Abstract
Seed longevity is a crucial issue for germplasm conservation and seed marketing. This trait is determined not only by environmental conditions, but also by genetic factors. Molecular mapping of responsible loci has been performed with several crops, but not with tobacco (Nicotiana tabacum L.). In the present study, we investigate 122 recombinant inbred lines derived from a cross between the cultivars Florida 301 and Hicks. Four germination-related traits were studied by examining seeds either untreated or after controlled deterioration (CD): total germination (TG, %), normal germination (%), time to reach 50 % of TG (h), and the area under the curve after 200 h of germination. Whereas Hicks exhibited high germination percentage and speed in untreated (fresh) seeds, Florida 301 seems to withstand the CD treatment better, having increased seed longevity. In total, four genomic regions located on four different linkage groups (LGs) were identified to be associated with the selected traits. Positive alleles for the individual traits were contributed by both parents. A major quantitative trait locus (QTL) for high percentage TG located on LG 8/18 appeared in both control and deteriorated seeds and was contributed by Hicks. In contrast, Florida 301 donated a favorable allele for germination speed on LG seven after CD only. The position of this locus compared well with a QTL detected in the same population, in a former study examining resistance against the black shank disease caused by Phytophthora nicotianae). The effects of environmental growing conditions of the mother plants on seed longevity are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lifespan, or longevity, of seeds is an important subject, not only for the seed industry but also for the ex situ conservation of germplasm collections. It is known that there are distinctive differences between crops. The first scientific papers on crop-specific differences in seed longevity appeared in the nineteenth century. Haberlandt (1873) reported that barley lost half of its germination capacity in 4 years, maize and oats lost half in 6 years, wheat lost practically all in 6 years, and rye lost all in 3 years. More recent studies, by Walters et al. (2005) and Nagel and Börner (2010), confirm that seed deterioration behavior is species specific. However, there are also hints of an intraspecific variability. Early studies on rice (Jones 1926) and soybeans (Burgess 1938) suggested that some varieties stored better than others under comparable conditions. Other examples of varietal differences in seed longevity for a range of crop species are described by Priestley et al. (1985).
In order to study the longevity of seeds in a reasonable period of time (not spending years) artificial ageing tests were established. Seeds are exposed for short periods to the two environmental variables which cause rapid seed weakening; high temperature and high relative humidity (RH). High vigour seed lots will withstand these extreme stress conditions and deteriorate at a slower rate than low vigour ones (Hampton and TeKrony 1995).
First attempts at determining the genetic loci responsible for seed storability were made for Arabidopsis (Bentsink et al. 2000) and rice (Miura et al. 2002). For Arabidopsis, the authors analyzed 162 recombinant inbred lines (RIL) of a bi-parental cross. Four putative quantitative trait locus/loci (QTL) affecting seed longevity were identified on three different chromosomes. Another RIL population comprising 114 lines was investigated by Clerkx et al. (2004). The authors detected three QTL on three different chromosomes, two of which were in highly comparable positions to those detected by Bentsink et al. (2000).
For rice, Miura et al. (2002) initiated a study on seed longevity using 98 backcross inbred lines. Analysis revealed three QTL. Zeng et al. (2006) and Xue et al. (2008) analysed 127 doubled haploid lines and 71 RILs, respectively, detecting three QTL each. All three studies pointed to rice chromosome 9 as the carrier of an important genomic region affecting seed longevity in comparable chromosomal regions.
In addition to the pilot examinations in Arabidopsis and rice, genetic studies on seed longevity also followed for soybean (Singh et al. 2008), barley (Nagel et al. 2009, 2014), maize (Revilla et al. 2009), wheat (Landjeva et al. 2010; Rehman Arif et al. 2012), lettuce (Schwember and Bradford 2010), and oilseed rape (Nagel et al. 2011).
Comprehensive studies on seed longevity for Nicotiana species were initiated by Agacka et al. (2013, 2014). Seeds stored at different temperatures (20, 0, −15/−18 °C) were investigated. It was demonstrated that decreasing the temperature from 20 to 0 °C increases the storability of seeds of selected Nicotiana species from about 10 to 30 years, and that decreasing it further, to −15/−18 °C, increases storability to more than 50 years, as measured by a germination threshold higher than 75 %. As in other species, intraspecific variation was noticeable.
