Abstract
Textile industry, a major trade in most developing countries, generates huge quantities of dye laden wastewater. Azo dyes, which are still used widely in textile industry are very toxic in nature and need to be removed from the wastewaters in a cost-effective manner. The conventional treatment methods are energy intensive and costly, making it necessary to explore low-cost methods for developing countries. The present study explores the potential application of an indigenous alkaliphilic bacterium Nesterenkonia lacusekhoensis EMLA3 for decolorizing eleven different types of azo dyes (Methyl red, Tartrazine, Ponceau S, Reactive red 35, Evans blue, Acid red 3R, Acid red, Violet C BL, Reactive violet, Red AG and Methyl orange) at high pH of wastewater. The strain showed remarkable ability to decolorize more than 90% of these azo dyes (100 mg l−1 each) within 72 to 192 h through biodegradation in nutrient medium. The bacterium showed good growth (in terms of absorbance at 600/660 nm in the presence of the dyes indicating its tolerance toward these pollutants. The bacterium not just decolorized dye mixture at high pH in presence of indigenous microorganisms of wastewater, but also decreased Chemical Oxygen Demand (COD). It gradually lowered pH of the wastewater from initial 11.0 to 8.4 during the treatment. The microbially treated textile wastewater when tested for seed germination and growth of Vigna radiata showed no phytotoxicity. Use of this indigenous microbe thus opens new opportunities for treating dye containing wastewaters in an ecofriendly and economic manner.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Globalization has led to a sharp shift in textile and clothing industry from developed nations to developing nations in the last two decades, with more than 60% exported clothing and apparels being manufactured presently in the developing countries. Textile industry is reported to use more than half of the world’s dyes and pigments (Raman & Kamani, 2016). Fast growth of textile industries in the past has led to a big challenge of treating the enormous quantities of dye containing wastewaters discharged into the environment (Chandanshive et al., 2016). Various types of dyes not only deteriorate the esthetic value of the water but also increase the Chemical Oxygen Demand (COD), which impairs effective light penetration required by primary producers for photosynthesis and growth of plants (Lellis et al., 2019). The dyes also enter the higher trophic levels through the aquatic food chain, and due to their toxic nature, affect aquatic life and human health as well.
Dyes are composed of chromophore and auxochrome groups like amine, carboxyl, sulfonate and hydroxyl, which provide color and its intensity (Pavko, 2011). Based upon the mode of application, main dye classes are identified as acid, basic, reactive, direct, disperse, vat, mordant and sulfur (Hunger et al., 2004; Saratale et al., 2011). While in accordance with the chemical structure and presence of the type of chromophore, the dyes are classified as azo, nitro, anthraquinone and sulfur dyes (Holkar et al., 2016).
Among various types of synthetic dyes, azo dyes are a prime group that constitutes 60–70% of all the colorants used (Sudha et al., 2014), which because of their mutagenic and carcinogenic nature pose serious health hazard (Lata et al., 2017). The azo dyes may have various substituents like chloro (–Cl), methyl (–CH3), nitro (–NO2), amino (–NH2), hydroxyl (–OH) carboxyl (–COOH). Azo dyes, due to their ease of production, cost effectivity, stable nature and variety of colors, in comparison to the natural dyes are popular, and more than 3000 varieties are available (Chang et al., 2004). They find extensive use in textile, paper, leather, food, cosmetic and pharmaceutical industries (Telke et al. 2009). Though many azo dyes have been banned because of their toxicity, yet many of them are still in use in the developing countries. During the process of manufacturing and after their usage in textile industries, about 10–15% of these dyes are lost that ultimately reach the environment, which even in concentration less than 1 mg l−1 are toxic and harmful for aquatic life and ecosystems (Singh et al., 2015). It is, therefore, extremely important to address the problem associated with dye containing textile wastewater by using strategies that are effective and economical so that the treated wastewater can find application in irrigation or reuse in textile industry itself (Chandran, 2016; Sghaier et al., 2019).
Conventional physico-chemical methods used for the removal of dyes from wastewater effluent have the inherent drawbacks of being economically unfeasible (as they require more energy and chemicals), being unable to completely remove the recalcitrant azo dyes and their organic metabolites, generating a significant amount of sludge that may cause secondary pollution problems (Forgacs et al., 2004; Zhang et al., 2004). However, microbial or enzymatic decolorization and degradation is an ecofriendly, cost-competitive alternative to chemical decomposition process that could help reduce water consumption compared to physico-chemical treatment methods (Rai et al., 2005; Verma & Madamwar, 2003).
Microorganisms including bacteria, fungi (Tochawng et al., 2019) and cyanobacteria (Afreen & Fatma, 2013) have been used by various researchers for decolorizing dye containing waters, most of which remove the color by biosorption process (Brik et al., 2006; Aksu et al., 2007; Kaushik et al., 2011). The biosorption process is relatively inexpensive, easy and environment friendly, hence, more emphasis is being given to biological removal of dyes from wastewaters using dead cells of microorganisms (Robinson et al., 2001; Shah et al., 2013). However, after biosorption, safe disposal of the microorganisms loaded with the pollutant is a major limiting issue (Chaukura et al., 2016).
Using live microbial cells for bioremoval of dyes and other contaminants from wastewaters face the challenge of reduced efficiency at high pH and toxicity due to pollutants in the textile wastewaters. For optimal performance of microorganisms, pH is considered as an important parameter that significantly influences efficiency of color removal and COD reduction through microbial bioremediation (Muhammad et al., 2008). The textile wastewater is generally alkaline due to use of detergents and caustic soda in the process of dyeing (Hussain et al., 2004), and their active azo dyes are known to bind with the cotton fibers under alkaline medium (Aksu et al., 2007). Due to stable and complex nature, the azo dyes are difficult to disintegrate, and this complexity increases in the presence of high pH (Singh et al., 2015; Ameenudeen et al., 2020). Using acid for neutralizing the pH of alkaline wastewaters is not only expensive, but also not safe (Mohan et al., 2002). While textile effluents have alkaline pH, most of the reported dye decolorizing microorganisms work in neutral pH range 6–7 (Selva Kumar et al., 2013; Dwivedi et al., 2017; Sangeetha & Ajitha, 2017).
Since alkaliphilic microorganisms are likely to perform well in dye containing alkaline wastewaters, it was thought worthwhile to explore the bioremediation potential of an alkaliphilic bacterium Nesterenkonia lacusekhoensis EMLA3, that had been isolated earlier by our research group from textile wastewater and was found to decolorize Methyl red at high pH and salinity under agitation at 200 rpm (Bhattacharya et al., 2017).
The present study was therefore, aimed at assessing the tolerance of the bacterium Nesterenkonia lacusekhoensis EMLA3 to a variety of azo dyes and particularly, its potential to decolorize the dyes individually and in mixture form at high pH in nutrient medium, simulated wastewater and real textile wastewater spiked with the 11 dyes. The study also aimed at having a preliminary insight into the decolorization process by studying changes in spectral properties like peak shift or new peaks of the dyes after treatment based on UV–visible spectra. The study further examined the overall bioremediation potential of the microorganism for COD reduction and moderation of pH with little additional costs. Degradation of azo dyes using microorganism may generate intermediate organic compounds that are toxic and carcinogenic in nature (Solís et al., 2012), which makes it important to test the treated wastewater for phytoxicity, if it is to be used for irrigation. Thus, phytoxicity assay was performed after microbial treatment of the wastewater using Vigna radiata seeds.
