Abstract
Fish can be a key strategy in addressing the global food and nutrition insecurity challenges. Fish nutrient data are critical for policy development targeting to reduce these problems. Despite this, much of the published studies on nutritional characteristics of fish have focused on marine ecology and there is little information on natural freshwater fish habitats like inland delta areas. This study investigated the nutrient composition of Okavango Delta fishes and their potential contribution to the recommended nutrient intake. Fish species sampled were Clarias ngamensis, Oreochromis andersonii, Schilbe intermedius, Marcusenius altisambesi, Brycinus lateralis, Barbus poechii and Tilapia sparrmanii. The nutrients analyzed were moisture, ash, proteins, Na, K, Ca, Mg, P and Zn. The small fishes (B. lateralis and B. poechii) had higher mineral content; Ca (643–968 mg/100 g), Mg (47–49 mg/100 g) and Zn (2.8–3.6 mg/100 g) compared to the larger species with average mineral content; Ca (73–460 mg/100 g), Mg (23–35 mg/100 g), Zn (1.2–2.9 mg/100 g). The most nutrient-dense species recognized were B. poechii and B. lateralis followed by the large species, M. altisambesi. These species illustrated the potential to contribute 80–121% Ca, 32–47% Mg and 70–150% Zn of the recommended nutrient intake to children (1–9 years), pregnant women and the elderly (65 + years). The study recommends prioritizing small and some large species (M. altisambesi and T. sparrmanii) for polyculture systems, primarily for securing food and nutrition security for local communities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As the world’s population explodes to almost 10 billion in 2050, world leaders and scientists alike are faced with the challenge of feeding the growing population; many of whom are in developing countries already plagued with hunger and malnutrition (UN, 2022). Globally over 800 million people are undernourished, about 50% of these people are in Asia while 30% are in Africa (WFP and WHO 2022). Furthermore, about 150 million children are stunted, 45 million wasted and over 2 billion people have micronutrient deficiency (WFP and WHO 2022). In addition, the triple burden of malnutrition, undernutrition, obesity and micronutrient deficiencies are the biggest contributors to poor health worldwide (Obiero et al., 2019). Recognizing this, the United Nations through the sustainable development goals (SDG) made a commitment to end all forms of food and nutritional insecurity by 2030. With more than 1 in 7 people having no access to protein and energy sufficient diets, food production will have to increase by 50% by 2050, if at all food and nutritional insecurity is to be a thing of the past (Lauria et al., 2018). While this is achievable, various challenges still exist at the center of which is how to improve the production and distribution of nutrient-dense food. Fish is a nutrient-dense food that is cheap and commonly available even in countries with food and nutrition insecurity (Cleaver, 2006). It is well known for its high nutritional quality with a very good supply of readily digestible proteins, polyunsaturated fatty acids, essential minerals and micronutrients such as iron, calcium, zinc, selenium, iodine, vitamin A, B and D (Fawole et al., 2007; Kawarazuka & Bene, 2011; Pirestani et al., 2009; Reksten et al., 2020a). These minerals are essential for biochemical functions of the human body e.g., calcium, phosphorus, and magnesium support bone development whereas zinc is necessary for reproductive health. Fish consumption is generally associated with good health as studies have shown that high fish intake reduces chances of getting cardiovascular diseases and regulates blood sugar levels, leading to better weight control and less chances of getting high blood pressure and type II diabetes (Mendivil, 2021). In Ghana, the addition of fish powder during weaning was shown to improve infant growth (Lartey et al., 1999). It has also been shown in Bangladesh, that boosting the national consumption of a small indigenous fish high in vitamin A achieved better health benefits at a lower cost than a national vitamin A wheat flour fortification program (Fiedler et al., 2016). Fish is also a valuable trading commodity at a global scale (Béné et al., 2015).
Unfortunately, global fish populations are declining due to high exploitation pressure (Costello et al., 2012) and competing uses of freshwater resources (Brander, 2007; Molden & De Fraiture, 2004). Sustainable aquaculture development can supplement global fish supply (FAO, 2021; Troell et al., 2014) and also relieve exploitation pressure on wild fish stocks (Bogmans & van Soest, 2022; De Silva et al., 2003; Diana, 2009). If developed and managed sustainably, aquaculture can make a crucial contribution to local diets and economies; especially in developing countries with a high prevalence of mineral and micronutrient deficiencies which contribute to increasing cases of perinatal and maternal mortality, child mortality, growth retardation, cognitive deficits and reduced immune function (Black et al., 2013; Golden et al., 2016). These health issues are directly linked to poverty and lack of enough food. Since fish is well recognized as a rich source of several nutrients, and is cheap and readily available to most poor communities, scientists recommend its inclusion in food-based strategies, specifically to assist in eliminating protein, mineral and micronutrient deficiencies that are prevalent in low and middle income countries (Hicks et al., 2019; Kawarazuka & Bene, 2011; Roos et al., 2007a). To promote the efficient utilization of fish as food and feed as well as to contribute to the development of effective and sustainable national food policies, comprehensive information on fish composition and nutrition is needed (Kolding et al., 2019).
Substantial research, mostly in Asia, has been done on fish nutrient composition and their potential contribution to micronutrient deficiencies in vulnerable groups (Andersen et al., 2016; Islam et al., 2023; Kongsbak et al., 2008; Roos et al., 2003, 2007a, 2007b; Thilsted, 2012). Generally, small indigenous species (SIS) are key sources of essential nutrients (Kolding et al., 2019; Thilsted et al., 1997) which are otherwise available to poor riparian households (Kawarazuka, 2010; Kawarazuka & Bene, 2011). These SIS are more valuable among young children during their formative stages of life (Bogard et al., 2015; Byrd et al., 2021; Kongsbak et al., 2008).
Research on fish nutrition has also contributed greatly to the expansion of aquaculture production (Rombenso et al., 2022). Different fish species with high potential for addressing food security have been explored, including, red seabream (Pagrus major), rainbow trout (Oncorhynchus mykiss), Nile tilapia (Oreochromis niloticus), rice field eels (Monopterus albus), Japanese sea bass (Lateolabrax japonicus), Asian seabass (Lates calcarifer), and dusky grouper (Epinephelus marginatus) (de Mello et al., 2022; Dong et al., 2020; Milián-Sorribes et al., 2021; Mzengereza et al., 2021; Shi et al., 2020; Xu et al., 2020; Yang et al., 2020).Various fish feed formulations have emerged with potential to maximize production performance, facilitate immune resilience, and generally improve fish health, while also minimizing the environmental impacts of aquaculture, specifically nutrient pollution (Milián-Sorribes et al., 2021; Rombenso et al., 2022). There have also been efforts on finding new aquafeed formulations which are cheaper and more available than marine-based ingredients (Turchini et al., 2019). While substantial research has thus far been done on fish nutrition, most work relates to marine ecology and strictly experimental and inland fish rearing sites. Little research has been conducted on important natural freshwater fish habitats like inland delta areas; yet these systems play a crucial role in promoting global food security (Thomas, 1996). They are a key source of mitigation against the so-called “hidden hunger” (Lynch et al., 2016). It is therefore critical to establish the nutritional status of fish species in such areas. This study uses the fish species of the Okavango Delta to contribute to global knowledge of fish nutritional status of inland delta fish species.
The Okavango Delta supports the largest fishery in Botswana, with at least 71 freshwater fish species (Merron & Bruton, 1995). In the Okavango, fish has a vital role in food, nutrition, and the socio-economic lives of the people, considered the poorest in the country. However, the overall national fish yield is relatively low, and the country has one of the lowest per capita fish consumption in Africa, estimated at 3.7 kg in 2016 (SADC FISHERIES, 2016), much lower than the global consumption of 20.3 kg (FAO, 2021). Recent development of a national aquaculture strategy is expected to facilitate the development of an aquaculture sub-sector, whose aim is to increase overall fish supply in Botswana (Mosepele, 2021). Fish nutritional content, especially of SIS, is of particular importance because this will contribute to policy formulation that highlights the critical role of these fish species in national food and nutrition security programs (Kolding et al., 2019).