In order to study the genetics of seed longevity in Nicotiana, we followed an approach that had been adopted for barley, wheat, and rapeseed (Nagel et al. 2009, 2011, 2014; Rehman Arif et al. 2012). Seed lots of a previously existing mapping population, developed for detecting QTL for distinctive characters, were used for investigating germination related traits. In the present study, we used seeds of RILs derived from a cross between tobacco (Nicotiana tabacum) cultivars Florida 301 and Hicks. Originally, this mapping population was developed with the aim of finding QTL associated with resistance to black shank, caused by Phytophthora nicotianae (Xiao et al. 2013).
Materials and methods
Plant material
A population of 122 RILs (F5:6) was derived from the cross Florida 301 × Hicks using single seed descent. Seed of the RILs was produced from greenhouse-grown plants in 2007, whereas seed of the parental lines originated from field plots in 2004. Seed were stored under ambient temperatures, but at a relative humidity of about 30 % until experiment was carried out in 2014. The linkage map, consisting of 339 markers distributed among 24 linkage groups (LGs), covered a total length of 1,176.54 cM (Xiao et al. 2013). The number of markers on each LG varied from 5 to 29. LG numbers were assigned as published by Bindler et al. (2011).
Experimental design
Seed germination for control treatment (C) and after controlled deterioration (CD) was performed for each entry in four replications (independent experiments) of 50 seeds per line on moistened filter paper (90 mm roundfilter, C 160; Munktell & FILTRAK GmbH, Germany) in Petri dishes placed in a climate chamber (Panasonic MLR-352-PE). Corresponding to ISTA (2014) the temperatures were 30 ± 2 and 20 ± 2 °C, during illumination (8 h) and darkness (16 h), respectively.
In order to determine the germination speed, germinated seeds (visible radical emergence) were counted daily within a germination period of 10 days. Based on these counts, the time needed to reach 50 % of total germination (TG) and the area under the curve (AUC; the integration of the fitted curve between t = 0 and a defined endpoint t = x) after 200 h of germination were determined using germination software GERMINATOR (Joosen et al. 2010). On day 10, the TG and the number of seedlings showing a normal appearance [normal germination (NG)] according to ISTA (2014) were recorded.
To achieve identical initial moisture content, seeds were placed into permeable paper bags and equilibrated at 22 ± 2 °C and 10 % (RH). One week before CD seeds were placed above 8.7 M unsaturated lithium chloride LiCl (47 % RH and 20 °C, calculated based on the Seed Information Database, Royal Botanic Gardens, Kew, UK) on plastic racks, which were positioned in airtight boxes. Afterwards, unsaturated LiCl solution was replaced by 7.1 M LiCl solution to reach RH of 60 % inside the boxes (Hay et al. 2008). The boxes were placed in ageing chambers at a temperature of 45 °C for 30 days. Temperature and time were chosen based on a pre-experiment performed with the parental lines only (data not shown).
QTL analysis was conducted using software QTL Cartographer 2.5 (Zeng 1994). QTL having LOD scores higher than three (major QTL) were considered. QTL having LOD >2, <3 were considered only when they appeared in positions of major QTL. Mapping was performed for traits (C and CD), %TG (%TG-C, %TG-CD), %NG (%NG-C, %NG-CD), time needed to reach 50 % of TG (T50-C, T50-CD), and AUC after 200 h of germination (AUC-C, AUC-CD).
Results
Analysis of phenotypic data
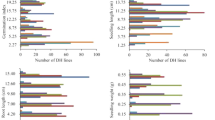
The distributions of the RILs, as well as the positions of the parents for the germination-related traits, are given in Figs. 1 and 2. Because the seeds of the parents had originated from a different regeneration year, a direct comparison with the RILs is not possible. However, one can assess the responses of both parents separately. Superior performance of Hicks as compared to Florida 301 under control conditions was observed for %TG and %NG (Fig. 1). After the CD, Florida 301 appeared to be tolerant whereas the %NG for Hicks declined indicating that parental lines differed in seed longevity.
Similar performance was observed when examining the germination speed traits T50 and AUC (Fig. 2). Control seeds of Hicks germinated faster (lower T50), reaching higher AUC as compared to Florida 301. After the deterioration treatment, however, the performance of the parental lines was reversed, again indicating the superior seed longevity of Florida 301.
The distributions of the RILs provide clear indication of quantitative inheritance of the traits under investigation before and after the deterioration treatment. The effects of the treatment were most pronounced for the germination speed traits, with a considerable increase in T50 and decrease in AUC.
