Abstract
Root-knot nematodes (Meloidogyne spp.) are destructive pests of crops. Pepper (Capsicum annuum L.) contains genes that control resistance to root-knot nematodes. Using suppression subtractive hybridization and RACE strategies, a nucleotide-binding site and leucine-rich repeat (NBS-LRR) family gene, CaRKNR (FJ231739), was isolated and cloned from the nematode-resistant pepper line HDA149. CaRKNR is a novel NBS-LRR gene with an open reading frame of 3600 bp that is homologous (70.45 % identity) to the gene Mi-1.2. After Meloidogyne incognita inoculation, real-time qPCR showed that the CaRKNR expression level was increased from 0.63 to 2.16 times. Using the virus-induced gene silencing system, the CaRKNR gene’s expression level was reduced significantly than controls, and the average numbers of galls and egg masses in silenced seedlings were 44.39 and 42.01, respectively, while the controls were 0.13. This study revealed that CaRKNR was induced by M. incognita and its expression correlated with pepper resistance against root-knot nematodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root-knot nematodes (Meloidogyne spp., RKNs) are root obligate endoparasites that infect numerous plant species and cause serious damage annually to agricultural crops (Abad et al. 2008). RKN second-stage juveniles (J2) infect roots, move to the root apex, and, upon reaching the zone of cell elongation and the developing vascular cylinder, ultimately become sedentary within the root. At this point, one or more plant cells situated around the head of the J2 are stimulated by repeated stylet probing to undergo mitosis without cytokinesis and differentiate into multi-nucleated giant cells. The giant cells act as sinks, diverting plant nutrients to provide metabolic energy for the nematode. In addition, the hyperplasia and hypertrophy of the surrounding cells lead to the typical root gall (Tytgat et al. 2000).
Breeding of disease resistance is the most efficient strategy for RKN management, especially in Solanaceous plants. Resistance genes are key to the plant’s defense against RKNs. The Mi-1.2 (Solanum lycopersicum) is a single dominant gene in tomato that has been cloned. It encodes a protein with a nucleotide-binding site and leucine-rich repeat motif (NBS-LRR) (Milligan et al. 1998), and confers resistance against three major RKNs, Meloidogyne arenaria, Meloidogyne javanica, and Meloidogyne incognita (Gilbert and McGuire 1956). Currently, RKN resistance in commercially available tomato cultivars is conferred only by the Mi gene (Huang et al. 2004).
In pepper, some RKN-resistance genes (R genes) have been discovered, but few have been cloned. Two nematode-resistant pepper cultivars (Carolina Wonder and Charleston Belle) have been reported to have the nematode-resistance gene N (Thies and Fery 1998). Using doubled-haploid pepper lines, six Me genes were found, which clustered on the P9 chromosome (Djian-Caporalino et al. 2007). HDA149 is a nematode-resistant pepper doubled-haploid line that carries the single dominant gene Me3, conferring strong RKN resistance (Djian-Caporalino et al. 2001) against three major RKNs, M. arenaria, M. javanica, and M. incognita (Djian-Caporalino et al. 2001), as well as the typical hypersensitive response to RKN infection (Bleve-Zacheo et al. 1998). In this research, we isolated a novel R gene, which was induced by RKN and involved in pepper (Capsicum annuum L.) resistance against M. incognita. These findings could be conducive to breeding new nematode-resistant pepper cultivars.
Using suppression subtractive hybridization (SSH) technology, a SSH cDNA library of pepper root responses to nematode infection was constructed, and a nucleotide-binding site and leucine-rich repeat (NBS-LRR) family gene, CaRKNR, was isolated. Based on the isolated gene fragment, the full-length sequence of CaRKNR was cloned, and the expression characteristics and functions were analyzed using reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) and virus-induced gene silencing (VIGS). These results will be useful in revealing the pepper nematode-resistant response, and in pepper breeding for RKN control.
Materials and methods
Biological materials
Pepper line HDA149 was obtained from INRA (Montfavet, France). Meloidogyne incognita was grown in a greenhouse at the Chinese Academy of Agricultural Science (Beijing, China). J2 juveniles freshly hatched from egg masses were used as inocula. The pepper HDA149 seedlings were grown in the greenhouse. After 21 days of sowing, single plants were transplanted into 10 × 15 cm (diameter/high) plastic pots that contained a pasteurized mix of sandy loam soil and fine washed river sand (2:1 by volume). The pots were placed in growth chambers programmed to maintain a temperature of 22 °C and 16/8-h (light/dark) cycles (Thies and Fery 1998). 10 days after transplant, each seedling was inoculated with 600 J2 of M. incognita. The controls were mock-inoculated with water. Plant roots were harvested 12, 24 and 36 h after inoculation. At each time point, 10 inoculated and 10 mock-inoculated plants were harvested, with three independent biological replicates. Roots were washed, gently dried and sectioned 1–2 cm above the growth tip, snap-frozen in liquid nitrogen, and stored at −80 °C for future use.
