh-7, was used to screen for recombinant individuals among 73 plants in the WA-LqxA/‘C9083’//‘C9083’ population in 2013; no recombinant plants were detected (Fig. 3a). In 2014, STSh-7 and RM171 were used to screen for recombinant individuals among 897 plants in the WA-LqxA/‘C9083’//‘C9083’ population. Two and one recombinants were identified by STSh-7 and RM171, respectively. Another nine markers were used to genotype three selected recombinants. One recombinant plant was detected by STS10-43, while the remaining eight markers did not detect any recombinant plants. Thus, the Rf gene was likely located between STS10-43 and RM171 (Fig. 3b). The location of the Rf gene was further delimited to an approximately 370-kb region between markers STSh-9 and RM171 on the long arm of chromosome 10 based on the ‘Nipponbare’ reference genome sequence. Additionally, previous studies concluded that Rf1a and Rf1b for BT-type CMS, Rf5 for HL-type CMS, and Rf4 for WA-type CMS are located in this mapping region (Hu et al. 2012; Komori et al. 2004; Wang et al. 2006). Moreover, ‘C9083’ carries Rf1a and Rf1b (Wang et al. 2006) (Fig. 3c). Thus, we confirmed that the Rf locus in ‘C9083’ for the fertility restoration of WA-type CMS plants was identical to the Rf1 locus.
Genetic mapping of the Rf gene. a Location of the Rf gene between STSh-7 and RM171 on chromosome 10. b The Rf gene was mapped between STS10-43 and RM171. c The Rf gene was delimited to a 370-kb region overlapped by AC068950, AC092489, AC068923, and AC079888. This region included Rf5 (Rf1a), Rf1b, and Rf4
The Rf4 gene helps restore the fertility of WA-type japonica lines
During 2014, Rf4 was cloned from two WA-type indica restorers, ‘Minhui63’ and ‘IR24’ (Kazama and Toriyama 2014; Tang et al. 2014). Additionally, the Rf locus in ‘C9083’ responsible for restoring the fertility of WA-type CMS was believed to include Rf4. To confirm the presence of Rf4 in ‘C9083’, we sequenced the Rf4 allele. A comparison with the ‘Minhui63’ and ‘IR24’ Rf4 nucleotide sequences revealed the ‘C9083’ Rf4 nucleotide sequence was identical to that of ‘IR24’. Thus, the ‘C9083’ Rf4 gene may be responsible for restoring the fertility of WA-LqxA plants. Furthermore, the mitochondrial gene orf352 (WA352) is associated with the WA-type CMS, and is highly expressed in young panicles (Luo et al. 2013). An earlier investigation indicated that Rf4 expression is associated with a decrease in WA352 mRNA levels (Tang et al. 2014). In 2016, we examined WA352 expression levels in the young panicles of BC5F1 plants harboring Rf4Rf4 or Rf4rf4, which were collected on August 15 and September 10, respectively. A qRT-PCR assay indicated that the WA352 transcript levels were lower in plants carrying Rf4Rf4 than in plants carrying Rf4rf4 (Fig. 4). Thus, Rf4 is likely involved in restoring the fertility of WA-type CMS japonica plants.
Genetic effect of Rf4 on the fertility restoration of WA-type japonica lines
In 2016, 60 plants of the BC5F1 population were sown on May 12, 20, and 27, June 5, 15, and 25, and July 5, 15, and 25, and flowered on August 3, 10, 15, 21, and 27, and September 5, 16, 21, and 28, respectively. All plants were genotyped with markers STSh-7 and RM171. Plants harboring Rf4Rf4 produced normal anthers and stainable pollen grains at all flowering dates. In contrast, plants harboring Rf4rf4 produced degenerated anthers and shrunken pollen grains if they flowered before August 21, but produced normal anthers and stainable pollen grains if they flowered after September 5. Additionally, plants of the BC5F1 population that flowered before August 21 had low natural spikelet fertility levels (< 5%) (Fig. 5a), while plants that flowered after September 16 had varying natural spikelet fertility levels (Fig. 5b). Of the plants flowering after September 16, we randomly selected 40 harboring Rf4Rf4 and 38 harboring Rf4rf4, and determined that the average natural spikelet fertility rates were 37.89 and 17.22% (Fig. 5c), respectively. These phenotypes indicated that Rf dosage effects and the temperature affected the fertility restoration of WA-CMS japonica lines.