QTL mapping
For the traits under consideration, seven major and two minor QTLs on four different LGs were detected (Table 1; Fig. 3). For the control seed lots, the highest LOD value (6.67) was observed for a QTL for %TG that explained 18.3 % of the variation for this trait on LG8/18. The favorable allele at this QTL was contributed by Hicks. One QTL affecting %NG was detected on LG 7, along with a minor locus for %TG. In each case the favorable allele was contributed by Florida 301. For the control, other major and minor QTL for T50 and AUC, respectively, were identified in identical positions on LG 23. The low germination speed was contributed by Florida 301 showing high and low values for T50 and AUC, respectively.
Positions of the QTL of control seeds C (above) and seeds after CD (below) for the traits % total germination (%TG-C, %TG-CD), % normal germination (%NG-C, %NG-CD), time needed to reach 50 % of total germination (T50-C, T50-CD), and area under the curve after 200 h of germination (AUC-C, AUC-CD). LODs are given below the arrows. Vertical bars indicate intervals LOD > 3 (major QTL; filled bars) and LOD > 2 (minor QTL; empty bars)
After CD, the QTL with highest LOD (4.83) was again detected for %TG on LG 8/18, in a similar position as identified for the seed lots from the C. This QTL explained 13.3 % of the phenotypic variation for this measured trait, and the favorable allele was contributed by Hicks. On LG 23, a QTL determining %NG and contributed by Florida 301 was identified in a position highly comparable to the one determining germination speed in the control material. Finally, a QTL controlling germination speed after treatment appeared on LG 6 for the traits T50 and AUC. The low speed of germination (high T50 and low AUC, respectively) was contributed by Hicks.
Discussion
The published results on seed longevity for Nicotiana are inconsistent. As early as 1907, Shamel and Cobey (cited in Kingcaid 1943) stated that fully matured and dry tobacco seed can retain viability when kept dry for 10 years, or, as has been observed in several cases, a much longer time. Also, Johnson et al. (1930; cited in Kingcaid 1943) reported that tobacco seed that was originally of good germinating capacity may germinate ‘satisfactorily’ at 10 years of age. More recently, Walters et al. (2005) compared the seed longevity of about 90 species, including N. tabacum, from different surveys and found the germination range to decline to 50 % between 10 and 81 years. Studies by Agacka et al. (2014) of N. tabacum and N. rustica stored at −15/−18 °C indicate seed longevity of greater than 50 years.
Here, we describe for the first time the genetic dissection of the seed longevity trait in N. tabacum. It is known that germination per se and seed longevity are very complex traits under polygenic control. In the present study, however, only four regions, located on four different LGs, were detected for the four traits considered, for untreated and treated seed lots. A major QTL for high germination percentage (TG) was detected on LG 8/18. This seems to be a genetic factor determining germination per se, independent of the quality of the seed, because it was detected when both control and deteriorated seeds were tested.
Another highly comparable region carrying a QTL, discovered for control seeds and after the treatment, was located on LG 23. For the untreated seed lots, this QTL is responsible for the high germination speed of Hicks, as shown by the presence of major and minor QTL for T50 and AUC, respectively. After the seed treatment, however, a QTL for high %NG of Florida 301 was found. It seems that the locus for germination speed became negligible after the CD treatment. This is confirmed by the phenotypic data shown in Fig. 2. While the speed of germination for was greater for the Hicks parent in the control seed lots, the opposite was observed after the CD treatment. Whether this contributed to the appearance of the Florida 301 QTL for high percentage of NG in the comparable region after the treatment remains unclear.
Two chromosomal regions, on LGs 7 and 6, were detected separately in control and CD experiments, respectively. On LG 7, a major and minor QTL for %NG and %TG, respectively, were detected at highly comparable positions. The positive alleles were contributed by Florida 301. In contrast to the Hicks locus on LG 8/18, identified in the control and CD experiments, the Florida 301 QTL is expressed only with the untreated seeds. A QTL for germination speed after CD treatment only was detected on LG 6. The higher speed was contributed by Florida 301which is confirmed by phenotypic data (Fig. 2) after CD treatment.