Several studies have established that nutrients in fish vary with fish species (Bogard et al., 2015; Egun & Oboh, 2022; Mohanty et al., 2015). Knowledge on fish nutrient composition will therefore guide appropriate and optimal utilization of each species. Studies have generated valuable information on the ecology of Okavango fishes but only one study (Mogobe et al., 2015) has so far investigated the essential mineral composition of Okavango fishes. However, this investigation was limited to Chanoga lagoon, which is at the distal end of the Delta. The aim of this study was to expand the previous work to other parts of the Okavango Delta and assess if the Okavango fish species have the potential to make a meaningful contribution to nutrition security. The objectives of this study were therefore to;—(1) assess proximate and mineral content of Okavango freshwater fish species, (2) assess potential contribution of Okavango fish species to the recommended mineral intakes for population groups at risk of nutrient deficiency (3) identify the most nutrient-dense species that could be recommended for both subsistence and commercial aquaculture development.
2 Materials and methods
2.1 Study site
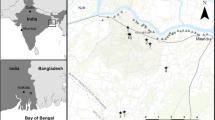
The Okavango Delta (Fig. 1) is one of the largest inland deltas of the world and the largest freshwater body in Botswana. It covers a total area of ~ 22,000 km2 (McCarthy et al., 1993). The Delta is sustained by seasonal flood waters via the Okavango River draining the central Angolan highlands. The Delta experiences high evapotranspiration such that the lower reaches of the system receive only 2% of the total water inflow, thus creating permanent swamps in the upper reaches and seasonal and occasional floodplains in the lower reaches. The Delta is a major tourist attraction in Botswana because of its high biodiversity of flora and fauna including approximately 71 fish species (Mbaiwa, 2015; Ramberg et al., 2006). The Delta supports small scale subsistence/artisanal, commercial and recreational fisheries with 3000–5000 resident fishers (Kolding et al., 2003). The fish provides high quality protein supplement to the diet of many subsistence people and form an important recreational and commercial fishery.
The fish samples were collected from the following 3 sampling locations in the Okavango Delta (Fig. 1): Nxaraga lagoon on the Boro River, Maun on Thamalakane River and Lake Ngami. The Nxaraga lagoon is in the seasonally flooded eco-region of the Delta and falls within the protected Moremi game reserve where public fishing is prohibited. Thamalakane River, the main surface outlet of the Delta runs through Maun, the biggest village in the Ngamiland tourism region. Lake Ngami, an endorheic depression located south of the Delta is an extremely popular fishing site for commercial fishers. All the 3 sites receive water during winter when the floods from Angola reach the Delta and start receding in the summer months (September—March). Fishing in Okavango is open from April to December every year and the area is popular with subsistence, commercial and recreation fishers. Wilson and others (Wilson and Dincer 1976) recognized five major habitats in the Okavango Delta. These habitats include the riverine floodplain, permanent swamp, seasonal swamp, rivers, and sump lakes. For this study fish samples were collected from three of the five habitat types: (1) Nxaraga lagoon representing the permanent swamps (2) Thamalakane River representing the seasonal river and (3) Lake Ngami, the sump lake. These three sites grade into one another such that the Boro River, which is the main outlet of the Okavango Delta, flows through the Nxaraga Lagoon and drains into the Thamalakane River at Matlapaneng. The Thamalakane River flows through Maun town and bifurcates 15 km downstream into the Boteti River and Nhabe River which drains into Lake Ngami. It is important to note that evapotranspiration takes a huge toll on the Delta’s water resources such that Maun and Lake Ngami may receive little or no river flow during low flood seasons.
2.2 Sampling
The fish samples were collected between February and March 2013 using a multi-filament fishing net consisting of several panels arranged from 16 to 150 mm mesh sizes. The multi-filament net was set overnight, from approximately 1800 h and lifted in the morning at 0600 h. Fish removed from each panel were identified according to species. Thereafter we recorded their weight, sex, and total length. All the fish were collected and transported in cooler boxes with ice to the Okavango Research Institute Environmental laboratory where they were cleaned with running tap water, rinsed with deionized water, and placed into new zip-lock plastic bags, kept frozen at about −18 °C until analysis.
2.3 Sample preparation
Preparation of the fish samples for analysis was done according to the standard AOAC official Method 937.07 (AOAC International, 2000). In brief, frozen fish samples were slowly thawed in the laboratory at room temperature. Large fish samples (≥ 130 mm) were descaled, eviscerated, head, tail, bones, and fins removed, and the remaining flesh rinsed with deionized water. The flesh was cut into small pieces and homogenized by grinding in a stainless-steel blender for further analyses. Small fish samples (< 130 mm) were homogenized whole after thorough rinsing with deionized water. In this work, 1–5 fish specimens for each species were homogenized and analyzed for each site investigated. Prior to analysis, samples were dried overnight in a laboratory oven (Scientific: Series 2000, South Africa) at 105 °C.
2.4 Proximate analysis
2.4.1 Protein content
Protein content of fish samples was estimated using the Kjeldahl method as described by (Ayanda et al., 2019). Briefly, about 1.25 g of homogenized fish sample was digested for 2 h in 20 ml sulfuric acid in the presence of selenium as a catalyst to yield ammonium sulfate. The ammonium was liberated by distillation and received in boric acid, then measured by back titration. The total protein content of the fish samples was estimated by multiplying the nitrogen content (liberated ammonium) by a conversion factor equal to 6.25 as shown below:
where CP is the crude protein, Vs and Vb are the titration volumes of the sample and blank, W is the weight of the sample, x is the normality of the acid, and 14.007 is the molecular weight of nitrogen.
2.4.2 Moisture content
Moisture content determination in the fish samples was achieved using the gravimetric method in a convection oven (Jaziri et al., 2021). About 1 g of homogenized fish sample was transferred into an oven and dried at 105 °C until constant weight. Moisture content calculated as follows:
where, W1 is the wet weight and W2 is the dry weight.
2.4.3 Ash content
Ash is the total inorganic (mineral) content in foods. The dry ash content of the fish samples was estimated according to AOAC method 920.153, also used by (Jaziri et al., 2021), where 1–5 g of each fish sample was weighed into crucibles of known weights and dried in an oven at 105 °C. After drying, the samples in crucibles were transferred to a general-purpose laboratory muffle furnace, (Carbolite CWF, Fischer Scientific, UK) and incinerated at 450 °C overnight. The following day, the crucibles with samples were removed from the muffle furnace, transferred to a desiccator, and left to cool down. After cooling, the samples were reweighed, and the ash content calculated using Eq. 3 as follows:
2.5 Mineral analysis
The mineral analysis involved determination of calcium (Ca), magnesium (Mg), zinc (Zn), potassium (K), sodium (Na) and phosphorus (P). Approximately 1.000 g of dry and homogenized sample was incinerated in the muffle furnace overnight at 450 °C. The resultant ash was treated with 5 mL of 6 M hydrochloric acid, boiled to near dryness on a hot plate, cooled and the residue re-dissolved in 10 mL of 0.1 M nitric acid. The solutions were left standing for 2 h to cool and quantitatively transferred into 50 mL volumetric flasks, topped to the mark with ultrapure water and the resulting solution used to analyze for Ca, Mg, Zn, K and Na. The first three elements (Ca, Mg and Zn) were analyzed using a Varian SpectrAA 220 Flame Atomic Absorption Spectrometer (Victoria, Australia) while K and Na were determined by flame photometry using Model 410 Flame Photometer from Sherwood Scientific Ltd (Cambridge, UK). These protocols were also used by (Kiczorowska et al., 2019). For analysis of phosphorus, samples were analyzed using the molybdenum blue phosphorus reaction method and determined spectrophotometrically at a wavelength of 823 nm with a PerkinElmer UV/Visible Lambda 20 Spectrometer (Norwalk, USA), according to the AOAC 986.24 method, also used by (Nagul et al., 2015). All values were reported in milligrams (mg) per 100 g of wet fish (i.e., mg/100 g ww).