Discussion
Wild abortive-type CMS has been used for breeding three-line indica hybrids since the 1970s in China, and the resulting hybrids are grown on 10% of the total cultivated rice area worldwide (Barclay 2010; Li et al. 2007). To date, WA-type japonica hybrids have not been developed because of the difficulties associated with breeding restorers for WA-type japonica CMS lines (Yang 1994). In WA-type indica hybrids, the fertility restoration of WA-type indica CMS lines is usually considered to be controlled by Rf3 and Rf4, with Rf4 having a greater effect (Ahmadikhah and Karlov 2006; Cai et al. 2013; Tang et al. 2014; Zhang et al. 1997, 2002). The development of three-line hybrid rice suggests that most BT-type japonica restorers likely carry Rf4 and are able to restore the fertility of WA-type japonica CMS lines. In the present study, most BT-type restorers were able to restore the normal development of pollen grains and anthers in WA-type japonica CMS lines, even though most of the testcrossed F1 plants had low spikelet fertility rates. Additionally, ‘C9083’, a BT-type japonica restorer, was unable to restore the fertility of WA-type CMS in the testcrossed F1 plants, but plants in the backcrossed populations produced normal anthers and stainable pollen grains. These results implied that BT-type restorers carry Rf genes for WA-type CMS, but most of these genes may only have minor effects on the fertility restoration of WA-type japonica CMS lines. Thus, we hypothesized that Rf4 may exist in BT-type japonica restorers to restore the fertility of WA-type CMS lines. However, we observed that this restoration differed in two nuclear backgrounds, possibly because of unknown genes in WA-type indica maintainers responsible for restoring the fertility of WA-type CMS lines. These genes may not exist in WA-type CMS japonica lines, which could influence the ability of Rf4 to restore the fertility of WA-type CMS lines.
To determine whether Rf4 is present in BT-type restorers and involved in restoring the fertility of WA-type CMS lines, WA-LqxA/‘C9083’//‘C9083’ populations were generated. A genetic analysis of the fertility restoration of WA-type CMS lines revealed that a ‘C9083’ genetic locus was related to the restoration of normal anthers and stainable pollen grains in WA-type CMS lines with a japonica genetic background. This locus was mapped between markers STSh-9 and RM171, with a physical distance of 370 kb. This region also included Rf1a and Rf1b for BT-type CMS and Rf4 for WA-type CMS (Huang et al. 2014; Komori et al. 2004; Wang et al. 2006). Sequencing the Rf4 allele confirmed that ‘C9083’ carries Rf4. Moreover, the expression of the target Rf genes decreased the expression of WA352, which is functionally the same as Rf4 (Tang et al. 2014). Thus, we inferred that Rf4 in ‘C9083’ is related to the fertility restoration of WA-type CMS lines, although we could not eliminate the possibility of other genes in this mapping region being involved. In this study, plants carrying Rf4rf4 in the BC1F1 or BC5F1 populations were sterile or had low fertility levels, indicating that all of the Rf genes had only minor effects on restoring the fertility of WA-type japonica CMS lines. To the best of our knowledge, this is the first study to confirm that the ability of Rf4 to restore the fertility of WA-type japonica CMS lines is poor. Most BT-type japonica restorers carrying only Rf4 exhibited a relatively limited ability to restore the fertility of WA-type japonica CMS lines. However, some BT-type restorers were able to restore the fertility of WA-type japonica CMS lines, implying that there may be diverse Rf loci in these BT-type restorers. Future studies will need to verify this possibility.