Construction of the SSH cDNA library
Total RNA was isolated according to the manufacturer’s instructions using Trizol (Invitrogen, Paisley, UK). Poly(A)+ RNAs were purified from total RNA using a mRNA isolation system (Promega, USA). The cDNAs were reverse-transcribed from ~2 μg purified mRNA of each sample. Double-stranded cDNAs were synthesized and amplified using the BD SMART PCR cDNA Synthesis Kit (Takara, Japan). A cDNA sample from root tips inoculated with RKN was used as a tester, while another sample from root tips mock-inoculated with water was used as a driver; both of these were used to constructed a SSH cDNA library. The cDNA subtractive hybridization and selective amplification of cDNA fragments were performed according to the user manual for the PCR-Select cDNA Subtractive Kit (Takara, Japan). The cDNA fragments specific to the tester were then amplified by a primary PCR consisting of 26 cycles with PCR primer 1 and a secondary PCR of 11 cycles using nested primers 1 and 2R (Table supplement 1, S1). The amplified cDNA fragments, purified with the QIAquick PCR Purification Kit (Qiagen, USA), were ligated into the pGEM T-easy vector (Promega, USA). Subsequently, the products were introduced into Escherichia coli strain Top10 (Tiangen, China). The subtracted cDNA library of HDA149 was constructed. Dot blot hybridization was carried out according to the manufacturer’s protocol (DIG Nonradioactive Nucleic Acid Labeling and Detection System kit, Roshi, USA). The differential screening of clones was performed according to the recommended protocol.
Microarray hybridization and signal analysis were carried out as described previously (González-Candelas et al. 2010). Only signal values 1.3 times greater than the corresponding background and derived from at least two replicate hybridizations were taken as valid measurements (López-pérez et al. 2014). Using Sanger dideoxy sequencing technology, these positive clones were sequenced by the Chinese National Human Genome Center (Sino Geno Max Co., Ltd, Beijing, China). All of the expressed sequence tags (ESTs) were submitted to the National Center of Biotechnology Information (NCBI) database of expressed sequence tags (dbEST) and published.
Isolation of CaRKNR and sequence analysis
The sequence of the full-length cDNA of CaRKNR was obtained by 5′ and 3′ rapid amplification of cDNA ends (RACE), using the 5′, 3′-RACE System for Rapid Amplification of cDNA Ends kit (Invitrogen,UK). In the 3′-RACE procedure, mRNAs are converted into cDNAs using reverse transcriptase and an oligo-dT adapter primer (AP). Specific cDNA is then amplified by PCR using a gene-specific primer (GSP3) that anneals to a region of known exon sequences and an AP that targets the poly(A) tail region (3′-AUAP). 5′-RACE uses an antisense gene-specific primer (GSP2) for the synthesis of specific cDNA by reverse transcriptase. Prior to PCR, a dT-tailing step attaches an adapter sequence to the unknown 5′ sequences of the cDNA. Specific cDNA is then amplified by PCR using a nest GSP (GSP1) that anneals in a region of known exon sequences and an AP that targets the 5′ terminus (5′-AUAP). The anchor and APs were selected based on the manufacturer’s recommendations.
The entire nucleotide sequences of CaRKNR clones were determined using an automatic DNA sequencer (ABI Prism, USA). With the complete ORF primers, CaRKNR-F and CaRKNR-R, the full-length sequence of CaRKNR cDNA was amplified. The GSP1-3, CaRKNR-F, and CaRKNR-R primer sequences are supplied in Table S1.
A phylogenetic analysis, including distance, parsimony, and bootstrap analyses, was performed using Mega (version 6.01). Neighbor-joining (NJ) was the primary method to infer phylogenetic relationships between the NBS genes (Meyers et al. 2003). Numbers on branches indicate the percentage of 1000 bootstrap replicates that support the adjacent node.
RT-qPCR
RT-qPCR was performed to determine the expression pattern of CaRKNR in resistant pepper line HDA149. Total RNA samples from root tips, stems, leaves, buds, and young fruits of HDA149 were reverse-transcribed using an oligo-dT nucleotide and the Super Script III Reverse Transcriptase (Invitrogen,UK). qPCR was performed using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, USA) with 1.1 software. The amplification program was as follows: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s, 60 °C for 30 s and 72 °C for 30 s, using qPCR SYBR Premix Ex Taq II for the fluorophore SYBR green with fluorescein (Takara, Japan). Standard curves were established with five serial dilutions of first-strand cDNA, ranging from 1 to 1/10,000. As a reference, the β-actin cDNA was amplified using the primers β-actin F1 and R2 (Table S1). The CaRKNR gene-specific primers used for qPCR are provided in Table S1. The relative abundance of transcripts was calculated by the comparative threshold cycle (CT) method (Applied Bio-systems, USA). RT-qPCR was carried out in triplicate for each sample.