Considering both the phenotypic and QTL mapping data, it can be concluded that the cultivar Hicks expresses a high germination percentage and speed in untreated (fresh) seeds, whereas Florida 301 seems to withstand the controlled seed deterioration treatment better, overtaking Hicks and, therefore, having greater seed longevity. Thus, good germination performance of fresh seeds does not necessarily translate into high seed longevity. This has already been revealed for other species. Nagel et al. (2010) investigated genebank collections of barley (Hordeum vulgare), wheat (Triticum aestivum), rye (Secale cereale), sorghum (Sorghum bicolor), oilseed rape (Brassica napus), and flax (Linum usitatissimum) stored between 26 and 33 years. Whereas high germination was found initially, accessions separated strongly after 20 years. Some of the ‘high germinating’ accessions dropped close to zero.
QTL associated with seed longevity in other crops, such as wheat and barley, often appear in genomic regions containing genes determining aspects of biotic and abiotic stress responses (Nagel et al. 2009, 2014; Rehman Arif et al. 2012). The Florida 301 × Hicks RILs of the present study have been used for mapping loci responsible for resistance to the black shank disease caused by P. nicotianae (Xiao et al. 2013). Above-ground symptoms of the disease are wilting and yellowing of the leaves, developments that can have a pronounced effect on seed development. One QTL, detected in field and greenhouse disease studies on LG 6, is comparable with the locus for germination speed detected in the present study. The favorable germination speed allele was contributed by Florida 301, the disease resistant parent.
The environmental growing conditions of the mother plants have a highly significant effect on seed quality and longevity, not only with respect to biotic and abiotic stresses. Nagel et al. (2014) investigated seeds of a barley association mapping population regenerated in the same growing season but on two different field plots separated by less than 800 m. Beside common marker-trait associations (loci) many ‘field specific’ loci were detected. Due to the identical regeneration year and the close proximity of the experiments, it was concluded that soil composition may have been one reason responsible for the differences. This was supported by a soil analysis, indicating a difference in nutrient contents, mainly phosphorus, potassium, manganese, and nitrogen.
In order to draw final conclusions about the genetics of seed germination/longevity and their interaction with the growing conditions (disease resistance) of the mother plants in Nicotiana, more studies need to be performed. Another mapping population developed from a cross between the black shank-resistant cultivar Beinhart-1000 and Hicks (Vontimitta and Lewis 2012), having one parent in common with the present study, may be a good choice for follow-up investigations.
References
Agacka M, Depta A, Börner M, Doroszewska T, Hay FR, Börner A (2013) Viability of Nicotiana spp. seeds stored under ambient temperature. Seed Sci Technol 41:474–478
Agacka M, Laskowska D, Doroszewska T, Hay FR, Börner A (2014) Longevity of Nicotiana seeds conserved at low temperatures in ex situ genebanks. Seed Sci Technol 42:355–362
Bentsink L, Alonso-Blanco C, Vreugdenhil D, Tesnier K, Groot SPC, Koornneef M (2000) Genetic analysis of seed-soluble oligosaccharides in relation to seed storability of Arabidopsis. Plant Physiol 124:1595–1604
Bindler G, Plieske J, Bakaher N, Gunduz I, Ivanov N, van der Hoeven R, Ganal M, Donini P (2011) A high density genetic map of tobacco (Nicotiana tabacum L.) obtained from large scale microsatellite marker development. Theor Appl Genet 123:219–230
Burgess JL (1938) Report on project to determine the percentage and duration of viability of different varieties of soybeans grown in North Carolina. Proc Assoc Off Seed Anal 23:69
Clerkx EJM, El-Lithy ME, Vierling E, Ruys GJ, Blankestijin-De Vries H, Groot SPC, Vreugdenhil D, Koornneef M (2004) Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol 135:432–443
Haberlandt F (1873) Die Keimfähigkeit unserer Getreidekörner, ihre Dauer und die Mittel ihrer Erhaltung (The germinating capacity of our cereals, their longevity and the means of preserving it). Wien Landw Ztg 22:126
Hampton JG, TeKrony DM (1995) Handbook of vigour test methods. International Seed Testing Association, Zürich, p 117