2.6 Potential contribution of fish to recommended nutrient intake (RNI)
The potential contribution of fish to the recommended mineral intake for selected population groups, considered vulnerable to mineral deficiency, were calculated using Eq. 4 and 5, and the World Health Organization (WHO) RNI reference values given in Table 1. For this work, the potential contribution of fish to mineral intake was considered for 3 vulnerable groups (children, pregnant women and the elderly). The FAO High Level Panel of Experts report on Food Security and Nutrition (HPLE Report 7, 2014) estimated that 150 g serving portion of fish per day is sufficient to supply a satisfactory level of proteins, lipids, iodine, calcium, phosphorus, and micronutrients essential for maintaining human nutritional needs. Therefore, in this study, the ideal standard serving portion of fish was taken to be 150 g for adults and 75 g for children, assuming children consume half the portion size for adults. The calculated potential contribution values thereof were compared with the actual contributions calculated using the current serving portions of 10 and 5 g for adults and children, obtained from the per capita consumption of 3.7 kg.
where NA is the nutrient content of the fish (g/g fish).
SP is the assumed standard portion of fish per day (g fish/day). NSP is the nutrient amount in a standard portion (mg nutrient). RNIi is the Recommended Nutrient Intake for a particular nutrient (see Table 1). %RNIi is the percentage contribution to daily RNI from an assumed standard portion. This method for calculating the contribution of fish to nutrient intake was used by (Byrd et al., 2020; Egun & Oboh, 2022; Kiczorowska et al., 2019; Reksten et al., 2020b).
2.7 Quality assurance
Sample blanks, containing reagents only, were included in each batch of analysis. Duplicates from each individual sample were weighed, digested and analyzed separately. For all species except for those where only 1 fish was caught, at least 2 individual fishes were analyzed to represent each species. Deionized water was used for rinsing and preparation of reagents. All reagents used were of analytical grade, manufactured by either Sigma Aldrich or Merck.
2.8 Statistical analysis
Statistical analysis was done using SPSS for windows (IBM SPSS Statistics version 28.000). The software was used to obtain descriptive statistics (means ± standard deviation) and test of significance at p ≤ 0.05. Charts were obtained using Sigma plots.
3 Results and discussion
The results for fish species caught and the comparison of nutrient composition in the 3 locations are given in Tables 2 and 3. Comparative analysis showed that most nutrients did not vary significantly between sampling sites, so the data were pooled and averages representing the Okavango freshwater ecosystem given in Tables 4 and 5. The distribution of fish in the Okavango is controlled mainly by the water flow, so there are no endemic species in the river system as observed by (Ramberg et al., 2006) which may explain the small variations observed between sites.
3.1 Fish species sampled in this study
Table 2 displays the fishes caught with their scientific, English, and local names. The 7 species sampled are among the most common species in the Delta. According to (Mosepele et al., 2022), the smallest fish species in the Okavango delta is about 30 mm in length while the largest is over 1 m. However, in this study, the catch varied from the smallest of 83 mm to the largest at 410 mm. Although previous fish surveys (Merron & Bruton, 1995) reported a total of 71 fish species in the Okavango Delta, only 7 species were caught in this study, namely Barbus poechii, Brycinus lateralis, Clarias ngamensis, Marcusenius altisambesi, Oreochromis andersonii, Schilbe intermedius and Tilapia sparrmanii using the multi-filament net (Table 2). This is consistent with the general spatial–temporal distribution of fish species diversity and abundance in the Delta as controlled by the flood regime, particularly the hydro-period and water depth in the system. A study on fisheries of the Okavango floodplain (Mosepele et al., 2022) reported that the permanently flooded upper delta lagoons have higher fish species richness compared to the seasonally flooded lower Delta lagoons. These authors attributed the difference in fish structure and distribution to the relative hydrological stability in the upper delta vs increased hydrological variability in the lower delta. As a result, the upper Delta has the highest number of artisanal and commercial fishers (Mosepele, 2000). The Delta’s hydrological regime is also strongly correlated to the temporal fish biomass across the Delta. According to (Mmopelwa et al., 2009) fish biomass across the Delta is highest between August and November and lowest between March and May. The period between March and May, which coincides with the sampling period for this study, is associated with the lowest water discharge across the system. It is observed that only two fish species (O. andersonii and M. altisambesi) out of the 7 species were caught at all the 3 sites – Nxaraga Lagoon (NXL), Thamalakane River (TML) and Lake Ngami (LNG). The species, B. poechii and B. lateralis are categorized as small (< 130 mm) fish species that are underutilized, although highly abundant and widespread in the Delta (Merron & Bruton, 1995). These small fishes are particularly important for household food consumption by artisanal fishers including mostly women and children because they have a low market value. The other larger species (C. ngamensis, O. andersonii, S. intermedius, M. altisambesi and T. sparrmanii) are considered to have a good commercial value.
3.2 Comparison of nutrient content of fish from the 3 sampling locations
Nutritional composition of freshwater fish has been reported to vary between different ecosystems (Jim et al. 2017). Scientists have also reported that nutrient composition of fish is influenced by several factors and among those are diet, surrounding environment, including the concentration of minerals in the water body (Simionov et al., 2019). This study presents nutrient data (Table 3) obtained from analysis of 7 freshwater fish species caught in 3 different locations of the Okavango Delta (Nxaraga Lagoon (NXL), Thamalakane River (TML) and Lake Ngami (LNG). Although these sampling sites are interconnected, belonging to the same ecosystem, they are far apart from each other, so environmental conditions, land use practices and water quality may vary and influence the fish composition and quality. Statistical data analysis using SPSS with sampling site as a factor showed only a few significant differences (p < 0.05) between sites. The small species (B. lateralis), caught in Nxaraga and Lake Ngami showed significant differences in Na and Ca content, higher in Lake Ngami compared to Nxaraga fish. One species that showed significant variations in all the nutrients across all the 3 sites was M. altisambesi. The analysis revealed that Na, K and Ca content in this species were significantly higher in fish caught from Lake Ngami and Thamalakane compared to Nxaraga, but Mg and Zn were higher in Nxaraga compared to the 2 sites. Another significant variation was observed in S. intermedius, which also had higher concentration of Na, K and Ca in fish from Thamalakane than Nxaraga, and again higher Mg and Zn content in fish from Nxaraga compared to Thamalakane. In general, significant differences in mineral content were observed between Nxaraga and the two sites (Thamalakane and Lake Ngami). Scientists (Mogobe et al., 2018; West et al., 2015) have observed an increasing salt concentration gradient downstream the Okavango River waters, with Thamalakane and Lake Ngami (downstream areas) having higher salt concentrations than the upstream, Nxaraga. This may explain why the concentrations of macro-elements in the two species (M. altisambesi and S. intermedius) were significantly higher in fish caught from Thamalakane and Lake Ngami than those from Nxaraga.