2 Materials
2.1 The bacterial strain and mother culture
The bacterial strain Nesterenkonia lacusekhoensis EMLA3 used in the present study was isolated from highly alkaline textile effluent sample (pH 13.0) collected from a local textile industry, NCR, Delhi, India. The strain was identified using phenotypic studies and 16 sRNA sequencing from Microbial Type Culture Collection Facility (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, India (Bhattacharya et al., 2017).
Mother culture was prepared by inoculating a loopful biomass of the culture in nutrient broth medium in an Erlenmeyer flask. The nutrient medium had the composition (g l−1): Peptone 5.0, NaCl 5.0, Yeast extract 1.5, Beef extract 1.5 and pH of the medium was maintained at 11.5. The flask containing the culture medium and the inoculum was incubated at 30 °C at 200 rpm in an orbital shaker (Innova, Burswick, USA). The culture after overnight (16 h) growth (O.D.600 nm = 2.653) was used for the dye removal studies.
2.2 Dyes and stock solution
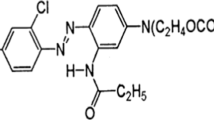
Eleven dyes used commonly in textile industry were selected, absorption maxima (λ max) of which are as follows: Methyl red, 430 nm; Tartrazine, 427 nm; Ponceau S, 520 nm; Reactive red 35, 504 nm; Evans blue, 606 nm; Acid red 3R, 506 nm; Acid red, 508 nm; Violet C BL, 547 nm; Reactive violet, 550 nm; Red AG, 498 nm; and Methyl orange, 464 nm.
Reactive violet 1 was obtained from local textile market of Ludhiana, and Acid red, Reactive red 35, Red AG, Acid red 3R and Violet C BL were procured from Khari Baoli, New Delhi, India, while Methyl red, Tartrazine, Ponceau S, Evans blue and Methyl orange were purchased from SRL, India. Analytical grade chemicals and culture media of Qualikems and Himedia, India were used, respectively.
Dye concentration in textile wastewater has been reported in the range of 10–50 mg l−1 (Karim et al., 2018) and up to 300 mg l−1 (Tony et al., 2009). Hence, dye concentration of 100 mg l−1 was selected to assess the tolerance of the bacterium for all the dyes in the study. Stock solution for each dye (1000 mg l−1) was prepared by dissolving 100 mg individual azo dye in 100 mL of distilled water, except methyl red, which was dissolved in a 1:1 (v/v) mixture of ethanol (99.9%) and distilled water. Stock solutions of the dyes were filter sterilized using sterile filter unit (0.22 micron, Himedia).
3 Methods
3.1 Bacterial growth in the presence of dyes
50 ml nutrient broth was spiked individually with the 11 azo dyes (100 mg l−1) and 2% (v/v) EMLA3 mother culture in 250 ml Erlenmeyer flasks. The flasks were incubated at room temperature (27 ± 2 °C) for 216 h. The samples were withdrawn at 24 h interval, and the growth profile of EMLA3 was studied by recording the absorbance at 600 nm using UV–Visible spectrophotometer (TechComp, Shanghai, China) to assess its tolerance to the dyes. At this wavelength, optical density of the bacterial cells is maximum based on the turbidity owing to light scattering from bacterium (Li et al., 2014). Actual absorbance due to bacterial cell growth was obtained by subtracting the readings of control (flask containing nutrient broth spiked with 100 mg l−1 of individual azo dye without bacterium) from that of experimental flasks (with the bacterium). For the initial absorbance, experimental flask with bacterium and dye containing medium was subtracted from the reading of control dye absorbance (without bacterium), to eliminate the absorbance due to presence of dye. For further readings of bacterial growth, the samples were collected at specific time intervals, and the obtained values were subtracted from that of the supernatant obtained after centrifugation of the sample. The control set-up containing only the dye and media were observed for 10 days to check if there was any degradative effect of abiotic factors (light with 9 h of photoperiod under tube light, temperature or pH) on the dye.
3.2 Individual dye removal from Nutrient broth
Preliminary testing of dye removal capability of the bacterium was done based on visual observations on color removal of individual dyes in nutrient broth solution inoculated with Nesterenkonia (Fig. 1). The flasks containing dye were tested both in static conditions and under agitation (200 rpm). Since significantly better results were obtained under stationary conditions for all the 11 dyes tested, further experiments were performed in static conditions, which also reduced the energy consumption. The capability of the microbe toward decolorization of dye(s) has been found to be dependent on their biological activity and chemical structure of the dye(s). Individual strains have been found to attack dye molecule at different positions (Prabha et al., 2017; Lellis et al., 2019). Microbes are known to behave differently with different types of dyes. For instance Brevibacillus used in a study by Alhassani et al. (2007) for Toluidine blue dye removal was more efficiently degraded under agitation as compared to static conditions. The results from a study by Chen et al. (2003) indicated that the decolorization of Red RBN dye by Aeromonas hydrophila was sensitive to the presence of oxygen. The study concluded that to achieve an effective color removal, agitation and aeration should be avoided. The same behavior was observed in present case also.
The eleven azo dyes (100 mg l−1) were added individually in different flasks, containing autoclaved nutrient broth medium (50 ml) and then inoculated with the bacterium (2% v/v). Initial pH of the medium was adjusted to 11.0 using 1 M NaOH or HCl, as per requirement. The flasks were incubated at room temperature for 216 h. Aliquots of samples were withdrawn at regular interval of 24 h followed by centrifugation at 10,000 rpm for 10 min to pellet the cell mass. The supernatants thus obtained were analyzed for color removal analysis. For determining percent decolorization of each dye, absorbance was measured at respective lambda max of individual dye. To confirm the role of the bacterial strain in the removal of azo dyes, an uninoculated flask was used as control for each azo dye.
The percentage removal of color of the azo dyes was calculated using the formula (1)
where Ai is initial absorbance, and Af is the final absorbance.
3.2.1 Spectral analysis of the degrading dyes
In order to examine whether during bacterial degradation the dyes are showing any shifts in spectral properties, the supernatants extracted at regular intervals as above were scanned at various wavelengths in the region of 200 to 700 nm using UV–Visible spectrophotometer, including both UV and visible region to understand the process of degradation, to see changes at each wavelength and to check for new peak formation, if any (Fig. 2). The range 200–700 nm was kept constant for every dye.
3.3 Bacterial treatment of simulated textile wastewater
The efficacy of the strain was also tested for treatment of simulated textile wastewater under static conditions and room temperature to test removal of the 11 azo dyes in mixture form (pH 11.0). The simulated wastewater was maintained at pH 11.0, and its composition was: NaCl 20 g l−1, COD 2448 mg l−1, Cu (II) 0.05 mM, Ni (II) 0.05 mM, Cr (VI) 0.05 mM, Azo dye mixture (each dye 10 mg l−1) (Bhattacharya et al., 2017). Chemical oxygen demand (COD) of the simulated wastewater was determined using standard refluxometric titration method (APHA 1998).
The simulated textile wastewater (50 ml) amended with azo dye mixture was inoculated with 50 ml mother culture pellet i.e., the pellet obtained by centrifugation of 50 ml of mother culture of the bacterium, followed by incubation at room temperature and static condition for 216 h. Samples were withdrawn after every 24 h for estimation of color and COD removal, during the treatment process of mixture of azo dyes with the bacterium. The change in pH during the treatment was also observed after every 24 h for 9 days using a pH meter (Decibel, Avadh Enterprises, India).
3.4 Dye decolorization studies in real textile wastewater
The mixture of the 11 azo dyes (10 mg l−1 each) was added to crude real textile wastewater (50 ml, pH 11.0) taken from the final discharge of textile mill and inoculated with 50 ml mother culture pellet of the bacterium. The reaction mixture was incubated at room temperature in static condition for 9 days. Treated samples of effluent were withdrawn at 24 h interval and analyzed for dye degradation and COD reduction.