Hay FR, Adams J, Manger K, Probert R (2008) The use of non-saturated lithium chloride solutions for experimental control of seed water content. Seed Sci Technol 36:737–746
ISTA (2014) International rules for seed testing. International Seed Testing Association, Bassersdorf
Johnson J, Murwin HF, Ogden WB (1930) The germination of tobacco seed. Wis Agric Exp Station Res Bull 104:15
Jones JW (1926) Germination of rice seed as affected by temperature, fungicides, and age. Agron J 18:576–592
Joosen RVL, Kodde J, Willems LAJ, Ligterink W, van der Plas LHW, Hilhorst HWM (2010) Germinator: a software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J 62:148–159
Kingcaid RR (1943) Effect of storage conditions on the viability of tobacco seed. J Agric Res 67:407–410
Landjeva S, Lohwasser U, Börner A (2010) Genetic mapping within the wheat D genome reveals QTLs for germination, seed vigour and longevity, and early seedling growth. Euphytica 171:129–143
Miura K, Lyn SY, Yano M, Nagamine T (2002) Mapping quantitative trait loci controlling seed longevity in rice (Oryza sativa L.). Theor Appl Genet 104:981–986
Nagel M, Börner A (2010) The longevity of crop seeds stored under ambient conditions. Seed Sci Res 20:1–12
Nagel M, Vogel H, Landjeva S, Buck-Sorlin G, Lohwasser G, Scholz U, Börner A (2009) Seed conservation in ex situ genebanks—genetic studies on longevity in barley. Euphytica 170:5–14
Nagel M, Rehman-Arif MA, Rosenhauer M, Börner A (2010) Longevity of seeds—intraspecific differences in the Gatersleben genebank collections. In: Proc 60th Tagung der Vereinigung der Pflanzenzüchter und Saatgutkaufleute Österreichs, Gumpenstein, Österreich, 24–26 Nov 2009, pp 179–181
Nagel M, Rosenhauer M, Willner E, Snowdon RJ, Friedt W, Börner A (2011) Seed longevity in oilseed rape (Brassica napus L.)—genetic variation and QTL mapping. Plant Genet Resour 9:260–263
Nagel M, Kranner I, Neumann K, Rolletschek H, Seal C, Colville L, Fernández-Marín B, Börner A (2014) Genome-wide association mapping and biochemical markers reveal that seed ageing and longevity are intricately affected by genetic background, developmental and environmental conditions in barley. Plant Cell Environ. doi:10.1111/pce.12474
Priestley DA, Cullinan VI, Wolfe J (1985) Differences in seed longevity at the species level. Plant Cell Environ 8:557–562
Rehman Arif MA, Nagel M, Neumann K, Kobiljski B, Lohwasser U, Börner A (2012) Genetic studies of seed longevity in hexaploid wheat using segregation and association mapping approaches. Euphytica 186:1–13
Revilla P, Butrón A, Rodríguez VM, Malvar RA, Ordás A (2009) Identification of genes related to germination in aged maize seed by screening natural variability. J Exp Bot 60:4151–4157
Schwember AR, Bradford KJ (2010) Quantitative trait loci associated with longevity of lettuce seeds under conventional and controlled deterioration storage conditions. J Exp Bot 61:4423–4436
Shamel AD, Cobey WW (1907) Tobacco Breeding. US Dept Agric Bur Plant Ind Bull 96:71
Singh RK, Raipuria RK, Bhatia VS, Rani A, Pushpendra, Husain SM, Chauhan D, Chauhan GS, Mohapatra T (2008) SSR markers associated with seed longevity in soybean. Seed Sci Technol 36:162–167
Vontimitta V, Lewis RS (2012) Mapping of quantitative trait loci affecting resistance to Phytophthora nicotianae in tobacco (Nicotiana tabacum L.) line Beinhart-1000. Mol Breed 29:89–98
Walters C, Wheeler LM, Grotenhuis JM (2005) Longevity of seeds stored in a genebank: species characteristics. Seed Sci Res 15:1–20
Xiao B, Drake K, Vontimitta V, Tong Z, Zhang X, Li M, Leng X, Li Y, Lewis RS (2013) Location of genomic regions contributing to Phytophthora nicotianae resistance in tobacco cultivar Florida 301. Crop Sci 53:473–481
Xue Y, Zhang SQ, Yao QH, Peng RH, Xiong AS, Li X, Zhu WM, Zhu YY, Zha DS (2008) Identification of quantitative trait loci for seed storability in rice (Oryza sativa L.). Euphytica 164:739–744
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Zeng DL, Guo LB, Xu YB, Yasukumi K, Zhu LH, Qian Q (2006) QTL analysis of seed storability in rice. Plant Breed 125:57–60
Acknowledgments
We thank Sibylle Pistrick and Gabriele Matzig for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agacka-Mołdoch, M., Nagel, M., Doroszewska, T. et al. Mapping quantitative trait loci determining seed longevity in tobacco (Nicotiana tabacum L.). Euphytica 202, 479–486 (2015). https://doi.org/10.1007/s10681-015-1355-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1355-x