Pathogen inoculations
To reveal the CaRKNR expression-level changes in pepper roots, 6-week-old pepper plants were challenged by different pathogens, RKN (M. incognita), tobacco mosaic virus (TMV), Ralstonia solanacearum, and Phytophthora capsici Leonian. These plant pathogens were stored in the disease lab of the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Science.
Meloidogyne incognita (1000 J2) were inoculated into the rhizosphere soil of each pepper seedling. The TMV inoculum, 1 mg TMV-infected tobacco leaves, were suspended in 5 ml phosphate buffer (50 mM) and then applied to the surface of fully expanded pepper plant leaves and rubbed with Carborundum. Ralstonia solanacearum was cultured in triphenyl tetrazolium chloride (TTC) liquid medium at 28 °C. The inocula were collected by centrifuge, re-suspended in sterilized water to 106 CFU ml−1, and then infiltrated into the leaves by needleless syringe. Phytophthora capsici was cultured on V8 agar medium for 5 days at 25 °C, the cultures were flooded with sterilized water, incubated at 25 °C for 45 min, refrigerated at 5 °C for 15 min, and then kept at room temperature. The suspension was passed through miracloth to separate the fungal material from pieces of agar, homogenized in a low-speed Warren blender for 5 min, and filtered through a double-layer cheesecloth to collect P. capsici zoospores (105 spores ml−1); these zoospores were used as inocula to spray on the leaves. The pathogens inoculation test included three independent replicates. These inoculated plants were incubated under 100 % relative humidity (Zheng et al. 2011). Plants mock-inoculated with sterile distilled water were controls. These pots were placed in growth chambers programmed to maintain a temperature of 22 °C, with a 16/8-h (light/dark) cycle (Thies and Fery 1998). At 36 h post-inoculation, sections of root tips (1–2 cm) were taken as samples for CaRKNR expression-level analysis, and total RNA isolation, DNAase treatment, cDNA synthesis, and RT-qPCR were performed as described above.
VIGS for CaRKNR
Using PCR (primers in Table S1), restriction enzymes and ligase, the CaRKNR cDNA fragments CaRKNR-1 (657 bp) and CaRKNR-2 (416 bp) were cloned into the BamHI-KpnI site of the pTV00 vector. pTV-CaRKNR1, pTV-CaRKNR2, and pTV00 (empty vector) were transformed into Agrobacterium tumefaciens strain GV3101, and the transformed cells were selected on YEB media containing 50 mg L−1 kanamycin, 15 mg L−1 tetracycline, and 50 mg L−1 rifampicin. These transformants were grown at 28 °C, centrifuged, re-suspended to an OD600 = 0.5 in buffer (10 mM MgCl2, 10 mM MES, and 200 µM acetosyringone), and shaken at 22–25 °C for 5 h. Transformant cultures were mixed at a 1:1 ratio with A. tumefaciens containing the tobacco rattle virus vector (TRV1, pBINRA6, OD600 = 0.5) (Liu et al. 2002).
The mixture was infiltrated into cotyledons of 6-week-old HDA149 pepper seedlings using a 5-ml needleless syringe. At the same time, mock inoculations using distilled water were performed as controls. The inoculated plants were transferred to a growth chamber maintained at 16 °C for 1 day with 60 % relative humidity, and then placed in a growth room at 25 °C with a light intensity of ~400 µmol m−2 s−1 in a 16/8-h light/dark cycle. For each experiment treatment, 10 chili pepper plants were inoculated and with three biological repeats. Ten days after infiltration, 90 positive silenced plants were selected by PCR (primers are listed in Table S1), and inoculated with 3000 J2 M. incognita per plant. Of these, 45 plants were used to measure the numbers of root knots and egg masses 8 weeks after nematode inoculation, and the other 45 were used for real-time qPCR analysis of the CaRKNR expression level. The VIGS test for CaRKNR was performed with three repeats. The numbers of galls and egg masses were analyzed using Duncan’s new multiple range method, available in the ANOVA section of the SAS9.1 software.