3.5 Phytotoxicity testing of dye laden wastewater following bacterial treatment
To assess the toxicity of azo dyes laden simulated and textile wastewater and change in toxicity after bacterial treatment, phytotoxicity assay was done with overnight soaked seeds of Vigna radiata as done by Ameenudeen et al. (2020). Germination experiments were performed in triplicates using 30 seeds of Vigna placed in each pre-sterilized Petri plate at room temperature (27 ± 2 °C), three of which were irrigated with 10 ml of distilled water, three with untreated dye mixture and three each with treated dye mixture in simulated or real textile wastewater. Growth of germinating seeds was recorded by measuring the length of roots and shoots every 24 h for 3 days. Comparisons of seed germination and seedling growth in distilled water, untreated wastewater and the microbe-treated wastewater were done to assess phytotoxicity of the dyes before and after degradation.
4 Results and discussion
4.1 Bacterial tolerance to the dyes
Effective application of the bacterium in bioremediation of the dye depends largely on the tolerance of the strain to the toxic dyes. Hence, growth response of the bacterium in the presence of each of the eleven azo dyes was studied. Interestingly, the cells of Nesterenkonia lacusekhoensis continued to grow in the presence of these dyes (100 mg l−l concentration), as may be seen in Fig. 3. Bacterial growth was measured based on absorbance of the cells at 600 nm (Li et al., 2014). Increase in absorbance indicated increased cell growth of the bacterium with time. Such increase was observed in the presence of all the azo dyes, and was maximum in the presence of Methyl orange, followed by that of Red AG, Acid red 3R, Reactive violet and Ponceau S indicating superior tolerance of the bacterium to these dyes. Only in the case of Evans blue, bacterial growth declined after 120 h. Though dyes are reported to be toxic to most of the organisms, however, some indigenous bacteria like Bacillus lentus B1337 (Oturkar et al., 2011), Halomonas sp. (Taran & Furoedin, 2013), Enterobacter sp. (Nasrin et al., 2019) and Klebsiella sp. (Xie et al. 2016) have demonstrated tolerance to some azo dyes like reactive red dyes, remazol black, red 3B and reactive black 5. Since the present bacterial strain was isolated from a textile effluent, it seems to have got acclimatized to different types of the dyes. Most commonly discharged dyes include the reactive, acidic, basic and anthraquinone dyes. The wastewater from which the bacterium had been isolated had pH 13.0; salinity: 365 mg l−1; COD: 850 mg l−1; Cu: 0.35 mg l−1; Fe: 2.0 mg l−1 (Bhattacharya et al., 2017), that seem to have acclimatized the microbial strain to the pollutants.
4.2 Microbial decolorization of azo dyes
4.2.1 Preliminary studies with static vs agitated cultures
Dye decolorization has been known to occur under strictly anaerobic, facultative anaerobic and aerobic conditions by different groups of bacteria. It has been noted that dye decolorizartion is much superior under anaerobic conditions, along with its occurrence under semi-anaerobic conditions.
Preliminary visual observations (Fig. 4) on decolorization of the eleven azo dyes (Acid red, Acid red 3R, Methyl red, Ponceau S, Tartrazine, Methyl orange, Evans Blue, Reactive red 35, Reactive violet 1, Violet C BL and Red AG) inoculated with alkaliphilic Nesterenkonia showed distinct color removal when kept in stationary conditions and high pH (11.0), whereas results were not positive for all the tested dyes when placed on rotary shaker. This could be due to competition between oxygen and azo dye for the reduced electron carriers under aerobic condition (Kalme et al., 2006; Saratale et al., 2010), leading to low decolorization during shaking.
No change in color of the uninoculated set of flasks containing individual azo dyes was observed in any of the cases indicating that the inoculated bacterial strain was involved in removal of color. These preliminary studies established that it would be possible to grow Nesterenkonia in the presence of all these azo dyes for decolorizing them at alkaline pH and under static conditions. Since the bacterial biomass remained colorless after the treatment, it indicated that dye decolorization was due to biodegradation rather than biosorption.
4.2.2 Dye decolorization from culture medium spiked with individual azo dyes
As absorbance of the individual dye solutions at their respective λ max were studied over a period of time after treatment with Nesterenkonia, a decline in absorbance of all the dyes at their respective λ max was observed indicating their decolorization by the bacterium under static condition. Dye decolorization by the bacterium was more than 90% in case of all the dyes tested, and this was generally achieved between 72 and 192 h of treatment. Reactive violet showed 100% color removal in 120 h (Fig. 5). In Reactive red 35 and Evans blue, maximum of 91–95% color removal was observed, which was, however attained within a much shorter period of 72–96 h.
General bacterial decolorization of azo dyes includes reductive cleavage of the azo bond under static/anaerobic conditions in the presence of azoreductase enzyme. This process involves transfer of electrons to azo dye, which acts as final electron acceptor. This leads to the appearance of colorless solution (Chang et al., 2000). Dye degradation under anaerobic conditions is thought to be relatively simple and non-specific process. Since anaerobic degradation has been observed in the current case, there is likely the role of azoreductase enzyme, which has the capability catalyze reductive cleavage of azo bonds (Chang et al., 2001).
Under anaerobic conditions, it is also reported that movement of azo dyes through biological membrane of the cell acts as a rate-limiting factor for dye decolorization (Kodam et al., 2005).
4.2.3 Shift in spectral properties of the biodegrading dyes
In order to examine whether during the decolorization by Nesterenkonia, there is any shift in spectral properties of the dyes, spectral scanning of the supernatants in the range 200–700 nm, using UV–Visible spectrophotometer was done at regular time intervals after removing the bacterial pellet, as shown in Figs. 5 and 6. As may be seen, the absorbance of the dyes at original λ max declined with time, in all the cases.
In case of Acid Red (Fig. 5), absorption values declined with time at the λmax (508 nm) indicating decolorization of the dye by the bacterium. A small new peak appeared at 322 nm at 72 h, and subsequently, another new peak appeared in the UV region (286 nm) at 96 h indicating production of new degradation metabolites that continue to show hypochromic shift (decreased absorbance at the respective λmax). However, the new peak declined with time showing that the new metabolite formed also got degraded (Fig. 5). The UV region was taken into account to check if there is generation of new peak with degradation as there is possibility of formation of new peaks with degradation of parent molecule.
Likewise, in case of all the other dyes, the bacterial treatment resulted in hypochromic shift of the dye that was gradual (up to 48–72 h) in some dyes like Violet C BL, Methyl red, Red AG and Tartrazine (Fig. 6), whereas in case of Methyl orange, it continued upto168h. Thereafter, absorbance at λmax diminished sharply indicating near complete color removal. Acid red and Evans blue, however, showed slow hypochromicity in the initial 24 h, followed by a sharp decline in absorbance at respective λmax of these dyes.
The color of all the eleven azo dyes was removed by more than 90%, but the rate of decolorization by the bacterium was found to be dye dependent. The degradation percentage of dyes depends on their complex aromatic structures, as they are resistant to light, biological activities and other degradative environmental factors (Kaushik & Malik, 2009). There is a variation in the microorganism’s dye degradation potential for each dye, as the dyes are molecularly different and variety of decolorization mechanisms apply on different types of dyes, significantly affecting decolorization rate (Park et al., 2007; Wilkolazka et al., 2002). Study by Nagai et al., (2002), showed that extracellular dye decolorization by Aspergillus niger ES-5 failed for four isolan dyes, due to their complex structure interfering with the active site of the enzyme. On the contrary, the presence of electron-withdrawing group present in the dye could elevate the redox potential of dye molecule, making it susceptible for enzymatic oxidation (Dias et al., 2003). Due to diverse structures of dyes, which include presence of different functional groups, decolorization capability varies (Hseuh & Chen, 2008). Azo dyes containing electron-releasing groups like amino group around the azo bond are said to be more vulnerable to color removal, than those with electron-withdrawing groups like sulpho groups (Cui et al., 2014).