3.3 Proximate composition of the fish species
Fish is mostly made up of proximate components, constituting 96% – 98% of the fish body and these include moisture, protein, minerals and fat (Ahmed et al., 2022; Ullah et al., 2022). These components vary with species, water bodies, geographic locations, and other internal and external conditions. The results of the proximate measurements obtained in this study are given in Table 3 and Fig. 2A. The mean ash, protein and moisture contents for the seven fish species analyzed here varied from 1.36 to 4.77%, 14.96–21.66% and 74–82% respectively (Table 3). Moisture content is an important measure of fish meat quality and also gives an indication for its processing and preservation needs (Jahan et al., 2021). As expected, the larger five fish species showed a significantly higher mean moisture content of 79.3 ± 0.3% compared to the small fish species (B. poechii and B. lateralis) at 74.4 ± 0.8%. By contrast, the mean ash content was significantly higher (p < 0.05) in small fish species at 4.62 ± 0.31% which were analyzed whole (including the viscera and bones) compared to the larger species (at 2.22 ± 0.12%) from which only the muscle tissue was analyzed. The mean ash, protein and water content observed in the seven Okavango fish species (Table 3) are similar to fish contents reported by other studies (Nolle et al., 2020; Paul et al., 2018; Ullah et al., 2022). For instance, (Nolle et al., 2020) reviewed a large number of published articles including more than 62 articles from approximately 26 countries involving more than 173 freshwater fish species. The supplementary data for (Nolle et al., 2020) indicated an average ash, protein and moisture contents of 2.2 ± 0.2, 17.2 ± 0.1 and 76.2 ± 0.2%, respectively. The 5 freshwater species studied by (Paul et al., 2018) had ash, protein and moisture contents of (1.88–2.57%), (13.93–15.41%), (70.82–76.11%) respectively, values a little bit lower compared to those obtained in this study. However, a study profiling nutritional content of selected freshwater fish in Bangladesh (Ullah et al., 2022) had ash content of (0.85–4.35%), moisture content of (77.35–81.16%) and protein of (15.62–18.73%). These values are close to the ones obtained in our study except for protein which is higher in the Okavango fish. One of the major factors contributing to the nutritional quality of fish food is attributed to their high protein content which are easily digestible and contain elevated levels of essential amino acids, such as arginine, leucine, methionine, and lysine. These biomolecules are important for both structural and functional activities including synthesis of muscle proteins, cell division, wound healing, production of enzymes and enhancing the immune system. In this study the crude protein content was higher, although not statistically significant, in the small fishes (at 20.1 ± 2.3%) than in the larger fish species at 16.5 ± 0.6% protein content. This therefore means that all the seven species can be used for nutrition security interventions in Botswana and other riparian countries. The Joint report (WHO/FAO/UNU 2007) and other studies (Wu, 2016) recommends a daily minimum intake of 0.8 g protein per kg body weight for healthy adults with minimal physical activity. The dietary requirement of protein for children (4–13 years), is a little bit higher, 0.9 g per kg body weight per day (Wu, 2016). If we assume an average body weight of 65 kg for an adult who consumes 100 g serving of Okavango fish containing 16.5% protein, then each serving will contribute about 32% to the RDA (Recommended Daily Allowance). On the other hand, children with average weight of 35 kg, consuming 50 g of any of these freshwater fish, will obtain 29% of their daily protein requirement. These values imply that these freshwater fishes are a reliable source of proteins. The small species which are harvested by women fishers are however more suitable for subsistence use due to their lower economic value. These species are also ideal for inclusion in food-based intervention strategies for malnourished populations due to their high protein content.
3.4 Mineral composition of common fish species
The results for the two electrolytes (Na and K), essential elements (Ca, Mg and Zn) and the structural element (P) are given in Table 4 and Fig. 2B. The mineral composition of fish varied across the species. Further analysis revealed that all minerals except K were significantly higher (p ≤ 0.05) in the smaller species compared to the larger species (Fig. 2B). Na and K are important for intracellular ion balance and maintaining acid–base balance in the human body (Eti et al., 2019). However, the World Health Organization (WHO, 2012a, 2012b) recommends a daily Na intake of less than 2000 mg and K intake of 3,510 mg per day. These guidelines were informed by several past scientific studies, including recent ones (Du et al., 2014; Grillo et al., 2019; Patel & Joseph, 2020) that associate a high Na, low K intake with the onset of hypertension and cardiovascular diseases. Research has established that Na and K work in tandem in our body system and food meals containing Na/K ratio of less than 1 is recommended (Ullah et al., 2022) for controlling blood pressure and associated diseases. The results of this study (Table 4, Fig. 2B) showed that all fish species studied here had a lower sodium and higher potassium concentration (sodium-to-potassium ratio of less than 1), with the highest sodium content of 127.8 ± 8.1 mg/100 g, obtained from B. lateralis, followed by M. altisambesi at 102.0 ± 3.5 mg/100 g, Na levels much lower than the maximum allowable. Larger fish species showed even lower amounts of Na contained in their fillet, for example C. ngamensis (78.5 ± 1.5 mg/100 g) and S. intermedius (83.2 ± 1.2 mg/100 g). Comparing Okavango fishes with other species, a study on nutrient composition of 19 marine fish species in Sri-Lanka (Reksten et al., 2020b) also obtained higher Na content (150 – 460 mg/100 g) in smaller species compared to larger species (42 – 61 mg/100 g). Furthermore, two freshwater species (Clarias gariepinus and Tilapia zilli) in Nigeria were reported to contain an average of 5 mg/100 g Na (Egun & Oboh, 2022) and another average of 5 mg/100 g was reported for some species in India by (Paul et al., 2018). The differences in Na content, especially between freshwater species may be attributed to the diverse ecology, different geographic locations, trophic levels, species investigated, as well as age and gender. There were large variations in Ca, Mg and Zn compositions among species (Table 4), ranging from 72.6 to 968.4 mg/100 g Ca, 23.5–49.5 mg/100 g Mg and 1.2–3.6 mg/100 g Zn. For all these three minerals, the highest concentrations were observed in the small fish species (B. lateralis and B. poechii) and the lowest from the large species C. ngamensis, T. sparrmanii and O. andersonii. The largest variation in Ca content was observed between the catfish (C. ngamensis) and the small species (B. poechii and B. lateralis) which contained 9 and 13 times more Ca than the catfish fillet. Another notable variation was observed in Mg and Zn contents between T. sparrmanii and B. poechii, in which the small species had twice the level of Mg and Zn in the larger tilapia. These observed differences in mineral content between the small and the large fish may be attributed to the fact that small fish were analyzed whole (viscera, bones, and flesh together) while only the fillet of the large species were analyzed. Several studies have also observed variations in mineral content between large and small fishes. Since the small species have shown higher mineral content, their integration into national diets may contribute significantly to mineral intake of the consumers as observed by (Byrd et al., 2020; Mohanty et al., 2019; Nolle et al., 2020; Reksten et al., 2020b). The mineral contents observed in this study compare very well with the results obtained by (Mogobe et al., 2015) for similar species caught from Chanoga Lagoon, an area located in the distal end of the Okavango Delta. The results also compare well with those obtained by (Nolle et al., 2020) in Zambia, for freshwater fishes where Ca content of small fishes ranged from 823 to 1324 mg/100 g, Mg 35–56 mg/100 g and Zn 2–5 mg/100 g. The closeness of the results may be due to the similarity of species investigated and the geographic proximity of the study areas. The amount of phosphorus in the Okavango fish species ranged from 226.9 to 1061 mg/100 g (Table 4, Fig. 2B) where B. lateralis had the highest concentration and C. ngamensis had the lowest.