Results and analysis
Full length of CaRKNR cDNA
Using SSH, a smeared PCR product was produced, ranging largely from 200 to 700 bp. Differential screening resulted in 1200 positive clones from the library. Based on the blot and sequencing, 211 ESTs were identified as either submergence-induced or highly expressed after differential screening of the HDA149 SSH cDNA library. These EST sequences were published on the NCBI dbEST database (JZ820466-JZ820676). The EST H-634 (JZ820592) was homologous to the nematode-resistance gene Mi-1 copy 2 (U65668.1) (Fig. S1), with 100 % coverage and 85 % identity. Thus, H-634 was confirmed as a candidate pepper nematode-resistance gene and named CaRKNR.
Based on the 610-bp nucleotide sequence of the CaRKNR gene fragment, the full-length cDNA sequence was obtained using RACE technology (GenBank accession number FJ231739). The full-length cDNA of CaRKNR is 4697 bp long, and includes a putative transcription start site, a potential open reading frame of 3600 bp, 123 bp 5′- and 974 bp 3′-untranslated regions, and a poly(A) tail. The potential open reading frame encodes 1199 amino acid residues (ACI43068.1).
Sequence alignment and phylogenetic analysis
The protein family (Pfam) blast (http://pfam.sanger.ac.uk/search) identified two Pfam-A matches to the CaRKNR sequence, a NB-ARC domain (467–747) and a LRR domain (894–954), which belonged to the gene families NB-ARC (PF00931) and LRR 8 (PF13855), respectively.
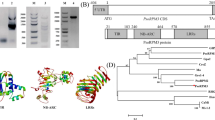
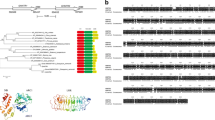
The CaRKNR protein sequence was blasted in NCBI (www.ncbi.nlm.nih.gov) using the BlastP program, and the first 38 proteins with maximum E values of 0, and 61 proteins with E values more than 2e-101, belonged to the AAA superfamily, which included a conserved NB-ARC domain. From these proteins, 23 were selected to construct a phylogenetic tree, which showed that CaRKNR and the disease resistance homolog Mi1-2 (AAC32252.1) in tomato (S. lycopersicum) were clustered together (Fig. 1). The homolog analysis (DNAman 6.0) showed that the protein sequence of CaRKNR was 70.45 %, identical to that of the RKN-resistance protein Mi-1.2. The motifs of 10 homologous genes were analyzed by MEME (version 4.9.1, http://meme.nbcr.net/), which identified three common motifs among these genes. The locations of motifs 1 and 2 overlapped with the conserve domains NB-ARC and LRR (Figs. 2, S2). This revealed that the CaRKNR gene belonged to the NBS-LRR gene family.
Phylogenetic tree analysis of a nucleotide-binding site and leucine-rich repeat (NBS-LRR) family protein from pepper (Capsicum annuum L.), CaRKNR, and some resistance proteins. A total of 23 proteins encoded by resistance genes were analyzed in this phylogenetic tree. The red dot indicates CaRKNR (ACI43068), and the other protein sequences were downloaded from NCBI (www.ncbi.nlm.nih.gov). Solanum lycopersicum sequences: AAC97933.1, AAC32252.1, AAC67238.1, AAC67237.1, AAC32253.1, ABI96216.1, CAD29725.1, CAD29726.1, CAD29729.1, AAG31014.1, AAG31013.1, AAG31017.1, AFN86172.1, AAG31016.1 and AAC49408.1; Capsicum annuum, ABE68835.1; Solanum bulbocastanum, AAZ95005.1; Solanum sp. VFNT, ABI96212.1; Solanum demissum, AAT39951.2; Solanum tuberosum, ABO93000.1; Nicotiana tabacum, ACE79482.1; and Solanum demissum, AAT40487.1. The bar shows 0.2 scale length
Analysis of three conserved motifs among the homologous proteins identified by MEME. Rectangles shaded with diagonal lines, spots, and grids indicate the locations of motifs 1–3, respectively. The line represents the sequences of homologous proteins and the ruler shows the lengths of the sequences. Protein sequences AAC97933.1, AAC32252.1, AAC67238.1, AAC67237.1, AAC32253.1, and ABI96216.1 are from Solanum lycopersicum, CaRKNR (ACI43068) and ABE68835.1 are from Capsicum annuum, AAZ95005.1 is from Solanum bulbocastanum, and ABI96212.1 is from Solanum sp. VFNT
Expression pattern analysis of CaRKNR
RT-qPCR was used to analyze CaRKNR expression levels in different tissues of resistant pepper line HDA149. The results indicated that CaRKNR was highly expressed in the bud, ~4.9 times greater than the reference gene β-actin, was weakly expressed in fruit, stem and root (0.2, 0.5 and 0.6 times the reference gene, respectively), and was undetectable in the leaves (Fig. 3).