Nesterenkonia decolorized all the eleven azo dyes, though varying in magnitude, which could be due to dye structure, variations in saturation of the cells with individual dye products, blockage of the active sites of microbial enzymes by the dye molecules or the inactivation of the transport system of the dye (Zhang et al., 2012). Methyl red was decolorized efficiently and rapidly by EMLA3, which may be due to the presence of amino group (Singh et al., 2015).
However, no definite association between chemical structure of the dyes and percent degradation could be drawn in the present case. Though Evans blue and Tartrazine, which had sulphonic groups took relatively more time to degrade, but some azo dyes that had sulphonic groups degraded fast in the presence of the microorganism.
Among the selected dyes, Methyl red consists of amino group, whereas Tartrazine, Ponceau S and Reactive red 35 show presence of sulpho groups. Others like Evans blue, Acid red, Acid red 3R, Methyl orange, Violet C BL and Reactive violet contain both the groups. In the present study, methyl red was decolorized efficiently and rapidly by EMLA3, which may be due to the presence of amino group. Though Acid red, Tartrazine, Reactive red 35 and Ponceau S possessed sulphonic group only, yet they showed fast decolorization and dyes like reactive violet and violet CBL, which had amino-sulphonic groups showed almost complete degradation. The results revealed that dye degradation was more when the bacterium entered log phase of growth from the lag phase after 24 h, indicating thereby dependence of dye decolorization capacity of the bacterial strain on the presence of increased number of cells in the active phase, for which tolerance of the bacterial strain to the azo dyes is an important pre-requisite.
EMLA3 strain decolorized 91–100% of the different dyes: Methyl red (97.7%), Tartrazine (98%), Ponceau S (95.3%), Acid red (98.5%), Acid red 3R (94.7%), Violet C BL (96.7%), Reactive violet (100%), Red AG (91.8%) in 120 h, Methyl orange (97.6% in 192 h), Reactive red 35 (91.5% in 96 h) and Evans blue (95% in 72 h) of incubation (Figs. 5, 6).
One interesting and significant finding of the present bacterial strain was its capability to decolorize the dyes at initial pH as high as 11.0, whereas in most of the earlier studies, different azo dyes were found to show better color removal at near neutral pH and decolorization was less when pH of the medium was alkaline (Lade et al., 2015; Sangeetha & Ajeetha, 2017). Decolorization of Methyl orange was reported to be as high as 96% by Klebsiella sp. under neutral pH, while that of Tartrazine was just 37% at the same pH (Cui et al., 2014). EMLA3 strain shows distinctly superior capability of decolorizing all the 11 azo dyes at initial pH 11.0 and that too under static conditions, within a short incubation period (72–192 h). While Cui et al. (2014) reported that the presence of 4 sulphonate groups in Ponceau S and its complex structure induce resistance to its degradation by microorganisms, the present bacterial strain removes 95% of this dye within a period of 120 h. Successful removal of naphthalene sulphonated Ponceau S has also been reported by Vijaykumar et al. (2007) using Kerstersia, but that is at pH 7.0. Removal of Methyl orange and Evans blue have been reported by Zabłocka-Godlewska (2015) using some other bacteria, but only at a low pH of 6.5. Another study at neutral pH has been reported by Carolin et al. (2020) with 98% removal of methyl orange dye (100 mg l−1) at pH 7, 30 °C. Anionic azo dye Methyl orange is reported to be a stable commercial dye resistant to biodegradation (Tantiwa et al., 2013). However, high removal of Methyl red high (80%) by Bacillus circulans at pH 7.5 (Patil et al., 2016), and 70% removal of Methyl orange was reported at pH 7, which declined with rise in pH (Shah et al., 2013). On a study for dye removal of Methyl orange and Ponceau S red using Franconibacter sp. exhibited more than 90% removal at pH 7, 37 °C at 100 mg l−1 dye concentration (Baena-Baldiris et al. 2020).
However, the present strain Nesterenkonia removes 97.6% of methyl orange dye at a high pH 11.0 in 192 h of incubation.
It is also important to note that degradation of dye(s) is reported mainly at neutral pH. A study by Kurade et al. (2013) showed disperse brown 118 dye degradation (96%) by Brevibacillus laterosporus within 48 h at pH 7, 40 °C, initial dye concentration of 50 mg l−1. Under most cases, biological activities have been observed under neutral pH (Chang & Lin, 2001). This makes important to explore the potential of alkaliphilic bacteria in dye containing wastewaters.
4.2.4 Moderation of pH during dye decolorization by the bacterium
Along with dye removal, a decline was observed in pH, which fell in the range 8.4–9.0 from an initial value of 11.0 after 48 h of incubation. The decrease in pH could be attributed to release of some acids produced during growth of the bacterial strain or some acidic metabolites of dyes (Bhattacharya et al., 2017). The natural reduction in pH of the wastewater during the treatment gives added advantage to the use of Nesterenkonia lacusekhoensis as the Federal Environmental Protection Agency (FEPA) regulations and MOEF notification, (2014) for dye and dye intermediate industry, under Environment Protection Act, 1986 specify that the pH of the effluent discharge should be in the range of 6.0–8.5 for disposal in surface water, pH 5.5–9.0 for marine disposal and on land for irrigation. A study conducted by Jain et al. (2014) on biosorption of dyes using Enterococcus faecalis strain R-16 showed biological neutralization and lead to a decrease in initial pH values from 12.0 to 7.0. Very few reports are available on biological neutralization of alkaline industrial effluent using alkaliphiles, where biological processes were developed for neutralization of highly alkaline chlor-alkali, beverage and textile industry wastewater (Jain et al., 2014). Using Nesterenkonia in the bioremediation process naturally decreases the pH to a closer proximity of the prescribed standards, thus presenting a solution for major environmental problem related to high pH of discharged industrial effluents.
4.3 Bioremediation of dye containing simulated wastewater
4.3.1 Decolorization of azo dye mixture
Following successful decolorization of all the eleven sulpho-aryl and amino-aryl azo dyes taken in the present study, it was thought important to examine the capability of the microbe Nesterenkonia lacusekhoensis for treating a mixture of these azo dyes (10 mg l−leach). No other source of carbon was added to the simulated wastewater containing the dye mixture.
The decolorization experiments continued for nine days, and change in absorbance was read every 24 h (Fig. 7). An uninoculated flask containing the dye mixture in simulated wastewater was also incubated under similar conditions that served as control. Percentage dye decolorization was calculated based on the change in absorbance at λ max of the dye mixture (475 nm) during the incubation period of nine days. The bacterium was found to decolorize 91.6% of the dye mixture in 5 days, which increased up to 95.4% in 9 days. While, no change in the absorption spectra was observed in the uninoculated flask. This confirms the role of EMLA3 strain in dye degradation.
There are very few reports where some microorganism or consortia have shown the capability to decolorize such mixtures of dyes. Kumar and Krishnaswamy (2016) using moderately alkaliphilic bacterial strain Serratia marcescens reported 80% removal of dual dye mixture (direct brown MR and direct yellow SG) at pH 9 in 5 days. A mixture of four dyes (Methyl orange, Methyl red, Congo red & Tartrazine) was decolorized up to 64% using Pseudomonas and a bacterial consortium at neutral pH (Shah et al., 2013). The present strain Nesterenkonia lacusekhoensis exhibited the capability of decolorizing the mixture of 11 azo dyes of different types by more than 95% and that too at the initial high pH of 11.0.