A study reported an average concentration of 210 mg/100 g P for some freshwater species in Europe (Steffens, 2006), which is much lower compared to the average obtained in this study (437 mg/100 g). Interestingly, the average concentration of phosphorus (1061 mg/100 g) in the small freshwater fish species, B. lateralis, was higher than levels obtained for some small marine fishes of Sri-Lanka which had an average of 697 mg/100 g (Reksten et al., 2020b). This shows that the small freshwater species are a reliable source of phosphorus. Phosphorus, calcium and magnesium play important roles in bone mass development and bone health, especially for growing children and the elderly population with fragile bones (Cuadrado-Soto et al., 2020). In this study the concentrations of Ca and P were similar in all the fish species studied (Table 4, Fig. 2B). To identify the species with a dense mineral content, all minerals analyzed were added up for each species. From Table 4, the two small species B. lateralis and B. poechii had the highest total mineral content of 2546 mg/100 g and 1761 mg/100 g respectively. Among the large fishes, M. altisambesi had the highest mineral content (1368 mg/100 g) followed by S. Intermedius (1078 mg/100 g). These findings further support the recommendation to prioritize small fish species for inclusion in food-based intervention strategies for populations with inadequate nutrient intake, since small species are easily accessible to most communities.
3.5 Correlation between moisture content and ash, protein, and mineral contents
Further analysis revealed strong and significant relationships between moisture content and all the proximate and mineral contents, except Na and K (Fig. 3; Table 6). Table 6 shows that %ash, %protein, Ca, Mg, Zn and P contents can be estimated from %water content of the species. The correlation coefficients (R2, Table 6) are all higher than 0.63, meaning that %water explained more than 63% of the total variation in each of the minerals in the fish species. Similar relationships between %water content and %protein, % lipids and %ash across various fish species have been reported by (Yeannes and Almandos 2003).
3.6 Potential contribution of fish species to the recommended nutrient intake
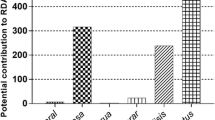
There is a global concern regarding the prevalence of inadequate intake of nutrients, specifically Ca, Zn, Mg, Fe and some vitamins in South Asia and Sub-Saharan Africa (Beal et al., 2017). The current contribution of the Okavango fish species to the recommended intake of Ca, Mg and Zn were estimated using the national per capita consumption of 3.7 kg/year for Botswana (SADC FISHERIES, 2016) which is one of the lowest fish per capita consumption in the region. This translates to a consumption of about 10 g of fish per day for adults and 5 g for children (assuming children eat 50% of an adult’s portion). The 10 g adult-size portion is 15 times smaller than the ideal serving portion of 150 g proposed by FAO and other researchers (Hansen et al., 2014; HLPE, 2014; Kiczorowska et al., 2019; Wird, 2016). The current contribution of fish species to the recommended mineral intake in this study is 0.6–8% for Ca, 1–3% Mg and 2–10% Zn for the different population groups. The results reflect a ridiculously small contribution, (less than 10%) from fish in supporting food and nutrition security in the country. The apparent poor utilization of fish may be attributed partly to the food culture, where the population is unfamiliar with fish as a nutrient-dense food and consumption is limited to the riparian communities only. However, the potential utilization and contributions of different fish species to the recommended daily mineral intake were calculated using the ideal fish consumptions of 150 g/day for adults and 75 g for children. The results are given in Fig. 4 for calcium, magnesium, and zinc, reflecting an overall higher contribution of mineral intake from small species compared to large species. Several studies have investigated the potential contribution of both marine and freshwater fishes to the recommended nutrient intake for several different population groups (Bogard et al., 2015; Egun & Oboh, 2022; Nolle et al., 2020; Reksten et al., 2020b) and most found that small fishes contributed more to RNI than larger species.
3.6.1 Calcium
Figure 4 reveals that the consumption of 150 g of small fish species (not considering other foods that may be eaten on the same day) will provide between 80 to 120% of the recommended daily intake of Ca to the vulnerable groups defined here (children, pregnant women, and the elderly). The large species, also rich in calcium (M. altisambesi, S. Intermedius and T. sparrmanii), have the potential to contribute close to 60%, 50% and 40% of Ca respectively. These projected values are fifteen times more than the current contribution range of 0.6–8% Ca to the recommended intakes. It is therefore important that fish is fully utilized and included in national diet diversification strategies to be accepted as a quality food, and for the resource to make a meaningful impact on nutrition security. The superiority of the small fishes (B. lateralis and B. poechii) in providing a high percentage of Ca to the daily requirement was also observed elsewhere by (Byrd et al., 2020; Nolle et al., 2020; Reksten et al., 2020b). The large species (C. Ngamensis and O. andersonii) had the lowest contribution of Ca, about 9% and 15% to RNI to all the three population groups. This finding underscores the key role that small fish species play in providing calcium to poor communities, in the Okavango Delta and elsewhere. Ca is important for bone formation and strengthening, critical for the growing fetuses in pregnant women, growing children and for the elderly who are prone to body imbalances resulting in falls and breaking of bones. The 3 fishes, B. lateralis, B. poechii and M. altisambesi are exploited predominantly by basket fishers and subsistence hook and line fishers, who happen to be mostly women and children (Mosepele, 2016; Mosepele et al., 2003). As mentioned earlier, because of their low market value, the small fishes that women and children catch are used mostly for household consumption. Our study shows that if these small fishes were to be consumed regularly in households, they would contribute significantly to household nutrition improvement.
3.6.2 Magnesium
According to (FAO/WHO 2005), the recommended daily intake of Mg for children, elderly and pregnant women are 79 mg/day, 207 mg/day and 220 mg/day respectively (Table 1). From the studied fish species, the potential contribution of Mg to the recommended daily intake ranged from 20 to 47% (Fig. 4). As with Ca the small fishes, B. lateralis and B. poechii could supply the highest amount of Mg to the population groups considered in this study, ranging between 46 and 33%. Magnesium is an important mineral, required as a cofactor for more than 300 enzymatic reactions and is thus needed for biochemical functioning of numerous metabolic pathways (Schwalfenberg & Genuis, 2017). Insufficient intake of this mineral has been associated with chronic inflammations, increased blood pressure and oxidative stress (De Baaij et al., 2015). This study has revealed that all the 7 species have the potential to provide a good amount of Mg, up to 47% of the children’s daily requirement.
3.6.3 Zinc
Zinc is an important trace nutrient and usually found to be deficient in low and middle-income populations (Gibson, 2011; Prasad, 2013). Currently 17% of the global population is zinc deficient (Golden et al., 2016) and zinc deficiency is associated with reproductive abnormalities, stunted growth, wasting and neuro-behavioral developmental problems (Brown et al., 2001; Deshpande et al., 2013). The results of this study showed that regular consumption of Okavango fish may contribute to adequate zinc supply for communities. Figure 4 shows that small species and large species have the potential to supply 100%–50% of the daily Zn requirement to children, pregnant women, and the elderly. Overall, our study shows that regular and adequate consumption of the fish species analyzed can reduce stunted growth, wasting and developmental problems associated with zinc deficiency in children. This observation is consistent with (Nnyepi et al., 2007) who reported that wasting and stunted growth among children (aged 0–5 years) in some riparian villages in the Okavango Delta were partly caused by lack of optimal utilization of the Delta’s fish resources.
4 Conclusions
The study revealed that freshwater fish are a reliable source of proteins and essential minerals, needed for human growth and body maintenance. The nutrient content varied significantly with species but the effect of environmental conditions on nutrient composition was inconclusive because of insufficient data. The study also revealed that sufficient consumption of any of the freshwater fish species can provide up to 120% of Ca, 50% Mg and 150% Zn of the daily mineral requirements to vulnerable population groups (children, pregnant women, and the elderly) who are prone to nutrient deficiency diseases. The small fish species showed a superior nutrient-dense content compared to the large species.