In the present study, the natural spikelet fertility rates of WA-LqxA plants and WA-LqxA/‘C9083’ F1 plants were very low (< 5%), indicating that outcrossing had little influence on the natural spikelet fertility rates of WA-type japonica CMS lines and the associated crosses. On average, the BC1F1 and BC5F1 plants carrying Rf4Rf4 were more fertile than the plants carrying Rf4rf4, indicating that the Rf dosage effects influenced the fertility restoration of WA-type CMS japonica lines. Additionally, plants carrying the same genotypes in the BC1F1 or BC5F1 populations exhibited different fertility levels at different temperatures, implying that the fertility restoration of WA-type CMS lines was mediated by the interactive effects of genetic and environmental factors. Similar phenotypes have also been observed in WA-type indica CMS lines, such as WA-LongtefuA, which is widely used in hybrids (Huang 1997).
In summary, we confirmed that Rf4 exists in BT-type japonica restorers and has minor effects on the fertility restoration of WA-type japonica CMS lines. Moreover, we revealed a dosage effect of Rf genes on the fertility restoration of WA-type CMS lines. The data presented herein may be valuable for breeding WA-type japonica restorer lines.
References
Ahmadikhah A, Karlov GI (2006) Molecular mapping of the fertility-restoration gene Rf4 for WA-cytoplasmic malesterility in rice. Plant Breed 125:363–367
Akagi H, Yokozeki Y, Inagaki A, Nakamura A, Fujimura T (1996) A codominant DNA marker closely linked to the rice nuclear restorer gene, Rf1, identified with inter-SSR fingerprinting. Genome 39:1205–1209
Barclay A (2010) Hybridizing the world. Rice Today 9:32–35
Cai J, Liao QP, Dai ZJ, Zhu HT, Zeng YF, Zhang ZM, Zhang GQ (2013) Allelic differentiations and effects of the Rf3 and Rf4 genes on fertility restoration in rice with wild abortive cytoplasmic male sterility. Biol Plant 52:274–280
Chen LT, Liu YG (2014) Male sterility and fertility restoration in crops. Annu Rev Plant Biol 65:579–606
Cheng SH, Zhuang JY, Fan YY, Du JH, Cao LY (2007) Progress in research and development on hybrid rice: a super-domesticate in China. Ann Bot 100:959–966
Fujii S, Toriyama K (2009) Suppressed expression of retrograde-regulated male sterility restores pollen fertility in cytoplasmic male sterile rice plants. Proc Natl Acad Sci USA 106:9513–9518
Fujimura T, Akagi H, Oka M, Nakamura A, Sawada R (1996) Establishment of a rice protoplast culture and application of an asymmetric protoplast fusion technique to hybrid rice breeding. Plant Tissue Cult Lett 13:243–247
Hu J, Wang K, Huang WC, Liu G, Gao Y, Wang JM, Huang Q, Ji YX, Qin XJ, Wan L, Zhu RS, Li SQ, Yang DC, Zhu YG (2012) The rice pentatricopeptide repeat protein RF5 restores fertility in Hong-Lian cytoplasmic male-sterile lines via a complex with the glycine-rich protein GRP162. Plant Cell 24:109–122
Huang RH (1997) Effect of different environmental factors on male-fertility stability of Longtepu A CMS lines. Fujian Sci Technol Rice Wheat 15:13–18
Huang JZ, E ZG, Zhang HL, Shu QY (2014) Workable male sterility systems for hybrid rice: genetics, biochemistry, molecular biology, and utilization. Rice 7:13
Huang QY, He YQ, Jing RC, Zhu RS, Zhu YG (2000) Mapping of the nuclear fertility restorer gene for HL cytoplasmic male sterility in rice using microsatellite markers. Chin Sci Bull 45:430–432
Huang WC, Hu J, Yu CC, Huang Q, Wan L, Wang LL, Qin XJ, Ji YX, Zhu RS, Li SQ, Zhu YG (2012) Two non-allelic nuclear genes restore fertility in a gametophytic pattern and enhance abiotic stress tolerance in the hybrid rice plant. Theor Appl Genet 124:799–807