The simulated wastewater spiked with the mixture of dyes showed peaks at 286 and 475 nm. Both the peaks falling under UV and another in visible range were monitored for spectral changes with time. Spectral scans of the wastewater after treatment with Nesterenkonia showed a hypochromic shift as decolorization proceeded with a fall in absorbance at 475 nm, but no significant change occurred in the absorbance peak at 286 nm (Fig. 7). Further, unlike the pattern of spectral scans observed for individual dyes undergoing decolorization by the bacterium, no new peaks were observed during degradation of dye mixture, indicating a modified response. The dark brown color of the simulated wastewater spiked with the dye mixture disappeared nine days after bacterial treatment leaving a light residual tinge. The bacterium interestingly decreased the pH of the simulated wastewater from an initial value of 11.0 to 8.1 during treatment, which is a desirable change conforming to discharge standards.
It is important to highlight that no external source of carbon was added in the reaction mixture to support or initiate the process of dye decolorization, whereas, majority of the microbial species are not able to use azo compounds as direct substrate and require some external substrate supply to improve the anaerobic decolorization process (Telke et al., 2009).
4.3.2 COD reduction
The initial COD of the reaction mixture was 2448 mg l−l, a value much higher than the standard discharge limits of 250 mg l−1 (CPCB 1995). COD was decreased to 1000 mg l−l in 9 days after treatment with EMLA3 under static conditions. Reduction in COD was greatly facilitated when the reaction mixture was shaken at 200 rpm for further 4 days, which decreased the COD to as low as 40 mg l−1. This may be attributed to vigorous oxidation of the reaction mixture likely to facilitate COD reduction (Joshi et al., 2004). Thus, after color removal, it is better to switch over to agitation phase in order to reduce the COD effectively for its bioremediation. Reduction of COD from the wastewater is also very important as that the efficiency of the dye removal is also influenced by high organic load (Joshi et al., 2004).
Since the bacterium Nesterenkonia showed distinct capability to use the dye mixture as a sole source of carbon (there was no other added carbon source) and could degrade the dyes even in the presence of NaCl and heavy metals like chromium, nickel and copper, which are designated as priority pollutants by USEP (Mahmoud, 2016), it would serve as an excellent microorganism to be used for bioremediation of wastewaters. To the best of our knowledge, this bacterium has only been explored for application in removal of Methyl red dye under agitation (Bhattacharya et al., 2017).
4.4 Decolorization of dye mixture and removal of COD from real textile wastewater
Since any trade effluent has more undefined composition, the dye degrading and COD removing efficacy of Nesterenkonia was also tested using real textile wastewater. The bacterium grew successfully in the dye containing real textile wastewater and decolorized the dyes. Bacterial cell growth measured at 600 nm continued up to 8 h (cell absorbance increased to 1.1079 from 0.5673) and then tended to stabilize. The bacterium effectively decolorized the dyes from the real textile effluent (Fig. 8). The wastewater spiked with the 11 azo dyes had absorbance maxima (λ max) at 475 nm, which declined after treatment with the bacterium, indicating decolorization of the dyes under static condition. In 8 h, 24% color was removed while removal improved to 66% in 24 h and up to 71–75% removal took place in subsequent hours. There was absolutely no decolorization of dyes from the dye spiked real textile wastewater in the control that was without Nesterenkonia sp., clearly showing the role played by the test bacterium in dye decolorization. Since the real textile wastewater (Characteristics: pH 10.0; Color: greenish; λmax: 606 nm; COD (Chemical oxygen demand: 2092 mg l−1) is complex in nature, some unknown interactions seem to influence the overall dye degradation process leading to little less dye removal (75%) compared to simulated wastewater (95%) after 9 days of treatment. Like that in simulated wastewater, here also the bacterium brought down the pH from initial 11.0 to 8.4 after 216 h. COD concentration of the wastewater also declined from initial 2680 mg l−1 to 440 mg l−1 by the end of 216 h when treated with the bacterium. The COD level could further be reduced to 200 mg l−l by subjecting the incubation mixture to agitation at 200 rpm for 48 h. The intermediates formed during the process of dye reduction are reduced to simple aromatic compounds due to hydroxylation and opening of the aromatic ring in the presence of oxygen (Pandey et al., 2007), supporting complete mineralization. Thus, in order to achieve effective wastewater treatment, an anaerobic process is generally followed by aerobic treatment can be sequenced to remediate dye containing wastewaters (You & Teng, 2009). Thus, in the present study, when anaerobic microbial treatment of 216 h was followed by an aerobic treatment of 48 h, the degradation seemed to be completed.
4.5 Non phytotoxic nature of the microbially treated textile wastewater
Since azo dyes are known to be toxic in nature, hence, besides removal of color by the microorganism, it is important to test the degraded residues for toxicity. The seeds of Vigna radiata failed to germinate in the untreated simulated textile wastewater (spiked with dye mixture) due to the presence of toxic contaminants. However, when the seeds were irrigated with dye laden simulated textile wastewater after treating it with Nesterenkonia, 100% germination was seen, and seedling growth also approximated that in distilled water, which served as control (Table 1). This suggests that the dye containing textile wastewater bioremediated by Nesterenkonia may be used for irrigating crop plants like Vigna radiata after trial testing. In real textile wastewater spiked with dye mixture, there was 30% more germination after treatment with the bacterium. The seeds provided with wastewater without bacterial treatment showed a little emergence of radical after 3 days, while plumule growth completely failed. Root growth, however, was quite good after bacterial treatment of the dye containing wastewater (Table 1). Vigna radiata seeds have earlier also been used to test the phytotoxicity of methylene blue, methyl red, Congo red and orange G, before and after treatment with Bacillus megaterium (Nair et al., 2017). Likewise, seed germination of Vigna radiata, which was very poor when irrigated with wastewater containing direct red 81 dye, tended to improve after being treated with Bacillus sp. DMS2 (Amin et al., 2020).
Excellent germination of Vigna radiata seeds irrigated with dye containing wastewater after treatment with Nesterenkonia may be seen in Fig. 9.
This method of treatment since being biological in nature supports the cost cut down in comparison to the conventional methods used in the treatment of such effluents. Since it does not involve agitation process during the dye removal process it cuts down the cost associated with the energy consumption. The high pH, which proposes problem in conventional systems, requiring dilution or neutralization of wastewater before treatment, adds up the cost and supports excessive water consumption increasing the cost of treatment. Along with this the amount of chemical intake in the conventional systems add on the cost of treatment. Whereas, in the case of biological treatment, the bacterial species used has fast regeneration time. The bacterium used in the study also back outs from the usage of external carbon source due to its capacity to efficiently decolorize dye mixture containing nutrient medium and textile wastewater without any extraneous carbon source; thus reducing the cost of operation.
5 Conclusions
The present study shows that the indigenous alkaliphilic isolate Nesterenkonia lacusekhoensis EMLA3 has the capability to successfully decolorize more than 90% of different types of azo dyes (including recalcitrant Methyl orange and Ponceau S), irrespective of whether they contain amino, sulphonic, aryl groups or both at the initial high pH 11.0, which is generally restrictive to microbial action. The microbe also decolorizes the mixture of these azo dyes (up to 95%) in simulated and real textile wastewater. Additionally, it reduces the COD and lowers down the pH to less than 8.4 during treatment. This eliminates the need of adding acids for neutralization, thus cutting down costs and hazards. The treated textile effluent containing degradation residues of the dye mixture was found non phytotoxic to Vigna radiata as revealed by seed germination and seedling growth, indicating that the microbe-treated wastewater is not toxic, though further studies on the degradation products of the dyes, and their toxicity, may be required before recommending their safe application in agriculture.