5 Policy implications
Since the small species are considered to have low economic value but are easily available to poor communities, who primarily feed on low nutrient staple diets, they can be used to diversify local diets and improve nutrition and health security in a sustainable manner. To ensure continuous availability of these nutrient-dense species, we recommend that B. lateralis and B. poechii be promoted for polyculture systems as part of aquaculture development aimed primarily at increasing production and securing access of fish to local communities. The large species can be used for supporting livelihoods and improving economic status of the poor through development of fishponds and other large scale fishery industries for fish export. The development of a national aquaculture strategy is expected to facilitate the development of an aquaculture sub-sector, which will ensure that fish becomes available to all people and not just limited to the Okavango communities, hopefully kick-starting positive fish consumption culture change. This study is supporting these strategies by providing nutritional data for guiding utilization and decision making.
Data availability
Data generated in this project is available from the corresponding author on reasonable request.
References
Ahmed, I., Jan, K., Fatma, S., & Dawood, M. A. (2022). Muscle proximate composition of various food fish species and their nutritional significance: A review. Journal of Animal Physiology and Animal Nutrition, 106(3), 690–719.
Andersen, A. B., Schmidt, L. K., Faurholt-Jepsen, D., et al. (2016). The effect of daily consumption of the small fish’Amblypharyngodon mola’or added vitamin A on iron status: A randomised controlled trial among Bangladeshi children with marginal vitamin A status. Asia Pacific Journal of Clinical Nutrition, 25(3), 464–471.
AOAC International (2000). AOAC Official Method 937.07. Fish and Marine products. Treatment and preparation of sample procedure AOAC Official Methods of Analysis. Arlington,VA
Ayanda, I. O., Ekhator, U. I., & Bello, O. A. (2019). Determination of selected heavy metal and analysis of proximate composition in some fish species from Ogun River. Southwestern Nigeria. Heliyon, 5(10), e02512.
De Baaij, J.H., Hoenderop, J.G., Bindels, R.J. (2015). Magnesium in man: Implications for health and disease. Physiological reviews
Beal, T., Massiot, E., Arsenault, J. E., Smith, M. R., & Hijmans, R. J. (2017). Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS ONE, 12(4), e0175554.
Béné, C., Barange, M., Subasinghe, R., et al. (2015). Feeding 9 billion by 2050: Putting fish back on the menu. Food Security, 7(2), 261–274. https://doi.org/10.1007/s12571-015-0427-2
Black, R. E., Victora, C. G., Walker, S. P., et al. (2013). Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet, 382(9890), 427–451. https://doi.org/10.1016/50140-6736(07)61690-0
Bogard, J. R., Thilsted, S. H., Marks, G. C., et al. (2015). Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. Journal of Food Composition and Analysis, 42, 120–133. https://doi.org/10.1016/j.jfca.2015.03.002
Bogmans, C. W., & van Soest, D. (2022). Can global aquaculture growth help to conserve wild fish stocks? Theory and empirical analysis. Natural Resource Modeling, 35(1), e12323.
Brander, K. M. (2007). Global fish production and climate change. Proceedings of the National Academy of Sciences, 104(50), 19709–19714.
Brown, K. H., Wuehler, S. E., & Peerson, J. M. (2001). The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food and Nutrition Bulletin, 22(2), 113–125.
Byrd, K. A., Thilsted, S. H., & Fiorella, K. J. (2020). Fish nutrient composition: A review of global data from poorly assessed inland and marine species. Public Health Nutrition, 24(3), 476–486. https://doi.org/10.1017/S1368980020003857
Byrd, K. A., Pincus, L., Pasqualino, M. M., Muzofa, F., & Cole, S. M. (2021). Dried small fish provide nutrient densities important for the first 1000 days. Maternal and Child Nutrition, 17(4), e13192.
Cleaver, K.M. (2006). Aquaculture: Changing the face of the waters meeting the promise and challenge of sustainable aquaculture. International Bank for Reconstruction and Development/The World Bank
Costello, C., Ovando, D., Hilborn, R., Gaines, S. D., Deschenes, O., & Lester, S. E. (2012). Status and solutions for the world’s unassessed fisheries. Science, 338(6106), 517–520.
Cuadrado-Soto, E., López-Sobaler, A. M., Jiménez-Ortega, A. I., et al. (2020). Usual dietary intake, nutritional adequacy and food sources of calcium, phosphorus, magnesium and vitamin D of Spanish children aged one to< 10 years. Findings from the EsNuPI Study Nutrients, 12(6), 1787.
de Mello, P. H., Araujo, B. C., Marques, V. H., et al. (2022). Long-Chain Polyunsaturated Fatty Acids n− 3 (n− 3 LC-PUFA) as Phospholipids or Triglycerides Influence on Epinephelus marginatus Juvenile Fatty Acid Profile and Liver Morphophysiology. Animals, 12(8), 951.
Deshpande, J. D., Joshi, M. M., & Giri, P. A. (2013). Zinc: The trace element of major importance in human nutrition and health. International Journal of Medical Science and Public Health, 2(1), 1–6. https://doi.org/10.5455/ijmsph.2013.2.1-6
Diana, J. S. (2009). Aquaculture production and biodiversity conservation. BioScience, 59(1), 27–38.
Dong, X., Wang, J., Ji, P., et al. (2020). Seawater culture increases omega-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFA) levels in Japanese sea bass (Lateolabrax japonicus), probably by upregulating Elovl5. Animals, 10(9), 1681.
Du, S., Neiman, A., Batis, C., et al. (2014). Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. The American Journal of Clinical Nutrition, 99(2), 334–343.
Egun NK, Oboh IP (2022) Potential contribution of captured fishes to the recommended nutrient intakes (RNIs): A case study of commercial fish species from Ikpoba reservoir, Edo State, Nigeria. Measurement: Food https://doi.org/10.1016/j.mea.food.2021.100014
Eti, M., Zakir, H., Quadir, Q., & Rahman, M. (2019). Protein and mineral contents in some fish species available in the Brahmaputra river of Bangladesh. European Journal of Nutrition and Food Safety, 11(1), 14–27.
FAO (2021) Shangai Declaration: Aquaculture for food and sustinable development. In https://aquaculture2020.org/uploads/shanghaideclaration Accessed 24 May 2022
FAO/WHO (2005) Vitamin and mineral requirements in human nutrition. 2nd Ed.
Fawole, O., Ogundiran, M., Ayandiran, T., & Olagunju, O. (2007). Proximate and mineral composition in some selected fresh water fishes in Nigeria. Internet Journal of Food Safety, 9, 52–55.
Fiedler, J. L., Lividini, K., Drummond, E., & Thilsted, S. H. (2016). Strengthening the contribution of aquaculture to food and nutrition security: The potential of a vitamin A-rich, small fish in Bangladesh. Aquaculture, 452, 291–303.
SADC FISHERIES (2016). SADC fisheries fact sheet. In SADC. https://www.sadc.int/files/9814/8724/5613/SADC_Fisheries_Fact_Sheet_Vol.1_No._1 Accessed 15 Apr 2022
Gibson, R.S. (2011). Strategies for preventing multi-micro nutrient deficiencies: a review of experiences with food-based approaches in developing countries. Combating micronutrient deficiencies: Food-based approaches pp. 7–27
Golden, C. D., Allison, E. H., Cheung, W. W., et al. (2016). Nutrition: Fall in fish catch threatens human health. Nature, 534(7607), 317–320. https://doi.org/10.1038/534317a
Grillo, A., Salvi, L., Coruzzi, P., Salvi, P., & Parati, G. (2019). Sodium intake and hypertension. Nutrients, 11(9), 1970.
Hansen, A. L., Dahl, L., Olson, G., et al. (2014). Fish consumption, sleep, daily functioning, and heart rate variability. Journal of Clinical Sleep Medicine, 10(5), 567–575. https://doi.org/10.5664/jcsm.3714
Hicks, C. C., Cohen, P. J., Graham, N. A., et al. (2019). Harnessing global fisheries to tackle micronutrient deficiencies. Nature, 574(7776), 95–98. https://doi.org/10.1038/s41586-019-
HLPE (2014). Sustainable fisheries and aquaculture for food security and nutrition. Committee on World Food Security, Food and Agriculture Organization of the United Nations.