RT-qPCR analysis of the CaRKNR expression level in different tissues of pepper HDA149. In this diagram, five bar graphs indicate the CaRKNR expression levels in bud, fruit, leaf, root, and stem samples of the pepper HDA149. Each bar graph represents the mean ± SD of three independent biological replicates
The pepper HDA149 was challenged by different pathogens, RKN (M. incognita), TMV, R. solanacearum, and P. capsici Leonian. The RT-qPCR revealed that the CaRKNR transcript level of the control was 0.467 time, while that of the RKN treatment was 1.523, 3.26 times higher than the control. The CaRKNR transcript levels after TMV, R. solanacearum, and P. capsici treatments were 0.492, 0.467 and 0.375 time, respectively (Fig. 4), and Duncan’s new multiple range method showed that the ANOVA sum of squares and mean square were 2.861 and 0.715, respectively (F = 91.65, P value < 0.0001). The CaRKNR transcript level in RKN plants was significantly higher than in plants receiving other treatments, but the differences among the control, TMV, R. solanacearum, and P. capsici treatments were not significant, indicating that the CaRKNR gene responded to RKN.
qPCR analysis of the CaRKNR expression level in pepper HDA149 challenged by four pathogens. Five bar graphs indicate the CaRKNR expression levels in pepper HDA149 root tissues and in the same tissue after the pepper plants were challenged by the pathogens Meloidogyne incognita, tobacco mosaic virus (TMV), Ralstonia solanacearum, and Phytophthora capsici Leonian. Each bar graph represents the mean ± SD of three independent biological replicates. The asterisk indicates significant difference at the P = 0.05 level by an ANOVA statistical analysis, the F value = 91.65, P value < 0.0001
VIGS of CaRKNR
8 weeks after nematode inoculation, CaRKNR VIGS effects were detected (Fig. 5). In the controls with mock Agrobacterium buffer and the pTV00 empty vector (negative control), the gall numbers were only 0.13 and 0.18, respectively. However, for the pTV-CaRKNR1 and pTV-CaRKNR2 treatments, the average numbers of galls were 50.22 and 38.56, respectively (Fig. 6). Compared with the control, the galls of CaRKNR-silenced seedlings were significantly increased (F = 71.31 and P < 0.0001). In addition, there was also a significant difference between the VIGS pTV-CaRKNR1 and pTV-CaRKNR2 treatments. The statistics of egg masses for VIGS treatments had a similar results with galls (F = 118.62 and P < 0.001). These showed that different cDNA fragments caused different VIGS effects. The VIGS effects revealed that CaRKNR has a RKN-resistance function.
Root galls and egg masses on pepper HDA149 seedlings after virus-induced gene silencing (VIGS) treatments. The numbers of galls and egg masses on the VIGS-treated seedlings (part a, pTV-CaRKNR1 and part b, pTV-CaRKNR2) were significantly greater than on the negative control (part c, empty pTV00) and mock (part d, buffer). The arrows indicate the gall and egg masses. CaRKNR: a nucleotide-binding site and leucine-rich repeat (NBS-LRR) family gene fragment from pepper (Capsicum annuum L.)
Gall and egg mass statistical analysis for different treatments in the CaRKNR VIGS test. Bar graphs indicate the numbers of galls and egg masses after different VIGS treatments, pTV-CaRNR1, pTV-CaRNR2, pTV00 and a mock control. The bar graphs shaded with spots indicate the gall quantity, and the bar graphs shaded with diagonals indicate the egg mass quantity. Each bar graph represents the mean ± SD of three independent biological replicates. The capital letters (A, B, C) on each bar graph indicate significant difference at P = 0.01 by ANOVA statistical analysis (Gall F value = 1097.70, P < 0.0001; Egg mass F value = 905.14, P < 0.0001), and the same letters are not significantly different
The effect of VIGS was analyzed at the RNA level by real-time RT-qPCR. After agro-infiltration, the normalized CaRKNR mRNA levels of the pTV00 and control plants were 0.63 and 0.57 times, respectively, but for the pTV-CaRKNR1 and pTV-CaRKNR2 treatments the levels were 0.27 and 0.41 times, respectively, reflecting decreases of 57.1 and 34.7 % compared with the controls. After RKN inoculation from 0 to 7 days, the CaRKNR mRNA level of control plants was increased significantly from 0.63 to 2.16. However, in silencing plants, the CaRKNR mRNA level showed only a slow increase (Fig. 7). These RT-qPCR results revealed that the CaRKNR mRNA in VIGS plants was silenced effectively.