Huang WC, Yu CC, Hu J, Wang LL, Dan ZW, Zhou W, He CL, Zeng YF, Yao GX, Qi JZ, Zhang ZH, Zhu RS, Chen XF, Zhu YG (2015) Pentatricopeptide-repeat family protein RF6 functions with hexokinase 6 to rescue rice cytoplasmic male sterility. Proc Natl Acad Sci USA 112:14984–14989
Itabashi E, Iwata N, Fujii S, Kazama T, Toriyama K (2011) The fertility restorer gene, Rf2, for Lead Rice-type cytoplasmic male sterility of rice encodes a mitochondrial glycine-rich protein. Plant J 65:359–367
Kazama T, Toriyama K (2014) A fertility restorer gene, Rf4, widely used for hybrid rice breeding encodes a pentatricopeptide repeat protein. Rice 7:28
Komori T, Ohta S, Murai NY, Kuraya Y, Suzuki S, Hiei Y (2004) Map-based cloning of a fertility restorer gene, Rf-1, in rice (Oryza sativa L.). Plant J 37:315–325
Li SQ, Yang DC, Zhu YG (2007) Characterization and use of male sterility in hybrid rice breeding. J Integr Plant Biol 49:791–804
Lincoln S, Daly M, Lander E (1992) Constructing genetic maps with MAPMAKER/EXP 3.0. Whitehead Institute Technical Report, Whitehead Institute, Cambridge
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Luo DP, Xu H, Liu ZL, Guo JX, Li HY, Chen LT, Fang C, Zhang QY, Bai M, Yao N, Wu H, Ji CH, Zheng HQ, Chen YL, Ye S, Li XY, Zhao XC, Li RQ, Liu YG (2013) A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat Genet 45:573–577
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76
Tang SZ, Zhang HG, Liang GH, Yan CJ, Liu QQ, Gu MH (2008) Reasons and countermeasures of slow development on Three-line Japonica hybrid rice. Hybrid Rice 23:1–5
Tang HW, Luo DP, Zhou DG, Zhang QY, Tian DS, Zheng XM, Chen LT, Liu YG (2014) The rice restorer Rf4 for wild-abortive cytoplasmic male sterility encodes a mitochondrial-localized PPR protein that functions in reduction of WA352 transcripts. Mol Plant 7:1497–1500
Wang ZH, Zou YJ, Li XY, Zhang QY, Chen LT, Wu H, Su DH, Chen YL, Guo JX, Luo D, Long YM, Zhong Y, Liu YG (2006) Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 18:676–687
Yang ZY (1994) Progresses in the breeding of japonica hybrid rice. Hybrid Rice 1994(z1):46–49
Yang ZY, Li ZB, Dong L, Zhu W, Cai Z, Qu LJ, Hua ZT (2016) Development and prospect of hybrid japonica rice in China. Chin Sci Bull 61:3770–3777
Yuan LP (1994) Increasing yield potential in rice by exploitation of heterosis. In: Virmanni SS (ed) Hybrid rice technology. New developments and future prospects. IRRI, Manila, pp 1–6
Zhang G, Lu Y, Bharaj TS, Virmani SS, Huang N (1997) Mapping of the Rf-3 nuclear fertility-restoring gene for WA cytoplasmic male sterility in rice using RAPD and RFLP markers. Theor Appl Genet 94:27–33
Zhang QY, Liu YG, Zhang GQ, Mei MT (2002) Molecular mapping of the fertility restorer gene Rf-4 for WA cytoplasmic male sterility in rice. Acta Genetica Sinica 29:1001–1004
Acknowledgements
This study was financially supported by the National Key Research and Development Program (2016YFD0101107), the National Basic Research Program of China (2013CBA01405), and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We thank Lesley Benyon, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
HZ analyzed the data and drafted the manuscript. XC and LZ evaluated the phenotypes and analyzed the data. HS and YG helped construct the BC population. MG helped design the study. ST designed the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Cheng, X., Zhang, L. et al. Rf4 has minor effects on the fertility restoration of wild abortive-type cytoplasmic male sterile japonica (Oryza sativa) lines. Euphytica 214, 49 (2018). https://doi.org/10.1007/s10681-018-2128-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-018-2128-0