Islam, M. R., Yeasmin, M., Sadia, S., Ali, M. S., Haque, A. R., & Roy, V. C. (2023). Small indigenous Fish: A potential source of valuable nutrients in the context of Bangladesh. Hydrobiology, 2(1), 212–234.
Jahan, S., Habib, A. H. M. S., Islam, S., Hasan, M. K., Begum, M., & Bardhan, S. (2021). Comparative Study of proximate and mineral composition of native and hybrid pangas(Pangasius Pangangasius, P.Hypophthalmus) at raw and fried stages. Journal of Asiatic Society of Bangladesh, Science, 47(1), 13–22. https://doi.org/10.3329/jasbsN47il.54183
Jaziri, A. A., Shapawi, R., Mokhtar, R. A. M., Noordin, W. N. M., & Huda, N. (2021). Chemical composition of lizardfish surimi by-product: Focus on macro and micro-minerals contents. Current Research in Nutrition and Food Science Journal, 9(1), 52–61.
Jim, F., Garamumhango, P., Musara, C. (2017). Comparative analysis of nutritional value of Oreochromis niloticus (Linnaeus), Nile tilapia, meat from three different ecosystems. Journal of Food Quality 2017
Kawarazuka, N., & Bene, C. (2011). The potential role of small fish species in improving micronutrient deficiences in developing countries: Building evidence. Public Health Nutrition, 14(11), 1927–1938. https://doi.org/10.1017/S1368980011000814
Kawarazuka, N. (2010). The contribution of fish intake, aquaculture, and small-scale fisheries to improving nutrition: A literature review. WorldFsh Center Working Paper No. 2106, Penang, Malaysia:WorldFish Center, 2010.
Kiczorowska, B., Samolińska, W., Grela, E. R., & Bik-Małodzińska, M. (2019). Nutrient and mineral profile of chosen fresh and smoked fish. Nutrients, 11(7), 1448. https://doi.org/10.3390/nu11071448
Kolding, J., Bills, I. R., Mosepele, K., Mmopelwa, T., & Nengu, S. (2003). Fisheries and Fish Biodiversity in the Okavango delta, Botswana; A rapid assessment. AquaRAP II: Rapid Assessment of the Aquatic Ecosystems of the Okavango Delta, Botswana, pp. 30-46.
Kolding, J., van Zwieten, P. A., Marttin, F., Funge-Smith, S., & Poulain, F. (2019). Freshwater small pelagic fish and fisheries in the main African great lakes and reservoirs in relation to food security and nutrition.
Kongsbak, K., Thilsted, S. H., & Wahed, M. A. (2008). Effect of consumption of the nutrient-dense, freshwater small fish Amblypharyngodon mola on biochemical indicators of vitamin A status in Bangladeshi children: A randomised, controlled study of efficacy. British Journal of Nutrition, 99(3), 581–597.
Lartey, A., Manu, A., Brown, K. H., Peerson, J. M., & Dewey, K. G. (1999). A randomized, community-based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. The American Journal of Clinical Nutrition, 70(3), 391–404.
Lauria, V., Das, I., Hazra, S., et al. (2018). Importance of fisheries for food security across three climate change vulnerable deltas. Science of the Total Environment, 640, 1566–1577.
Lynch, A. J., Cooke, S. J., Deines, A. M., et al. (2016). The social, economic, and environmental importance of inland fish and fisheries. Environmental Reviews, 24(2), 115–121.
MaI, Y., & MaE, A. (2003). Estimation of fish proximate composition starting from water content. Journal of Food Composition and Analysis, 16(1), 81–92. https://doi.org/10.1016/S0889-1575(02)00168-0
Mbaiwa, J. E. (2015). Ecotourism in Botswana: 30 years later. Journal of Ecotourism, 14(2–3), 204–222. https://doi.org/10.1080/14724049.2015.1071378
McCarthy, T., Ellery, W., & Ellery, K. (1993). Vegetation-induced, subsurface precipitation of carbonate as an aggradational process in the permanent swamps of the Okavango (delta) fan. Botswana. Chemical Geology, 107(1–2), 111–131.
Mendivil, C. O. (2021). Fish consumption: A review of its effects on metabolic and hormonal health. Nutrition and Metabolic Insights, 14, 11786388211022378.
Merron, G. S., & Bruton, M. N. (1995). Community ecology and conservation of the fishes of the Okavango Delta. Botswana. Environmental Biology of Fishes, 43(2), 109–119.
Milián-Sorribes, M. C., Tomás-Vidal, A., Peñaranda, D. S., et al. (2021). Estimation of phosphorus and nitrogen waste in rainbow trout (Oncorhynchus mykiss, Walbaum, 1792) diets including different inorganic phosphorus sources. Animals, 11(6), 1700.
Mmopelwa, G., Mosepele, K., Mosepele, B., Moleele, N., & Ngwenya, B. (2009). Environmental variability and the fishery dynamics of the Okavango Delta, Botswana: The case of subsistence fishing. African Journal of Ecology, 47, 119–127.
Mogobe, O., Mosepele, K., & Masamba, W. R. L. (2015). Essential mineral content of common fish species in Chanoga, Okavango Delta Botswana. African Journal of Food Science, 9(9), 480–486. https://doi.org/10.5897/AJFS2015.1307
Mogobe, O., Mosimanyana, E., Masamba, W. R. L., & Mosepele, K. (2018). Monitoring of water quality of the upper Okavango Delta. Bioivesity & Ecology, 6, 106–111.
Mohanty, B. P., Karunakaran, D., Mahanty, A., et al. (2015). Database on nutritional composition of food fishes from India. Current Science, 109(11), 1915–1917.
Mohanty, B. P., Mahanty, A., Ganguly, S., Mitra, T., Karunakaran, D., & Anandan, R. (2019). Nutritional composition of food fishes and their importance in providing food and nutrition security. Food Chemistry, 293, 561–570. https://doi.org/10.1016/j.foodchem.2017.11.039
Molden, D., De Fraiture, C. (2004). Investing in water for food, ecosystems and livelihoods. Stockholm: International Water Management Institute (Discussion Draft, Blue Paper)
Mosepele, K. (2000). Preliminary length based stock assessment of the main exploited stocks of the Okavango Delta fishery Bergen: Department of fisheries and Marine Biology. University of Bergen.
Mosepele, K. (2016). Dynamics of the seasonal floodplain fishery of the Okavango Delta, Botswana. Water Resource Management, 2016, 836–924. https://doi.org/10.2316/P.2016.836-024
Mosepele, K., Kolding, J., Bokhutlo, T., Mosepele, B. Q., & Molefe, M. (2022). The Okavango Delta: Fisheries in a fluctuating floodplain system. Frontiers in Environmental Science, 10, 854835.
Mosepele, K., Mmopelwa, T., Mosepele, B. (2003). Characterization and monitoring of the Okavango Delta artisanal fishery Environmental Monitoring of Tropical and Subtropical Wetlands Maun (Botswana): Harry Oppenheimer Okavango Research Center Okavango Report Series. pp 391–413
Mosepele, K. (2021). Botswana aquaculture strategy. GIZ, Gaborone
Mzengereza, K., Ishikawa, M., Koshio, S., et al. (2021). Growth performance, growth-related genes, digestibility, digestive enzyme activity, immune and stress responses of de novo Camelina meal in diets of red seabream (Pagrus major). Animals, 11(11), 3118.
Nagul, E. A., McKelvie, I. D., Worsfold, P., & Kolev, S. D. (2015). The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Analytica Chimica Acta, 890, 60–82. https://doi.org/10.1016/j.aca.2015.07.030
Nnyepi, M., Ngwenya, B., & Mosepele, K. (2007). Food (in) security and child nutrition in Ngamiland. Managing knowledge, technology and development in the era of information revolution, pp. 281–291.