Pepper HDA149 CaRKNR mRNA level analysis in different VIGS treatments. The bar graphs in the diagram indicate the CaRKNR expression levels from 0 to 7 days after RKN inoculation; bars shaded with gray, spots, diagonal lines, and a grid indicate the control, pTV00, pTV-CaRKNR1 and pTV-CaRKNR2 treatments, respectively
Discussion
CaRKNR is a novel resistance gene belonging to the NBS-LRR family
Using SSH technology, CaRKNR was isolated and cloned from the nematode-resistant pepper HDA149. Its encoded protein has a domain structure, including a central conserved region with NBS and C-terminal LRR domains, which are characteristics of the NBS-LRR family of resistance genes (Belkhadir et al. 2004). NBS-LRR motifs are common in nematode R genes, such as Mi-1, Hero A, Gpa2, Gro1, and Ma (Milligan et al. 1998; Ernst et al. 2002; Van der Vossen et al. 2000; Paal et al. 2004; Claverie et al. 2004; Chen et al. 2007). The CaRKNR protein was homologous to the Mi-1.2 protein, and 70.45 % of the sequence was identical. Mi-1.2 from tomato is a member of the NBS-LRR family that has been cloned and is located on chromosome 6 (Ammiraju et al. 2003). It offers resistance to the RKNs M. javanica, M. incognita, and M. arenaria, and triggers a localized tissue necrosis or hypersensitive response (Milligan et al. 1998). At present, nine genes of the Mi family have been reported in tomato (Jablonska et al. 2007). Mi-1 was the first RKN-resistance gene to be cloned (Milligan et al. 1998), and was used exclusively for 60 years in breeding tomatoes resistant to RKN. Thus, finding and exploiting novel nematode-resistant genes is important to control RKNs.
In pepper, six Me genes have been reported, and they cluster in a single genomic region within a 28-cM interval on chromosome P9. This genomic area is colinear to chromosome T12 of tomato and chromosome XII of potato (Djian-Caporalino et al. 2007). Not all of the Me genes have been cloned, but it was reported that HDA149 had a single dominant nematode resistance gene Me3 that induced localized cell necrosis in infected plants (Bleve-Zacheo et al. 1998). CaRKNR was cloned from HDA149, but it was necessary to determine whether it was a homolog of Me3 or another Me gene in pepper. Recently, the genome sequences of pepper were published, providing an important basis for CaRKNR gene mapping to determine the relationships among the pepper’s nematode-resistant genes (Kim et al. 2014; Qin et al. 2014). With the program Blast, CaRKNR was searched in the pepper Zunla1 (Capsicum annuum L.) genome database (accession ASJU00000000, release 2.0) (http://peppersequence.genomics.cn), results showed that CaRKNR was mapped on the chromosome 6, the Expect = 0.0, Identities = 99.5 %, covered from 219,292,195 to 219,295,794 bp of chromosome 6. Similar, in the Pepper CM334 (Capsicum annuum cv.) genome (AYRZ00000000) CDS database (V1.55) and chromosome database (V1.55), CaRKNR was also mapped on the chromosome 6, the alignment gene was CA00g56860, and Expect = 0.0, Identities = 99.7 %. From these results we putative that CaRKNR is on chromosome 6, not 9, don’t belong to Me family genes, but a novel NBS-LRR gene member from pepper.
CaRKNR is involved in M. incognita resistance
VIGS is an effective technology for the functional characterization analysis of genes involved in nematode resistance. Mi silencing correlated with the resistance-breaking phenotype and successful nematode development (Valentine et al. 2004). The study of a TRV-Mi construct indicated that the heat-stable resistance gene Mi-9 is mediated by a homolog of Mi-1 (Jablonska et al. 2007). Using VIGS, we found that the gene silencing of CaRKNR resulted in increased production of galls and egg masses in the pepper HDA149. The average number of galls after the two VIGS treatments (pTV-CaRKNR1 and pTV-CaRKNR2) was 44.39, and the egg mass was 42.01, which was significantly more than the empty vector control (0.13) or mock control (0.18), indicating that the resistance of pepper HDA149 against nematodes was weakened. At the same time, real-time RT-qPCR showed that the normalized mRNA level of CaRKNR was decreased in the silenced plants. At 7 days post-RKN inoculation, the average level was 0.95 time, but the control was 2.16 times, indicating that the VIGS treatments were effective in decreasing the CaRKNR expression level. The results of the VIGS tests and qPCR analysis demonstrated that CaRKNR functions are involved in the pepper’s M. incognita resistance.
The expression characteristics analysis showed that the CaRKNR gene responds to RKN and that after the inoculation of nematodes, the expression level was significantly increased. However, the qPCR data indicated that CaRKNR was more highly expressed in the bud tissue than in the root tissue, which is interesting. Since the bud tissue undergoes active growth and metabolism, the high CaRKNR expression level could be related to this activity. Mi-1 conferred resistance to RKNs, and the Mi gene was also involved in resistance to some potato aphids (Macrosiphum euphorbiae) and the sweet potato whitefly (Bemisia tabaci) (Rossi et al. 1998; Nombela et al. 2003). Whether the CaRKNR gene’s high expression level in buds has a resistance function remains to be determined.