The use of Nesterenkonia lacusekhoensis EMLA3, leads to reduction of both environmental and public health impacts of the toxic dye containing textile wastewaters at a low-cost. Use of such bioremediation methods can be extremely important for the developing countries that cannot afford to adopt expensive conventional treatment techniques and very often, the poor community engaged in the textile and dyeing industry living in the vicinity face the brunt of toxic dye contaminants entering into the groundwater and nearby surface waters, impacting their health and the environment.
Availability of data and material
The data have been generated after laboratory experiments on the azo dyes procured from local markets.
References
Afreen, S., & Fatma, T. (2013). Laccase production and simultaneous decolorization of synthetic dyes by cyanobacteria. International Journal of Innovative Research in Science, Engineering and Technology, 2(8), 3563–3568.
Aksu, Z., Kilic, N. K., Ertuğrul, S., & Dönmez, G. (2007). Inhibitory effects of chromium (VI) and remazol black B on chromium (VI) and dyestuff removals by Trametes versicolor. Enzyme Microbial Technology, 40(5), 1167–1174. https://doi.org/10.1016/j.enzmictec.2006.08.024
Alhassani, H., Rauf, M., & Ashraf, S. (2007). Efficient microbial degradation of toluidine blue dye by Brevibacillus sp. Dyes and Pigments, 75(2), 395–400. https://doi.org/10.1016/j.dyepig.2006.06.019
Ameenudeen, S., Unnikrishnan, S., & Ramalingam, K. (2020). Statistical optimization for the efficacious degradation of reactive azo dyes using Acinetobacter baumannii JC359. Journal of Environmental Management. https://doi.org/10.1016/j.jenvman.2020.111512
Amin, S., Rastogi, R.P., Chaubey, M.G., Jain, K., Divecha, J., Desais, C., & Madamwar, D. (2020). Degradation and toxicity analysis of a reactive textile diazo dye-direct red 81 by newly isolated Bacillus sp. DMS2. Frontiers in Microbiology, 11, 2280. https://doi.org/10.3389/fmicb.2020.576680.
Baena-Baldiris, D., Montes-Robledo A., & Baldiris-Avila, R. (2020). Franconibacter sp., 1MS: A new strain in decolorization and degradation of azo dyes ponceau S red and methyl orange. ACS Omega, 5(43), 28146–28157. https://doi.org/10.1021/acsomega.0c03786.
Bhattacharya, A., Goyal, N., & Gupta, A. (2017). Degradation of azo dye methyl red by alkaliphilic, halotolerant Nesterenkonia lacusekhoensis EMLA3: Application in alkaline and salt rich dyeing effluent treatment. Extremophiles, 21(3), 479–490. https://doi.org/10.1007/s00792-017-0918-2
Brik, M., Schoeberl, P., Chamam, B., Braun, R., & Fuchs, W. (2006). Advanced treatment of textile wastewater towards reuse using a membrane bioreactor. Process Biochemistry, 41(8), 1751–1757. https://doi.org/10.1016/j.procbio.2006.03.019
Carolin, C. F., Kumar, P. S., & Joshiba, G. J. (2020). Sustainable approach to decolourize methyl orange dye from aqueous solution using novel bacterial strain and its metabolites characterization. Clean Technologies and Environmental Policy, 23(2), 1–9. https://doi.org/10.1007/s10098-020-01934-8
Chandanshive, V. V., Rane, N. R., Gholave, A. R., Patil, S. M., Jeon, B., & Govindwar, S. P. (2016). Efficient decolorization and detoxification of textile industry effluent by Salvinia molesta in lagoon treatment. Environmental Research, 150, 880–896. https://doi.org/10.1016/j.envres.2016.05.047
Chandran, D. (2016). A review of the textile industries waste water treatment methodologies. International Journal of Scientific & Engineering Research, 7(1), 392–403.
Chang, J. S., Chen, B. Y., & Lin, Y. S. (2004). Stimulation of bacterial decolorization of an azo dye by extracellular metabolites from Escherichia coli strain NO3. Bioresource Technology, 91(3), 243–248. https://doi.org/10.1016/s0960-8524(03)00196-2
Chang, J. S., Chou, Y. P., & Chen, S. Y. (2001). Decolorization of azo dyes with immobilized Pseudomonas luteola. Process Biochemistry, 36(8–9), 757–763. https://doi.org/10.1016/S0032-9592(00)00274-0
Chang, J. S., & Kuo, T. S. (2000). Kinetics of bacterial decolorization of azo dye with Escherichia coli NO3. Bioresource Technology, 75(2), 107–111. https://doi.org/10.1016/S0960-8524(00)00049-3
Chang, J. S., & Lin, Y. C. (2001). Decolorization kinetics of recombinant E.coli strain harboring azo dye decolorization determinants for Rhodococcus sp. Biotechnology Letters, 23, 631–636. https://doi.org/10.1023/A:1010306114286
Chaukura, W., Gwenzi, W., Tavengwa, N., & Manyuchi, M. M. (2016). Biosorbents for the removal of synthetic organics and emerging pollutants: Opportunities and challenges for developing countries. Environmental Development, 19, 84–89. https://doi.org/10.1016/j.envdev.2016.05.002
Chen, K. C., Wu, J. Y., Liou, D. J., & Hwang, S. C. J. (2003). Decolorization of the textile dyes by newly isolated bacterial strains. Journal of Biotechnology, 101(1), 57–68. https://doi.org/10.1016/s0168-1656(02)00303-6
CPCB. (1995). Pollution control, acts, rules and modifications issued their under Central Pollution Control Board, New Delhi.
Cui, D.G., Zhao, M., & Han, S. (2014). Decolourization of azo dyes by a newly isolated Klebsiella sp. strain Y3 and effects of various factors on biodegradation. Biotechnology, Biotechnological Equipment, 28(3), 478–486. https://doi.org/10.1080/13102818.2014.926053.
Dias, A. A., Bezerra, R. M., Lemos, P. M., & Pereira, A. N. (2003). In vivo and laccase-catalysed decolourization of xenobiotic azo dyes by a basidiomycetous fungus: Characterization of its ligninolytic system. World Journal of Microbiology and Biotechnology, 19, 969–975. https://doi.org/10.1023/B:WIBI.0000007331.94390.5c
Dwivedi, P., & Tomar, R. S. (2017). Microbial degradation and decolorisation of azo and anthraquinone textile dyes. International Journal of Pharm Bio Sciences, 8(2), 989–998. https://doi.org/10.22376/ijpbs.2017.8.2.b989-998.
Forgacs, E., Cserhati, T., & Oros, G. (2004). Removal of synthetic dyes from wastewaters: A review. Environment International, 30(7), 953–971. https://doi.org/10.1016/j.envint.2004.02.001
Holkar, C. R., Jadhav, A. J., Pinjari, D. P., Mahamuni, N. M., & Pandit, A. B. (2016). A critical review on textile wastewater treatments: Possible approaches. Journal of Environment Management, 182, 351–366. https://doi.org/10.1016/j.jenvman.2016.07.090
Hseuh, C., & Chen, B. (2008). Exploring effects of chemical structure on azo dye decolorisation characteristics by Psuedomonas luteola. Journal of Hazardous Materials, 154(1–3), 703–710. https://doi.org/10.1016/j.jhazmat.2007.10.083
Hunger, K., Gregory, P., & Miederer, P. (2004). Important chemical chromophores of dye classes. Industrial Dyes, Chemistry, Properties, Applications. https://doi.org/10.1002/3527602011.ch2
Hussain, J., Hussain, I., & Arif, M. (2004). Characterization of textile wastewater. Journal of Industrial Pollution Control, 20, 137–144.