Nolle, N., Genschick, S., Schwadorf, K., Hrenn, H., Bradner, S., & Biesalski, K. H. (2020). Fish as a source of (micro)nutrients to combat hidden hunger in Zambia. Food Security, 12, 1385–1406. https://doi.org/10.1007/s12571-020-01060-9
Obiero, K., Meulenbroek, P., Drexler, S., et al. (2019). The contribution of fish to food and nutrition security in Eastern Africa: Emerging trends and future outlooks. Sustainability, 11(6), 1636.
Patel, Y., & Joseph, J. (2020). Sodium intake and heart failure. International Journal of Molecular Sciences, 21(24), 9474.
Paul, B. N., Bhowmick, S., Chanda, S., Sridhar, N., & Giri, S. S. (2018). Nutrient profile of five freshwater fish species. SAARC Journal of Agriculture, 16(2), 25–41. https://doi.org/10.3329/sja.vl6i2.40256
Pirestani, S., Ali Sahari, M., Barzegar, M., & Seyfabadi, S. J. (2009). Chemical compositions and minerals of some commercially important fish species from the South Caspian Sea. International Food Research Journal, 16(1), 39–44.
Prasad, A. S. (2013). Discovery of human zinc deficiency: Its impact on human health and disease. Advances in Nutrition, 4(2), 176–190.
Ramberg, L., Hancock, P., Lindholm, M., et al. (2006). Species diversity of the Okavango delta Botswana. Aquatic Sciences, 68(3), 310–337. https://doi.org/10.1007/s00027-006-0857-y
Reksten, A. M., Somasundaram, T., Kjellevold, M., et al. (2020a). Nutrient composition of 19 fish species from Sri Lanka and potential contribution to food and nutrition security. Journal of Food Composition and Analysis, 91, 103508.
Reksten, A. M., Somasundaram, T., Kjellevold, M., et al. (2020b). Nutrient composition of 19 fish species from Sri Lanka and potential contribution to food and nutrition security. Journal of Food Composition and Analysis. https://doi.org/10.1016/j.jfca.2020.103508
Rombenso, A., Araujo, B., & Li, E. (2022). Recent advances in fish nutrition: Insights on the nutritional implications of modern formulations. Animals, 12(13), 1705.
Roos, N., Islam, M. M., & Thilsted, S. H. (2003). Small indigenous fish species in Bangladesh: Contribution to vitamin A, calcium and iron intakes. The Journal of Nutrition, 133(11), 4021S-4026S.
Roos, N., Wahab, M. A., Chamnan, C., & Thilsted, S. H. (2007a). The role of fish in food-based strategies to combat vitamin A and mineral deficiencies in developing countries. The Journal of Nutrition, 137(4), 1106–1109. https://doi.org/10.1093/jn/137.4.1106
Roos, N., Wahab, M. A., Hossain, M. A. R., & Thilsted, S. H. (2007b). Linking human nutrition and fisheries: Incorporating micronutrient-dense, small indigenous fish species in carp polyculture production in Bangladesh. Food and Nutrition Bulletin, 28(2), S280–S293.
Schwalfenberg, G. K., & Genuis, S. J. (2017). The importance of magnesium in clinical healthcare. Scientifica. https://doi.org/10.1155/2017/4179326
Shi, Y., Zhong, L., Liu, Y., et al. (2020). Effects of dietary andrographolide levels on growth performance, antioxidant capacity, intestinal immune function and microbioma of rice field eel (Monopterus albus). Animals, 10(10), 1744.
De Silva SS, Moehl J, Satia B, Bartley D, Subasinghe R (2003) Inland fisheries and aquaculture: A synergy for sustainable food fish production. FAO Fisheries Circular 886
Simionov, I. A., Cristea, V., Petrea, S. M., Mogodan, A., Nicoara, M., Baltag, E. S., & Faggio, C. (2019). Bioconcentration of essential and nonessential elements in black sea turbot (psetta maxima maeotica linnaeus, 1758) in relation to fish gender. Journal of Marine Science and Engineering, 7(12), 466.
Steffens, W. (2006). Freshwater fish-wholesome foodstuffs. Bulgarian Journal of Agricultural Science, 12(2), 320.
Thilsted, S. H., Roos, N., & Hassan, N. (1997). The role of small indigenous fish species in food and nutrition security in Bangladesh. Naga ICLARM Quarterly, 20(3), 82–84.
Thilsted, S., (2012) The potential of nutrient-rich small fish species in aquaculture to improve human nutrition and health.
Thomas, D. H. (1996). Fisheries tenure in an African floodplain village and the implications for management. Human Ecology, 24, 287–313.
Troell, M., Naylor, R. L., Metian, M., et al. (2014). Does aquaculture add resilience to the global food system? Proceedings of the National Academy of Sciences, 111(37), 13257–13263.
Turchini, G. M., Trushenski, J. T., & Glencross, B. D. (2019). Thoughts for the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. North American Journal of Aquaculture, 81(1), 13–39.
Ullah, M. R., Rahman, M. A., Haque, M. N., Sharker, M. R., Islam, M. M., & Alam, M. A. (2022). Nutritional profiling of some selected commercially important freshwater and marine water fishes of Bangladesh. Heliyon, 8(10), e10825.
UN (2022). In Department of Economic and Social Affairs PD (Eds).
West DT, VanAs JG, VanAs LL. (2015) Surface water quality in the Okavango Delta panhandle Botswana. African Journal of Aquatic Science. 16;40(4):359–72.
WFP, WHO (2022) The state of food security and nutrition in the world 2022.
WHO. (2012a). Guideline: Potassium intake for adults and children. World Health Organisation.
WHO. (2012b). Guideline: Sodium intake for adults and children. World Health Organisation.
WHO/FAO/UNU (2007). Protein and amino acid requirements in human nutrition. World Health Organization technical report series, (935), 1.
Wilson, B. H., & Dincer, T. (1976). An introduction to the hydrology and hydrography of the Okavango Delta. In Symposium on the Okavango Delta (pp. 33-48). Botswana Soc. Gaborone Botswana.
Wird, T., (2016). Fishing for proteins: How marine fisheries impact on global food security up to 2050. A global prognosis. International WWF Centre for Marine Conservation, Hamburg, Germany
Wu, G. (2016). Dietary protein intake and human health. Food and Function, 7(3), 1251–1265.
Xu, C., Suo, Y., Wang, X., Qin, J. G., Chen, L., & Li, E. (2020). Recovery from hypersaline-stress-Induced immunity damage and intestinal-microbiota changes through dietary β-glucan supplementation in Nile tilapia (Oreochromis niloticus). Animals, 10(12), 2243.
Yang, R., Han, M., Fu, Z., et al. (2020). Immune responses of Asian seabass Lates calcarifer to dietary Glycyrrhiza uralensis. Animals, 10(9), 1629.
Acknowledgements
Appreciation for assistance goes to all field personnel of the Okavango Research Institute Monitoring Unit. Analytical work was conducted at the Okavango Research Institute Environmental Laboratory. Special thanks go to two research assistants, Mr Wakongwa Toro and Simon Bogosi who assisted with sample analysis. This project was supported by the Okavango Research Institute. The authors would like to appreciate the contribution made by reviewers in providing guidance to improve the quality of the manuscript.
Funding
Open access funding provided by University of Botswana.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by OM and all authors commented on all versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflict of interest in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mogobe, O., Mazrui, N.M., Gondwe, M.J. et al. Nutrient composition of common fish species in the Okavango Delta: potential contribution to nutrition security. Environ Dev Sustain 26, 19731–19753 (2024). https://doi.org/10.1007/s10668-023-03434-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-023-03434-3