Conclusion
CaRKNR, a novel NBS-LRR family gene, was cloned from the nematode-resistant pepper HDA149. A RT-qPCR analysis showed that the expression of CaRKNR was induced by M. incognita in pepper roots. The VIGS tests indicated that CaRKNR expression levels were decreased effectively in root, and that the numbers of galls and egg masses were significantly increased compared with the control. Thus, the pepper resistance to nematodes was weakened. This study revealed that CaRKNR was involved in pepper resistance against M. incognita, which may be important in pepper breeding.
Abbreviations
- ARC:
-
APAF-1, R proteins, and CED-4
- Blast:
-
Basic local alignment search tool
- LRR:
-
Leucine-rich repeat
- NB:
-
Nucleotide binding
- NJ:
-
Neighbour-joining
- ORF:
-
Open reading frame
- Pfam:
-
Protein family
- RACE:
-
Rapid amplification of cDNA end
- RKN:
-
Root-knot nematode (Meloidogyne spp.)
- RT-qPCR:
-
Reverse transcriptase quantitative polymerase chain reaction
- SSH:
-
Suppression subtractive hybridization
- VIGS:
-
Virus induced gene silencing
References
Abad P, Gouzy J, Aury JM, Castagnone-Sereno P, Danchin EG, Deleury E, Perfus-Barbeoch L, Anthouard V, Artiguenave F, Blok VC (2008) Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26:909–915
Ammiraju JS, Veremis JC, Huang X, Roberts PA, Kaloshian I (2003) The heat-stable root-knot nematode resistance geneMi-9 from Lycopersicon peruvianum is localized on the short arm of chromosome 6. Theor Appl Genet 106:478–484
Belkhadir Y, Subramaniam R, Dangl JL (2004) Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr Opin Plant Biol 7:391–399
Bleve-Zacheo T, Bongiovanni M, Melillo MT, Castagnone-Sereno P (1998) The pepper resistance genes Me1 and Me3 induce differential penetration rates and temporal sequences of root cell ultrastructural changes upon nematode infection. Plant Sci 133:79–90
Chen R, Li H, Zhang L, Zhang J, Xiao J, Ye Z (2007) CaMi, a root-knot nematode resistance gene from hot pepper (Capsium annuum L.) confers nematode resistance in tomato. Plant Cell Rep 26:895–905
Claverie M, Dirlewanger E, Cosson P, Bosselut N, Lecouls A, Voisin R, Kleinhentz M, Lafargue B, Caboche M, Chalhoub B (2004) High-resolution mapping and chromosome landing at the root-knot nematode resistance locus Ma from Myrobalan plum using a large-insert BAC DNA library. Theor Appl Genet 109:1318–1327
Djian-Caporalino C, Pijarowski L, Fazari A, Samson M, Gaveau L, O’byrne C, Lefebvre V, Caranta C, Palloix A, Abad P (2001) High-resolution genetic mapping of the pepper (Capsicum annuum L.) resistance loci Me3 and Me4 conferring heat-stable resistance to root-knot nematodes (Meloidogyne spp.). Theor Appl Genet 103:592–600
Djian-Caporalino C, Fazari A, Arguel M, Vernie T, VandeCasteele C, Faure I, Brunoud G, Pijarowski L, Palloix A, Lefebvre V (2007) Root-knot nematode (Meloidogyne spp.) Me resistance genes in pepper (Capsicum annuum L.) are clustered on the P9 chromosome. Theor Appl Genet 114:473–486
Ernst K, Kumar A, Kriseleit D, Kloos DU, Phillips MS, Ganal MW (2002) The broad-spectrum potato cyst nematode resistance gene (Hero) from tomato is the only member of a large gene family of NBS-LRR genes with an unusual amino acid repeat in the LRR region. Plant J 31:127–136
Gilbert JC, McGuire DC (1956) Inheritance of resistance to severe root knot from Meloidogyne incognitain commercial type tomatoes. Proc Am Soc Hortic Sci 68:437–442
González-Candelas L, Alamar S, Sánchez-Torres P, Zacarías L, Marcos J (2010) A transcriptomic approach highlights induction of secondary metabolism in citrus fruit in response to Penicillium digitatum infection. BMC Plant Biol 10:194–211
Huang X, McGiffen M, Kaloshian I (2004) Reproduction of Mi-virulent Meloidogyne incognita isolates on Lycopersicon spp. J Nematol 36(1):69–75