Jain, R. M., Mody, K. H., Keshri, J., & Jha, B. (2014). Biological neutralization and biosorption of dyes of alkaline textile industry wastewater. Marine Pollution Bulletin, 84(1–2), 83–89. https://doi.org/10.1016/j.marpolbul.2014.05.033
Joshi, M., Bansal, R., & Purwar, R. (2004). Color removal from textile effluents. Indian Journal of Fibre and Textile Research, 29, 239–259.
Kalme, S. D., Parshetti, G. K., Jafhav, S. U., & Govindwar, S. P. (2006). Biodegradation of benzidine based dye direct blue-6 by Psuedomonas desmolyticum NCIM 2112. Bioresource Technology, 98(7), 1405–1410. https://doi.org/10.1016/j.biortech.2006.05.023
Karim, M. A., Dhar, K., & Hossain, M. T. (2018). Decolorization of textile reactive dyes by bacterial monoculture and consortium screened from textile dyeing effluent. Journal of Genetic Engineering and Biotechnology, 16(2), 375–380. https://doi.org/10.1016/j.jgeb.2018.02.005
Kaushik, A., Mona, S., & Kaushik, C. P. (2011). Integrating photobiological hydrogen production with metal-dye bioremoval from simulated textile wastewater. Bioresource Technology, 102(21), 9957–9964. https://doi.org/10.1016/j.biortech.2011.08.011
Kaushik, P., & Malik, A. (2009). Fungal dye decolourization: Recent advances and future potential. Environment International, 35(1), 127–141. https://doi.org/10.1016/j.envint.2008.05.010
Kodam, K. M., Soojhawon, I., Lokhande, P. D., & Gawai, K. R. (2005). Microbial decolorization of reactive azo dyes under aerobic conditions. World Journal of Microbiology and Biotechnology, 21, 367–370. https://doi.org/10.1007/s11274-004-5957-z
Kumar, R. L., & Krishnaswamy, V. G. (2016). Removal of mixed azo dyes by a moderately alkaliphilic bacterial strain isolated from textile effluent contaminated site. Journal of Microbiology and Microbial Technology, 1(1), 1–9. https://doi.org/10.13188/2474-4530.1000005
Kurade, M. B., Waghmode, T. R., Kabra, A. N., & Govindwar, S. P. (2013). Degradation of xenobiotic textile dye, disperse brown 118, by Brevibacillus laterosporus. Biotechnology Letters, 35, 1593–1598. https://doi.org/10.1007/s10529-013-1253-z
Lade, H., Kadam, A., Paul, D., & Govindwar, S. (2015). Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI Journal, 14, 158–174. https://doi.org/10.17179/excli2014-642
Lata, J., Mehta, C., Devi, N., & Chand, D. (2017). A Study on the decolourization of methyl red by Acremonium sclerotigenum. International Journal of Research in Advent Technology, 5(9), 14–22.
Lellis, B., Fávaro-Polonio, C. Z., Pamphile, J. A., & Polonio, J. S. (2019). Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnology Research and Innovation, 3(2), 275–290. https://doi.org/10.1016/j.biori.2019.09.001
Li, Z., Tang, H., Yuan, W., Song, W., Niu, Y., Yan, L., Yu, M., Dai, M., Feng, S., Wang, M., Liu, T., Jiang, P., Fan, Y., & Wang, Z. (2014). Ag nanoparticle-ZnO nanowire hybrid nanostructures as enhanced and robust antimicrobial textiles via a green chemical approach. Nanotechnology, 25(14), 145702. https://doi.org/10.1088/0957-4484/25/14/145702
Mahmoud, M. S. (2016). Decolorisation of certain reactive dye from aqueous solution using baker’s yeast (Saccharomyces cerevisiae) strain. HBRC Journal, 12(1), 88–98. https://doi.org/10.1016/j.hbrcj.2014.07.005
Mohan, S. V., Rao, N. C., Prasad, K. K., & Karthikeyan, J. (2002). Treatment of simulated reactive yellow 22 (Azo) dye effluents using Spirogyra species. Waste Management, 22(6), 575–582. https://doi.org/10.1016/S0956-053X(02)00030-2
Muhammad, A., Shafeeq, A., Butt, M. A., Rizvi, Z. H., Chughtai, M. A., & Rehman, S. (2008). Decolorisation and removal of COD and BOD from raw and biotreated textile dye bath effluent through advanced oxidation processes (AOPS). Brazilian Journal of Chemical Engineering, 25(3), 453–459. https://doi.org/10.1590/S0104-66322008000300003
Nagai, M., Sato, T., Watanabe, H., Saito, K., Kawata, M., & Enei, H. (2002). Purification and characterization of an extracellular laccase from the edible mushroom Lentinulaedodes and decolourization of chemically different dyes. Applied Microbiology and Biotechnology, 60(3), 327–335. https://doi.org/10.1007/s00253-002-1109-2
Nair, L. K., Begum, M., & Ragunathan, R. (2017). Biodegradation of azo dyes using Bacillus megaterium and its phytotoxicity study. IOSR Journal of Environmental Science, Toxicology and Food Technology, 11(7), 12–20. https://doi.org/10.9790/2402-1107011220
Nasrin, T., Saha, A., Mohanta, M., Chaity, A., Rahman, S. K., Ruhi, R., Sarker, S., & Haque, Md. (2019). Decolourization of azo dye by indigenous bacteria and its impact on seed germination. International Journal of Bioscience, 14(6), 197–210. https://doi.org/10.12692/ijb/14.6.197-210.
Oturkar, C. C., Nemade, H. N., Mulik, P. M., Patole, M. S., Hawaldar, R. R., & Gawai, K. R. (2011). Mechanistic investigation of decolorisation and degradation of reactive red 120 by Bacillus lentus B1377. Bioresource Technology, 102(2), 758–764. https://doi.org/10.1016/j.biortech.2010.08.094.
Pandey, A., Singh, P., & Iyengar, L. (2007). Bacterial decolorization and degradation of azo dyes. International Biodeterioration and Biodegradation, 59(2), 73–84. https://doi.org/10.1016/j.ibiod.2006.08.006
Park, C., Lee, M., Lee, B., Kim, S. W., Chase, H. A., Lee, J., & Kim, S. (2007). Biodegradation and biosorption for decolorization of synthetic dyes by Funaliatrogii. Biochemical Engineering Journal, 36, 59–65. https://doi.org/10.1016/j.bej.2006.06.007
Patil, N. P., Dineshkumar, B. A., Kapdnis, B. P., & Gaikwad, V. B. (2016). Biodegradation of model azo dye methyl red and other textile dyes by isolate Bacillus circulans NPP. Journal of Pure Applied Microbiology, 10(4): 2793–2800. https://doi.org/10.22207/JPAM.10.4.38.
Pavko, A. (2011). Fungal decolourization and degradation of synthetic dyes some chemical engineering aspects. Book: Waste Water-Treatment and Reutilization. https://doi.org/10.5772/16120
Prabha, S., Gogoi, A., Mazumdar, P., Ramanathan, A. L., & Kumar, M. (2017). Assessment of the impact of textile effluents on microbial diversity in Tirupur district. Tamil Nadu. Applied Water Science, 7(5), 2267–2277. https://doi.org/10.1007/s13201-016-0394-3
Rai, H., Bhattacharya, M., Singh, J., Bansal, T. K., Vats, P., & Banerjee, U. C. (2005). Removal of dyes from the effluent of textile and dyestuff manufacturing industry: A review of emerging techniques with reference to biological treatment. Critical Reviews in Environmental Science and Technology., 35(3), 219–238. https://doi.org/10.1080/10643380590917932
Raman, C. D., & Kanmani, S. (2016). Textile dye degradation using nano zero valent iron: A review. Journal of Environment Management, 177, 341–355. https://doi.org/10.1016/j.jenvman.2016.04.034
Robinson, T., McMullan, G., Marchant, R., & Nigam, P. (2001). Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77(3), 247–255. https://doi.org/10.1016/s0960-8524(00)00080-8
Sangeetha, K., & Ajitha, S. (2017). Biodegradation of textile dyes using bacteria isolated from the textile eflluent. International Multidisciplinary Innovative Research Journal, 1(2), 1–9.
Saratale, R. G., Saratale, G. D., Chang, J. S., & Govindwar, S. P. (2010). Decolorisation and biodegradation of reactive dyes and dye wastewater by a developed bacterial consortium. Biodegradation, 21(6), 999–1015. https://doi.org/10.1007/s10532-010-9360-1
Saratale, R. G., Saratale, G. D., Chang, J. S., & Govindwar, S. P. (2011). Bacterial decolorization and degradation of azo dyes: A review. Journal of the Taiwan Institute of Chemical Engineers, 42(1), 138–157. https://doi.org/10.1016/j.jtice.2010.06.006
Selvakumar, S., Manivasagan, R., & Chinnappan, K. (2013). Biodegradation and decolourization of textile wastewater using Ganoderma lucidum. 3 Biotech 3, 71–79. https://doi.org/10.1007/s13205-012-0073-5.
Sghaier, I., Guembri, M., Chouchane, H., Mosbah, A., Ouzari, H. I., Jaouani, A., Cherif, A., & Neifar, M. (2019). Recent advances in textile wastewater treatment using microbial consortia. Journal of Textile Engineering and Fashion Technology, 5(3), 134–146. https://doi.org/10.15406/jteft.2019.05.00194
Shah, M. P., Patel, K. A., & Darji, A. M. (2013). Microbial degradation and decolorization of methyl orange dye by an application of Pseudomonas Sp. ETL-1982. International Journal of Environmental Bioremediation and Biodegradation, 1(1), 26–36. https://doi.org/10.12691/ijebb-1-1-5.
Singh, R. L., Singh, P. K., & Singh, R. P. (2015). Enzymatic decolorization and degradation of azo dyes-A review. International Biodeterioration and Biodegradation, 104, 21–31. https://doi.org/10.1016/j.ibiod.2015.04.027
Solís, M., Solís, A., Pérez, H. I., Manjarrez, N., & Flores, M. (2012). Microbial decolouration of azo dyes: A review. Process Biochemistry, 47(12), 1723–1748. https://doi.org/10.1016/j.procbio.2012.08.014
Sudha, M., Saranya, A., Selvakumar, G., & Sivakumar, N. (2014). Microbial degradation of azo dyes: A review. International Journal on Current Microbiology and Applied Science, 3, 670–690.
Tantiwa, N., Seesuriyachan, P., & Kuntiya, A. (2013). Strategies to decolorize high concentrations of methyl orange using growing cells of Lactobacillus casei TISTR 1500. Bioscience Biotechnology and Biochemistry., 77(10), 2030–2037. https://doi.org/10.1271/bbb.130352
Taran, M., & Furoedin, N. (2013). Decolorisation of remazol black-B by Halomonas sp. PTCC1417 isolated from Urmia lake: Optimization by Taguchi methodology. Biological Journal of Microorganisms, 2(6), 1–10.
Telke, A. A., Kalyani, D. C., Dawkar, V. V., & Govindwar, S. P. (2009). Influence of organic and inorganic compounds on oxidoreductive decolorization of sulfonated azo dye C.I. reactive orange 16. Journal of Hazardous Materials, 172(1), 298–309. https://doi.org/10.1016/j.jhazmat.2009.07.008.
Tochhawng, L., Mishra, V. K., Passari, A. K., & Singh, B. P. (2019). Endophytic Fungi: Role in dye decolorization. Advances in Endophytic Fungal Research. https://doi.org/10.1007/978-3-030-03589-1_1
Tony, B. D., Goyal, D., & Khanna, S. (2009). Decolorization of textile azo dyes by aerobic bacterial consortium. International Biodeterioration and Biodegradation, 63, 462–469.
Verma, P., & Madamwar, D. (2003). Decolorization of synthetic dyes by a newly isolated strain of Serratia maerascens. World Journal of Microbiology and Biotechnology, 19, 615–618. https://doi.org/10.1023/A:1025115801331
Vijaykumar, M. H., Vaishampayan, P. A., Shouche, Y. S., & Karegoudar, T. B. (2007). Decolorization of napthalene-containing sulfonated azo dyes by Kerstesia sp. strain VKY1. Enzyme Microbial Technology, 40, 204–211. https://doi.org/10.1016/j.enzmictec.2006.04.001
Wilkolazka, A. J., Rdest, J. K., Malarczyk, E., Wardas, W., & Leonowicz, A. (2002). Fungi and their ability to decolourize azo and anthraquinonic dyes. Enzyme Microbial Technology, 30(4), 566–572. https://doi.org/10.1016/S0141-0229(02)00022-4
Xie, X., Liu, N., Yang, B., Yu, C., Zhang, Q., Zheng, X., Xu, L., Li, R., & Liu, J. (2016). Comparison of microbial community in hydrolysis acidification reactor depending on different structure dyes by Illumina MiSeq sequencing. International Biodeterioration & Biodegradation International Biodeterioration & Biodegradation, 111, 14–21. https://doi.org/10.1016/j.ibiod.2016.04.004.
You, S. J., & Teng, J. Y. (2009). Anaerobic decolorization bacteria for the treatment of azo dye in a sequential anaerobic and aerobic membrane bioreactor. Journal of the Taiwan Institute of Chemical Engineers, 40(5), 500–504. https://doi.org/10.1016/j.jtice.2009.01.007
Zablocka-Godlewska, E., Przytas, W., & Grabinska-Sota, E. (2015). Dye decolourisation using two Klebsiella strains. Water, Soil and Air Pollution, 226(1), 2249. https://doi.org/10.1007/s11270-014-2249-6
Zhang, Q., Jing, Y. H., Shiue, A., Chang, C., Chen, B., & Hsueh, C. (2012). Deciphering effects of chemical structure on azo dye decolorization/degradation characteristics: Bacterial vs. photocatalytic method. Journal of the Taiwan Institute of Chemical Engineers, 43(5), 760–766. https://doi.org/10.1016/j.jtice.2012.03.001.
Zhang, F., Yediler, A., Liang, X., & Kettrup, A. (2004). Effects of dye additives on the ozonation process and oxidation by-products: A comparative study using hydrolyzed CI reactive red 120. Dyes and Pigments, 60(1), 1–7.
Acknowledgements
Authors thank GGS Indraprastha University, New Delhi for research grant under Faculty Research Grant Scheme (FRGS) to AK and fellowship (STRF) to YP.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by the second and corresponding author. The methodology was set up by all the three authors, and the investigation was done by the first author.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prabhakar, Y., Gupta, A. & Kaushik, A. Using indigenous bacterial isolate Nesterenkonia lacusekhoensis for removal of azo dyes: A low-cost ecofriendly approach for bioremediation of textile wastewaters. Environ Dev Sustain 24, 5344–5367 (2022). https://doi.org/10.1007/s10668-021-01661-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-021-01661-0