Jablonska B, Ammiraju JS, Bhattarai KK, Mantelin S, de Ilarduya OM, Roberts PA, Kaloshian I (2007) The Mi-9 gene from Solanum arcanum conferring heat-stable resistance to root-knot nematodes is a homolog of Mi-1. Plant Physiol 143:1044–1054
Kim S, Park M, Yeom SI, Kim YM, Lee JM, Lee HA, Seo E, Choi J, Cheong K, Kim KT (2014) Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat Genet. doi:10.1038/ng.2877
Liu Y, Schiff M, Dinesh-Kumar S (2002) Virus-induced gene silencing in tomato. Plant J 31:777–786
López-pérez M, Ballester AR, González-candelas L (2014) Identification and functional analysis of Penicillium digitatum genes putatively involved in virulence towards citrus fruit. Mol Plant Pathol. doi:10.1111/mpp.1217
Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR encoding genes in Arabidopsis. Plant Cell Online 15:809–834
Milligan SB, Bodeau J, Yaghoobi J, Kaloshian I, Zabel P, Williamson VM (1998) The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell Online 10:1307–1319
Nombela G, Williamson VM, Muniz M (2003) The root-knot nematode resistance gene Mi-1.2 of tomato is responsible for resistance against the whitefly Bemisia tabaci. Mol Plant Microbe Interact 16:645–649
Paal J, Henselewski H, Muth J, Meksem K, Menéndez CM, Salamini F, Ballvora A, Gebhardt C (2004) Molecular cloning of the potato Gro1-4 gene conferring resistance to pathotype Ro1 of the root cyst nematode Globodera rostochiensis, based on a candidate gene approach. Plant J 38:285–297
Qin C, Yu C, Shen Y, Fang X, Chen L, Min J, Cheng J, Zhao S, Xu M, Luo Y, Yang Y, Wu Z, Mao L, Wu H, Ling-Hu C, Zhou H, Lin H, González-Morales S, Trejo-Saavedra DL, Tian H, Tang X, Zhao M, Huang Z, Zhou A, Yao X, Cui J, Li W, Chen Z, Feng Y, Niu Y, Bi S, Yang X, Li W, Cai H, Luo X, Montes-Hernández S, Leyva-González MA, Xiong Z, He X, Bai L, Tan S, Tang X, Liu D, Liu J, Zhang S, Chen M, Zhang L, Zhang L, Zhang Y, Liao W, Zhang Y, Wang M, Lv X, Wen B, Liu H, Luan H, Zhang Y, Yang S, Wang X, Xu J, Li X, Li S, Wang J, Palloix A, Bosland PW, Li Y, Krogh A, Rivera-Bustamante RF, Herrera-Estrella L, Yin Y, Yu J, Hu K, Zhang Z (2014) Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. PNAS 111(14):5135–5140
Rossi M, Goggin FL, Milligan SB, Kaloshian I, Ullman DE, Williamson VM (1998) The nematode resistance gene Miof tomato confers resistance against the potato aphid. Proc Natl Acad Sci USA 95:9750–9754
Thies JA, Fery RL (1998) Modified expression of the N gene for southern root-knot nematode resistance in pepper at high soil temperatures. J Am Soc Hortic Sci 123:1012–1015
Tytgat T, Meutter JD, Gheysen G, Coomans A (2000) Sedentary endoparasitic nematodes as a model for other plant parasitic nematodes. Nematology 2:113–121
Valentine T, Shaw J, Blok VC, Phillips MS, Oparka KJ, Lacomme C (2004) Efficient virus-induced gene silencing in roots using a modified tobacco rattle virus vector. Plant Physiol 136:3999–4009
Van Der Vossen EA, Der Voort V, Rouppe JN, Kanyuka K, Bendahmane A, Sandbrink H, Baulcombe DC, Bakker J, Stiekema WJ, Klein-Lankhorst RM (2000) Homologues of a single resistance-gene cluster in potato confer resistance to distinct pathogens: a virus and a nematode. Plant J 23:567–576
Zheng J, Zou X, Mao Z, Xie B (2011) A novel pepper (Capsicum annuum L.) WRKY gene, CaWRKY30, is involved in pathogen stress responses. J Plant Biol 54:329–337
Acknowledgments
We thank INRA Versailles for providing the pepper HDA149. This work was supported by the National Science Foundation of China (30971905, 31101425), Agro-scientific Research in the Public Interest (201103018) and the China Agriculture Research System (CARS-25).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mao, Z., Zhu, P., Liu, F. et al. Cloning and functional analyses of pepper CaRKNR involved in Meloidogyne incognita resistance. Euphytica 205, 903–913 (2015). https://doi.org/10.1007/s10681-015-1438-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1438-8